Abstract
Context
Understanding the factors contributing to maintaining biodiversity is crucial to mitigate the impact of anthropogenic disturbances. Representing large proportions of green area in highly modified landscapes, residential gardens are often seen as local habitats that can contribute to larger networks of suitable environments at the landscape scale.
Objectives
We investigated the impact of the landscape context on butterfly communities observed in residential gardens, taking into account garden characteristics, land-use types and presence of linear features in the surrounding landscape. We examined how species traits affected butterflies’ response to landscape context and habitat quality.
Methods
We performed a cross-scale study, based on citizen science data documenting butterfly species composition and abundance in 920 gardens across France. We examined the effect of garden quality, the area of different land-use types and the length of linear elements measured at three scales within the surrounding landscape. Species were grouped according to their habitat preference and mobility.
Results
Urbanization negatively affected total species richness and the abundance of butterfly in each group. This was related to declining habitat quality and reduced area of suitable habitat in the surrounding landscape. The magnitude of this effect, however, was negatively correlated with mobility, a trait related to habitat preference. The spatial scale at which landscape context best explained variation in butterfly abundance changed with species’ habitat preference.
Conclusions
This study highlights the importance of preserving high quality habitats in altered landscapes and considering species’ mobility and habitat preference when assessing the impact of landscapes on butterfly communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss and alteration of natural habitats induced by land-use changes is one of the most important threats to terrestrial biodiversity (Sala et al. 2000; Rands et al. 2010; Pimm et al. 2014). While current distribution of protected areas is biased toward regions with low human pressure (Joppa and Pfaff 2009), understanding the importance of maintaining networks of suitable habitats (e.g. green veining) in human dominated landscapes has become a central theme in ecology and conservation planning (Margules and Pressey 2000; Ekroos et al. 2014). By shaping composition and configuration of natural and semi-natural habitats, human activities have a profound impact on extinction, colonization processes and species diversity. A large body of work has documented the effects of habitat loss on population persistence and diversity of plants (Dupré and Ehrlén 2002; Petit et al. 2004), mammals (Frey-Ehrenbold et al. 2013), birds (Watson et al. 2005) and insects (Hopfenmüller et al. 2014). In Europe, butterflies have strongly declined over the last decades, showing their sensitivity to global change (Van Swaay et al. 2013).
Among land-use changes, urbanization and intensive farming have been repeatedly identified to be the most detrimental to butterflies (Ekroos et al. 2010). Nevertheless, some human activities may also create new habitats that can contribute to maintain wildlife by providing refuges and resources in highly modified landscapes (Wood and Pullin 2002; Carrier and Beebee 2003; McFrederick and LeBuhn 2006; Loram et al. 2007; Öckinger et al. 2009). This is the case of residential gardens that often represent a substantial proportion of the vegetated area in otherwise sealed urban areas. Public and private gardens can provide larval host-plants and contribute to maintain continuous nectar resources throughout the season (Gaston et al. 2005; Matteson and Langellotto 2010). In France, half the population owns a garden which represents more than 1,300,000 hectares (Agreste 2014). Yet, because access to residential gardens is generally restricted, few studies have examined the contribution of such gardens to butterfly conservation. Identifying and understanding the factors that contribute to maintaining insect diversity in these highly modified environments is crucial as these landscape features might provide essential habitats that can mitigate the adverse effect of human activities on biodiversity in highly anthropogenized landscapes. Butterfly diversity has been found to be favoured by diversity of native plants in anthropogenic environments (Burghardt et al. 2009) and depends on management practices in private gardens (Muratet and Fontaine 2015). In contrast with local resources, site area and patch isolation seem to have a more limited influence on butterflies (Thomas et al. 2001; Pöyry et al. 2009).
Structure and composition of butterfly communities mainly depend on the amount of habitat in surroundings (Krauss et al. 2003a, b; Bergman et al. 2004; Flick et al. 2012) and, to a lesser extent, on habitat fragmentation and landscape features facilitating or limiting species dispersal (Bergman et al. 2004; Flick et al. 2012). Compared to local features, however, the importance of garden surroundings is unclear and it has been argued that butterfly populations in gardens can be either well or poorly connected to the wider countryside populations (Bergerot et al. 2011; Lizée et al. 2012). One explanation for these equivocal results lies in the complexity of the effect of landscape context on meta-community dynamics and butterfly dispersal. Linear features such as hedges and road verges can also play a role either as habitats or corridors connecting gardens to semi-natural habitats (Munguira and Thomas 1992; Ries et al. 2001; Saarinen et al. 2005), even if the positive effect of road verges can be hindered by mortality due to high traffic volume on highways and roads (Skórka et al. 2013). The combined effect of local and regional attributes on butterfly diversity stresses the importance of considering multiple scales when investigating the impact of landscape change on local species assemblages.
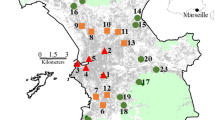
Here we perform a multi-scale study to assess the combined impact of garden quality and landscape structure on composition and abundance of butterfly communities in 920 private gardens distributed across France and four biogeographic regions (Fig. 1). We considered groups of species based on habitat preference and mobility, two traits that have been shown to influence species response to their environment (Bergman et al. 2004; Kuussaari et al. 2007). More specifically, we investigated the effect on butterfly abundance and richness of (a) garden area and resource availability and (b) amount of semi-natural habitats and urbanized area in the surrounding landscape. We expected abundance of butterflies and total species richness to increase primarily with habitat quality and, to a lesser extent, with the area of habitats and the availability of corridors in the surrounding landscape, especially the area of semi-natural habitats and the length of road verges. We also hypothesized that habitat specialists and low-dispersing butterflies would be more sensitive to local and landscape features than habitat generalists and high-dispersing species.
Material and method
Butterfly Data
Butterfly data were obtained through a nationwide butterfly monitoring scheme in private gardens called “Observatoire des Papillons des Jardins” (OPJ, obj.mnhn.fr). This citizen science program was initiated in 2006 by Vigie Nature at the French National Museum of Natural History (http://vigienature.mnhn.fr/) to gather butterfly counts in private gardens and backyards. Analyses were conducted on a total of 920 gardens distributed across France and surveyed at least once since March 2006 (Fig. 1).
The OPJ monitoring scheme protocol is based on the identification and count of butterflies from a closed list of 9 species and 19 species groups (morphospecies, see Table 1). All studied species/morphospecies are widespread across France and equally likely to be found in any region. Butterfly survey were performed between March 1st and October 31th and reported monthly. Using an online reporting system, volunteers reported the monitoring frequency (daily, weekly, or monthly) and the maximum number of individuals of each species/morphospecies observed simultaneously in the garden during that month, Monitoring frequency was then used to account for variation in sampling effort over the season. To reduce heterogeneity in count data due to variation between species and species-groups detectability, monthly abundances above 10 (0.4 % of all data) were truncated to a maximum count of 10 (Julliard et al. 2006). In our analyses, we removed Macroglossum stellatarum (the only Heterocera in the closed list) and four species (Aporia crataegi, Cacyreus marshalli, Callophrys rubi and Brintesia circe) for which phenological comparison with (expert) atlas data indicated frequent species misidentifications in the OPJ dataset (results not shown). Although identification errors may still persist in the remaining dataset, we are confident that by grouping species according to two ecological traits (habitat preference and mobility), our analyses were robust to bias related to potential misidentification as confounded species generally share the same traits. This was shown for both habitat preference (Kuussaari et al. 2007) and mobility (Stevens et al. 2013).
For a given garden, month and year, we calculated the cumulative (maximum) abundance of all species for each of the six butterfly groups considered (three habitat specialization classes, three classes of mobility). As the group of small blue lycaenids included common species preferring either forest edges (e.g. Celastrina argiolus) or grasslands (e.g. Polyommatus icarus), its abundance was used to calculate both the abundance of forest and grassland species. As a proxy of species richness, we used the total number of species/morphospecies seen in each garden at each month of observation. Habitat preference (semi-natural grasslands, field margins or forest edges) was based on the classification provided by Kuussaari et al. (2007), and their mobility (high, medium, low) on the mean dispersal distance (MDD) provided in Stevens et al. (2013). Based on species’ MDD, we defined three levels of mobility, where low mobility species have a MDD lower than 200 m, medium mobility between 200 and 500 m, and high mobility above 500 m.
Garden characteristics
For each garden, volunteers provided information about the garden area (0, 20, 100, 200, 400, 600, 800, 1000, 1500, 2000, 2500, 3000, 4000, 5000 and 6000 m) and the presence of specific garden elements (lawn, pavement, pond for instance) as well as a list of plants species known to positively influence butterflies as either nectar resources or larval host plants. Based on these local variables, we calculated an index of garden naturalness and an index quantifying the potential nectar offer. The index of garden naturalness, determined by the presence of ivy, nettle, bramble and fallow land, was calculated as the total number of these elements found in the garden. Garden nectar offer was estimated from the attractiveness coefficient of flowers for butterflies (Bergerot et al. 2010), using the sum of the coefficients of all the flowers listed in Appendix Table 1 and present in the garden.
Landscape structure and composition
To quantify the structure and the composition of the landscape surrounding each garden, we collected information from digitized land cover maps from the BD-Topo® (French National Geographical Institute 2013) [http://professionnels.ign.fr/bdtopo] and the (Registre Parcellaire Graphique 2011 (RPG), produced by Agence de Service et Paiement in compliance with the European Common Agricultural Policy legislation). Specifically, we computed the total area of grasslands, croplands and woodlands within buffers of 100, 500 and 2000 m radii around the center of each garden. Urbanization was calculated from the soil sealing raster file (pixels of 20 × 20 m) available from the European Environment Agency (EEA 2010). Soil sealing value was contained between 0 and 100, corresponding to the percentage of impervious area in the pixel. An index of urbanization was then calculated as the total urbanized area in the buffer considering all pixels with impervious area percentage above 0. Because different linear features may have contrasted effect on butterflies, we distinguished a priori favorable (trails, roads) from a priori unfavorable elements (highways) and calculated their respective cumulative length in the different radii. We excluded from the calculation (1) roads in urban areas because they rarely have vegetated verges and therefore play limited role for butterflies, and (2) trails and roads in forests because their impact on butterflies is highly dependent on canopy openness, a feature that could not be estimated with available GIS layers.
Climatic data
As our study covers large bioclimatic gradients and because butterflies species distributions are known to be strongly dependent on climatic conditions (Menéndez et al. 2007; Schweiger et al. 2014), we included in our models three variables that encompass the combined effect of geographic and climatic drivers on butterfly’s distribution across France. To avoid problems related to model over-fitting, we restricted our selection to three main variables, namely elevation (m), annual precipitation (mm), and minimal temperature (°C) of the coldest month that we extracted from the WorldClim database (Hijmans et al. 2005).
Statistical analyses
Because garden and landscape variables were highly correlated, we applied a principal components analysis (PCA) on the standardized variables to synthesize the information in reduced orthogonal dimensions for each buffer size. From the PCA, we retained the first two principal components (representing respectively 55, 49 and 48 % of the variance for 100, 500 and 2000 m scales, Table 2).
We modeled butterfly abundance and species richness with generalized linear mixed models (GLMM), using a Poisson distribution for the error term. In our models, we included “garden” as a random effect, and fixed effects were: the positions of the gardens on the two axes of the PCA, an interaction term between latitude and longitude to account for potential spatial gradient in the butterfly abundance data, an interaction term between the month and the year of the observation to account for seasonal and yearly variations in abundance, a quadratic effect of garden elevation to account for potential hump-shaped relationship (Wagner et al. 2013) and the monthly monitoring frequency to account for sampling effort. The full model fitting butterfly abundance or species richness (X iym ) in garden i for year y and month m was defined by:
where α is the intercept, β the coefficients of each explanatory variables, lat = latitude, long = longitude, y = year, m = month, vf = visit frequency, prec = precipitation, temp = temperature, elev = elevation and PCA1 and PCA2 the two axes of the PCA.
We determined the best model for each response variable by sequentially deleting explanatory variables from the complete model, starting with interactions and the least significant terms. To ease interpretation and comparison across scales, values of continuous variables (latitude, longitude, PCA1 and PCA2) were standardized and centered before model selection (Schielzeth 2010). We applied this model selection procedure for each spatial scale. All statistical analyses were computed with the software “R software 3.0.1.” (R Core Team 2013) and the package « lme4 » (Bates et al. 2013).
Results
The total dataset comprised 220,133 individual butterflies recorded during 20,640 observation events, in the 920 gardens. The number of species recorded per garden and per month ranged from 0 to 23 and the number of individuals from 0 to 184. Mobility was correlated with habitat preference (X 2 = 17.4, df = 4, p value = 0.002): grassland species were generally characterized by low mobility, while field margin species showed high mobility and forest edge species medium mobility (Appendix Table 2). Analyses were done for both habitat specialization and mobility, but to avoid redundancy, we only show the results for habitat specialization.
Spatial scales
After AIC-based model selection, six models were retained to explain how butterfly abundance and species richness relate to landscape and local variables measured at three different scales:
AIC comparison showed that the set of variables included in the best models was relatively consistent across scales, but that the spatial extent (i.e. buffer size) at which the effect of landscape structure on abundance was strongest varied between butterfly groups (Table 3). For the abundance of grassland species, the best fit was obtained within 100 m radius. This contrasted with field margin species for which the best fit was observed for 2000 m buffers. For forest-edge species, we found no clear difference between buffers of 100 and 2000 m radii. For species richness, landscape structure measured within 100 m buffers had lower explanatory power compared to measures extracted from larger buffers. Nevertheless, we could not identify an optimal radius as model fits were relatively equivalent between buffers of 500 and 2000 m radius.
Local and landscape variables
Gardens were distributed along two main environmental gradients (Table 2). The first PCA axis represented a gradient of urbanization where “high-quality” gardens characterized by large area, enhanced naturalness and high nectar offer were associated to rural landscapes and “low-quality” gardens characterized by small area, reduced naturalness and low nectar offer to urbanized areas. The second axis represented a gradient opposing landscapes characterized by large proportion of forested areas to landscapes having large areas of cropland. Linear features were either not important or strongly confounded with cropland (100 m) or forested areas (500 and 2000 m).
There was a strong negative effect of the urban gradient on grassland, forest edge and -to a lesser extent- field-margin species abundance, but also on the global species richness (Table 4 and Fig. 2). Increases in cropland area had a negative effect on the abundance of forest-edge species, but not the others. The effect of cropland area on species richness changed across scales, the effect being positive at the 500 m scale and negative at the 2000 m scale.
Discussion
Our results provides clear evidence of the influence of the surrounding landscape on butterfly species assemblages in private gardens, highlighting the negative impact of urbanization on garden butterflies, but also showing that the magnitude of this effect varies with species’ habitat preference and mobility. By contrast, the response of the butterfly species assemblages to the composition of the non-urban matrix was less systematic.
As expected, the spatial scale at which landscape factors affected butterflies abundance varied between species groups and was mainly related to species’ habitat preference and mobility. Nevertheless, we could not distinguish and measure the relative effect of habitat preference and dispersal capacity as these two factors were highly correlated, habitat generalists and specialists showing respectively high and low mobility. Mobility in butterflies is often shown to be associated with other life-history traits such as diet specialization, niche breadth or reproduction (Komonen et al. 2004), leading to what is known as a “dispersal syndrome” (Stevens et al. 2013). The correlation between habitat preference and species mobility we observed was also shown by Turlure et al. (2010) where species mobility is related to the spatial distribution of nectar plants and its overlap with larval host plants, species mobility being reduced when nectar resources are more widespread. Field margin species may have greater dispersal ability because their resources (host plants, nectar) tend to be more scattered over the agricultural landscapes and less predictable over seasons and years due to more frequent and intensive disturbances compared to grasslands or forest edges. While mobile species may benefit of the enhanced gene flow between populations (Stevens et al. 2012), greater dispersal capacity may also contribute to avoid inbreeding and competition with kin or of density-dependent processes such as competition or parasitism (Clobert et al. 2012). Conversely, dispersal is costly and associated with greater mortality risks, especially if the outcome of the dispersal event is strongly uncertain (Clobert et al. 2012). This may explain why grassland specialists tend to have lower mobility. To adequately disentangle the effect of species mobility from the influence of habitat preference, we would need to analyze a set of species that covers a wider range of mobility within each group of habitat.
Butterfly abundance was depleted by urbanization in every species groups, most likely due to reduced area of semi-natural habitats (semi-natural grasslands, forest edges and field margins), garden area and lower food and host plant resources in the garden (i.e. decrease of the garden variables). This is consistent with other studies focusing on butterfly abundance in urban areas, showing that species richness and abundance increased with vegetated area and declined with impervious areas, proportion of built area being a better predictor of butterfly diversity than the amount of vegetated area (Lizée et al. 2012). The effect of local habitat condition such as host plant density, nectar resource, vegetation condition (i.e. naturalness in our case) and site area was also found in both urban (Di Mauro et al. 2007; Williams 2011) and semi-natural landscapes (Matter and Roland 2002). Nevertheless, the literature on the effect of habitat area in the surrounding landscape on local butterfly abundance is equivocal. While Krauss et al. (2003a) and Botham et al. (2015) demonstrated a positive relationship between habitat area and butterfly abundance, others found no correlation between butterfly abundance and habitat area (e.g. Flick et al. 2012). In our study, due to the high correlation between grassland area, garden variables and urbanized area, we were unable to partial out and identify the most important factor between local habitat quality, urbanized area and other landscape variables. In fact, these factors are likely to interact and result in an overall effect on butterfly diversity and abundance along a gradient of urbanization (Di Mauro et al. 2007).
Here we showed that the strength of the effect of urban gradient varied across species groups. Thus, grassland (i.e. poorly mobile) species were more impacted than forest-edge species (i.e. moderately mobile) and field margin species (i.e. highly mobile). The spatial scales at which the models best explained species response were consistent with their dispersal capacity of each group. Grassland specialists were more influenced by the variation measured in the narrow surrounding (100 m radius), whereas the response of species with high mobility (i.e. field margin species) was better explained by variation observed within the larger landscape. The trait-dependency that we observed in species responses to landscape context was also documented other habitat and landscape types (Brückmann et al. 2010; Ekroos et al. 2013; Perović et al. 2015). While highly mobile species are more prone to move between patches than low mobility species, the difference in the effect size observed between species can also, at least partly, be explained by the deferred cost of dispersal (Stamps et al. 2005; Bonte et al. 2012). This suggests that in our study, the variation in species groups’ response to the urban gradient resulted from the combined effect of the difference in garden’s attractiveness and species’ mobility, both being strongly determined by species life history traits. In urban environments, such constraints seem to be more detrimental for specialist species than generalists.
As expected, abundance of forest species was higher in landscapes with larger proportion of forested area, suggesting that the local abundance this butterfly group is directly impacted by the amount of habitat in the surrounding landscape. Such effect, however, was not detected for grassland and field-margin species. This was surprising, considering that previous studies have shown the positive effect of habitat area on the abundance of specialist and generalist species (Steffan-Dewenter and Tscharntke 2000; Botham et al. 2015). The negative correlation observed between garden quality and grassland area measured within a 100 m buffer may partly explain the non-detection of an effect of habitat area on grassland butterflies’ abundance. Also indicating the relative importance of local habitat quality for grassland species, a factor that may shade the effect habitat area in the surrounding landscape. For field margin species, the apparent lack of effect of the habitat availability in the large surrounding (2000 m surrounding) is potentially associated with their high mobility and capacity to move between more isolated patches.
In the same way as its affected butterfly abundance, the number species was significantly lower in garden embedded in more urbanized landscape. Butterfly species richness is indeed known to be affected by urbanization (Di Mauro et al. 2007), as well as by nectar resources, host plants and habitat area (Steffan-Dewenter and Tscharntke 2000; Thomas et al. 2001; Pöyry et al. 2009). More surprising is the contrasting influence of the forested area on total species richness, being negative at 500 m and becoming positive measured at 2000 m scale. This apparent contradiction is most likely due to the difference in the covariance pattern observed between landscape and garden variables at the two different scales. While the positive effect of woodland area on total species richness has been evidenced in other studies (Shreeve and Mason 1980; Marini et al. 2009; Brückmann et al. 2010), it would be sensible, as a complement, to also assess the effect of land-use change on richness of specific functional and ecological groups. In fact, changes in habitat area is very likely to affect species in different ways with respect to their mobility, feeding niches and reproductive rates (Öckinger et al. 2010). Because of the relatively low number of species considered in our study, we could not partition the effect of land-use change on species richness between the different groups. Nevertheless, as species richness was best explained by variable measured within buffers of 500 and 2000 m, our results suggest that variation of species richness observed in residential gardens is mainly determined by species with relatively high level of mobility.
The number of citizen programs related to biodiversity monitoring and conservation is rapidly increasing worldwide, although only a fraction is being used for scientific publications (Theobald et al. 2015). Although it has been proven that such programs generally deliver information as reliable as professional monitoring (Lewandowski and Specht 2015), this may not always be true and biases such as observer effects have been reported (Williams et al. 2015). In our case, comparison of the butterfly data partly delivered by non-experts with more expert data indicated that some species were reliably detected and identified while other species were not, leading us to shorten the initial list of species. As a result, analysis of citizen science data should always be conducted with caution and we recommend systematically comparing citizen data with external references before analyzing such data. In addition, citizen programs should implement protocols asking participant to inform a number of variables related to sampling effort and local environment as they proved to be influential variables.
Conclusion
The differences in response to landscape context and local quality between species according to functional and ecological traits stress the need to consider species traits in butterfly studies. Urbanization appeared to be the most important driver of total species abundance and species richness in private gardens, by simultaneously decreasing the area and the quality of habitat. While being detrimental for all species groups, the strongest effect of urbanization was observed for grassland species, a group of butterflies that is characterized by limited mobility and that is known to be declining at an alarming rate in Europe (Van Swaay et al. 2013). Considering that urban areas are continuously growing worldwide, this threat is likely to increase in the future (Prokop et al. 2011) and calls for immediate and more integrated conservation actions in urban areas.
Using data stemming from non-expert volunteers enable us to conduct one of the first large scale study on butterfly communities in private gardens. Citizen science based monitoring programs are a unique way to generate datasets that cover large spatial extent and areas where access is usually restricted. Nevertheless, such programs come with the drawback of depending on relatively simple sampling designs that restrict the hypothesis that can be tested. As a complement, further studies should adopt sampling designs that would enable to better quantify and disentangle the effect of garden quality and urbanization. Such studies would provide a real measure of the benefit of improving local habitat quality in altered landscapes and how these actions can compensate for habitat loss induced by urbanization. Our study also stresses the need to better understand the role of private gardens in ecological processes such as food resources and egg-laying spots. Comparing residential gardens to nearby semi-natural habitats in terms of butterfly communities, biotic and abiotic conditions could help to advise concrete conservation actions in gardens to maintain and potentially increase butterfly diversity and abundance in anthropogenized landscapes.
References
Agreste (2014) La statistique, l’évaluation et la prospective agricole. http://agreste.agriculture.gouv.fr/. Accessed June 2015
Bates D, Maechler M, Bolker B (2013) lme4: linear mixed-effects models using S4 classes
Bergerot B, Fontaine B, Renard M, Cadi A, Julliard R (2010) Preferences for exotic flowers do not promote urban life in butterflies. Landsc Urban Plan 92:98–107
Bergerot B, Fontaine B, Julliard R, Baguette M (2011) Landscape variables impact the structure and composition of butterfly assemblages along an urbanization gradient. Landscape Ecol 26:83–94
Bergman K-O, Askling J, Ekberg O, Ignell H, Wahlman H, Milberg P (2004) Landscape effects on butterfly assemblages in an agricultural region. Ecography 27:619–628
Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, Lehouck V, Matthysen E, Mustin K, Saastamoinen M, Schtickzelle N, Stevens VN, Vandewoestijne S, Baguette M, Barton K, Benton TG, Chaput-Bardy A, Clobert J, Dytham C, Hovestadt T, Meier CM, Palmer SCF, Turlure C, and Travis JMJ (2012) Costs of dispersal. Biol Rev 87:290–312
Botham MS, Fernandez-Ploquin EC, Brereton T, Harrower CA, Roy DB, Heard MS (2015) Lepidoptera communities across an agricultural gradient: how important are habitat area and habitat diversity in supporting high diversity? J Insect Conserv 19:403–420
Brückmann SV, Krauss J, Steffan-Dewenter I (2010) Butterfly and plant specialists suffer from reduced connectivity in fragmented landscapes. J Appl Ecol 47:799–809
Burghardt KT, Tallamy DW, Gregory Shriver W (2009) Impact of native plants on bird and butterfly biodiversity in suburban landscapes. Conserv Biol 23:219–224
Carrier J-A, Beebee TJC (2003) Recent, substantial, and unexplained declines of the common toad Bufo bufo in lowland England. Biol Conserv 111:395–399
Clobert J, Baguette M, Benton TG, Bullock JM, Ducatez S (2012) Dispersal ecology and evolution. Oxford University Press, Oxford
Di Mauro D, Dietz T, Rockwood L (2007) Determining the effect of urbanization on generalist butterfly species diversity in butterfly gardens. Urban Ecosyst 10:427–439
Dupré C, Ehrlén J (2002) Habitat configuration, species traits and plant distributions. J Ecol 90:796–805
EEA (2010) Soil sealing data in aggregated spatial resolution (20 × 20 m). http://www.eea.europa.eu/data-and-maps/data/eea-fast-track-service-precursor-on-land-monitoring-degree-of-soil-sealing. Accessed April 2013
Ekroos J, Heliölä J, Kuussaari M (2010) Homogenization of lepidoteran communities in intensively cultivated agricultural landscapes. J Appl Ecol 47:459–467
Ekroos J, Rundlöf M, Smith HG (2013) Trait-dependent responses of flower-visiting insects to distance to semi-natural grasslands and landscape heterogeneity. Landscape Ecol 28:1283–1292
Ekroos J, Olsson O, Rundlöf M, Wätzold F, Smith HG (2014) Optimizing agri-environment schemes for biodiversity, ecosystem services or both? Biol Conserv 172:65–71
Flick T, Feagan S, Fahrig L (2012) Effects of landscape structure on butterfly species richness and abundance in agricultural landscapes in eastern Ontario, Canada. Agric Ecosyst Environ 156:123–133
Frey-Ehrenbold A, Bontadina F, Arlettaz R, Obrist MK (2013) Landscape connectivity, habitat structure and activity of bat guilds in farmland-dominated matrices. J Appl Ecol 50:252–261
Gaston KJ, Smith RM, Thompson K, Warren PH (2005) Urban domestic gardens (II): experimental tests of methods for increasing biodiversity. Biodivers Conserv 14:395–413
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hopfenmüller S, Steffan-Dewenter I, Holzschuh A (2014) Trait-specific responses of wild bee communities to landscape composition. configuration and local factors. PLoS One 9(8):e104439. doi:10.1371/journal/pone.0104439
Joppa LN, Pfaff A (2009) High and far: biases in the location of protected areas. PLoS One 4(12):e8273. doi:10.1371/journal/pone.0008273
Julliard R, Clavel J, Devictor V, Jiguet F, Couvet D (2006) Spatial segregation of specialists and generalists in bird communities. Ecol Lett 9:1237–1244
Komonen A, Grapputo A, Kaitala V, Kotiaho JS, Päivinen J (2004) The role of niche breadth, resource availability and range position on the life history of butterflies. Oikos 105:41–54
Krauss J, Steffan-Dewenter I, Tscharntke T (2003a) How does landscape context contribute to effects of habitat fragmentation on diversity and population density of butterflies? J Biogeogr 30:889–900
Krauss J, Steffan-Dewenter I, Tscharntke T (2003b) Local species immigration, extinction, and turnover of butterflies in relation to habitat area and habitat isolation. Oecologia 137:591–602
Kuussaari M, Heliölä J, Pöyry J, Saarinen K (2007) Contrasting trends of butterfly species preferring semi-natural grasslands, fields margins and forest edges in northern Europ. J Insect Conserv 11:351–366
Lewandowski E, Specht H (2015) Influence of volunteer and project characteristics on data quality of biological surveys. Conserv Biol 29(3):713–723
Lizée MH, Manel S, Mauffrey JF, Tatoni T, Deschamps-Cottin M (2012) Matrix configuration and patch isolation influences override the species–area relationship for urban butterfly communities. Landsc Ecol 27:159–169
Loram A, Tratalos J, Warren PH, Gaston KJ (2007) Urban domestic gardens (X): the extent & structure of the resource in five major cities. Landscape Ecol 22:601–615
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:243–253
Marini L, Fontana P, Battisti A, Gaston KJ (2009) Agricultural management, vegetation traits and landscape drive orthopteran and butterfly diversity in a grassland–forest mosaic: a multi-scale approach. Insect Conserv Divers 2:213–220
Matter SF, Roland J (2002) An experimental examination of the effects of habitat quality on the dispersal and local abundance of the butterfly Parnassius smintheus. Ecol Entomol 27:308–316
Matteson KC, Langellotto GA (2010) Determinates of inner city butterfly and bee species richness. Urban Ecosyst 13:333–347
McFrederick QS, LeBuhn G (2006) Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol Conserv 129:372–382
Menéndez R, González-Megías A, Collingham Y, Fox R, Roy DB, Ohlemüller R, Thomas CD (2007) Direct and indirect effects of climate and habitat factors on butterfly diversity. Ecol Soc Am 88:605–611
Munguira ML, Thomas JA (1992) Use of road verges by butterfly and burnet populations, and the effect of roads on adult dispersal and mortality. J Appl Ecol 29:316–329
Muratet A, Fontaine B (2015) Contrasting impacts of pesticides on butterflies and bumblebees in private gardens in France. Biol Conserv 182:148–154
Öckinger E, Dannestam A, Smith HG (2009) The importance of fragmentation and habitat quality of urban grasslands for butterfly diversity. Landscape Urban Plan 93:31–37
Öckinger E, Schweiger O, Crist TO, Debinski DM, Krauss J, Kuussaari M, Petersen JD, Pöyry J, Settele J, Summerville KS, Bommarco R (2010) Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol Lett 13:969–979
Perović D, Gámez-Virués S, Börschig C, Klein A-M, Krauss J, Steckel J, Rothenwohrer C, Erasmi S, Tscharntke T, Westphal C (2015) Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J Appl Ecol 52:505–513
Petit S, Griffiths L, Smart SS, Smith GM, Stuart RC, Wright SM (2004) Effects of area and isolation of woodland patches on herbaceous plant species richness across Great Britain. Landscape Ecol 19:463–471
Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Science 344:1246752
Pöyry J, Paukkunen J, Heliölä J, Kuussaari M (2009) Relative contributions of local and regional factors to species richness and total density of butterflies and moths in semi-natural grasslands. Oecologia 160:577–587
Prokop G, Jobstmann H, Schönbauer A (2011) Report on best practices for limiting soil sealing and mitigating its effects. Study contracted by the European Commission. DG Environment, Brussels
R Core Team (2013) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rands MRW, Adams WM, Bennun L, Butchart SHM, Clements A, Coomes D, Entwistle A, Hodge I, Kapos V, Scharlemann JPW, Sutherland WJ, Vira B (2010) Biodiversity conservation: challenges beyond 2010. Science 329:1298–1303
Ries L, Debinski DM, Wieland ML (2001) Conservation value of roadside prairie restoration to butterfly communities. Conserv Biol 15:401–411
Saarinen K, Valtonen A, Jantunen J, Saarnio S (2005) Butterflies and diurnal moths along road verges: does road type affect diversity and abundance? Biol Conserv 123:403–412
Sala OE, Chapin III FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, LeRoy Poff N, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Schweiger O, Harpke A, Wiemers M, Settele J (2014) CLIMBER: climatic niche characteristics of the butterflies in Europe. Zookeys 367:65–84
Shreeve TG, Mason CF (1980) The number of butterfly species in woodlands. Oecologia 45:414–418
Skórka P, Lenda M, Moroń D, Kalarus K, Tryjanowski P (2013) Factors affecting road mortality and the suitability of road verges for butterflies. Biol Conserv 159:148–157
Stamps JA, Krishnan VV, Reid ML (2005) Search costs and habitat selection by dispersers. Ecology 86:510–518
Steffan-Dewenter I, Tscharntke T (2000) Butterfly community structure in fragmented habitats. Ecol Lett 3:449–456
Stevens VM, Trochet A, Van Dyck H, Clobert J, Baguette M (2012) How is dispersal integrated in life histories: a quantitative analysis using butterflies. Ecol Lett 15:74–86
Stevens VM, Trochet A, Blanchet S, Moulherat S, Clobert J, Baguette M (2013) Dispersal syndromes and the use of life-histories to predict dispersal. Evol Appl 6:630–642
Theobald EJ, Ettinger AK, Burgess HK, DeBey LB, Schmidt NR, Froehlich HE, Wagner C, HilleRisLambers J, Tewksbury J, Harsch MA, Parrish JK (2015) Global change and local solutions: tapping the unrealized potential of citizen science for biodiversity research. Biol Conserv 181:236–244
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proceedings 268:1791–1796
Turlure C, Schtickzelle N, Baguette M (2010) Resource grain scales mobility and adult morphology in butterflies. Landscape Ecol 25:95–108
Van Swaay C, Van Strien A, Harpke A, Fontaine B, Stefanescu C, Roy D, Kühn E, Õnuao E, Regan E, Švitra G, Prokofev I, Heliölä J, Settele J, Pettersson L, Botham M, Musche M, Titeux N, Cornish N, Leopold P, Juillard R, Verovnik R, Öberg S, Popov S, Collins S, Goloschchapova S, Roth T, Brereton T, Warren M (2013) The european grassland butterfly indicator 1990–2011. European Environment Agency
Wagner K, Krauss J, Steffan-Dewenter I (2013) Butterfly diversity and historical land cover change along an altitudinal gradient. J Insect Conserv 17:1039–1046
Watson JEM, Whittaker RJ, Freudenberger D (2005) Bird community responses to habitat fragmentation: how consistent are they across landscapes. J Biogeogr 32:1353–1370
Williams MR (2011) Habitat resources, remnant vegetation condition and area determine distribution patterns and abundance of butterflies and day-flying moths in a fragmented urban landscape, south-west Western Australia. J Insect Conserv 15:37–54
Williams RL, Stafford R, Goodenough AE (2015) Biodiversity in urban gardens: assessing the accuracy of citizen science data on garden hedgehogs. Urban Ecosyst 18(3):819–833
Wood BC, Pullin AS (2002) Persistence of species in a fragmented urban landscape: the importance of dispersal ability and habitat availability for grassland butterflies. Biodivers Conserv 11:1451–1468
Acknowledgments
We thank all the volunteers who spend time recording insects in their gardens and uploading their observation data. We also thank Noe Conservation, which support and feedback the volunteer network and without whom this work would not have been possible. RS is acknowledging support from the FRB and EDF SA (FRB-CESAB project LOLA-BMS). Finally many thanks to Christie Le Coeur and Karine Princé for their support and help. This study was supported by the LEVANA project funded by the French Ministry of Environment (Program DIVA 3, Contract 12-MBGD-DIVA-5-CVS-030).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Olivier, T., Schmucki, R., Fontaine, B. et al. Butterfly assemblages in residential gardens are driven by species’ habitat preference and mobility. Landscape Ecol 31, 865–876 (2016). https://doi.org/10.1007/s10980-015-0299-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-015-0299-9