Abstract
Context
Habitat conversion for agriculture is a major driver of global biodiversity loss, partly because of homogeneity within agri-ecosystems. Anthropogenic landscapes can also increase habitat heterogeneity and primary productivity, however, augmenting regional biodiversity, as species that exploit resources associated with human activities expand their ranges into novel ecological regions.
Objectives
We used birds as a model in the Kruger to Canyons Biosphere, South Africa, to ask whether agriculture can add habitat components and bird species complementary to those already present, and whether habitat variables and bird functional traits can be used to identify bird species most likely to respond to such habitat changes.
Methods
We surveyed birds and measured habitat structure in 150 fixed-radius point counts each in natural habitat and mango orchards, and assessed relationships between habitat variables and bird functional traits.
Results
Despite mango orchards having greater vertical height structure because of tall (average 20 m) Casuarina windbreaks, they were missing the low-scrub (1–2 m woody cover) component of natural vegetation. We found that species whose life-history traits and ecological attributes were associated with structures missing from mango orchards were correspondingly absent from the orchards, which translated into the exclusion of 35 % of the bird species; bird assemblages within mango orchards were only a subset of those found in natural habitat.
Conclusions
These findings suggest that knowledge of habitat structure, along with species’ functional traits can provide a predictive framework for effects that anthropogenic habitats may have on regional biodiversity, and allow management to reduce negative effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The greatest drivers of global biodiversity loss are unsustainable management of forests and habitat loss through land conversion for agricultural purposes (Secretariat of the Convention on Biological Diversity 2010). Agriculture represents the largest use of land and occupies ~38 % of the world’s terrestrial surface (Foley et al. 2011). Agriculture impacts biodiversity through its effects on landscape ecology at both habitat (Benton et al. 2003) and landscape heterogeneity scale (Weyland et al. 2012). Landscape heterogeneity is a measure of the diversity of habitat types at a larger scale, e.g., between farms and natural areas in the landscape, whereas habitat heterogeneity, or complexity, reflects the finer scale structural diversity within different habitats (Benton et al. 2003), which increases biodiversity by providing various niches and resources (Benton et al. 2003; Tscharntke et al. 2005; Weyland et al. 2012; Bonthoux et al. 2012).
Understanding how spatial patterns influence species distributions across various scales is the principal aim in landscape ecology. Spatial heterogeneity is regarded as the central causal factor in species richness and distribution in ecological systems (Pickett and Cadenasso 1995). Many studies show that species diversity and abundance are negatively correlated with percentage of land used for intensive human activity and levels of homogeneity within agroecosystems at both local (habitat) and regional (landscape) scales (Benton et al. 2003; Bremer and Farley 2010; Jones et al. 2012; Skórka et al. 2013). Other studies, however, indicate that biodiversity can increase in response to land-use practices (Bonthoux et al. 2012), particularly if the practice introduces novel components of habitat that can be exploited (Tscharntke et al. 2005; Bremer and Farley 2010; Jones et al. 2012; Carvalheiro et al. 2012). Examples of such increases have been documented in response to habitat transformation associated with agriculture (Okes et al. 2008; Child et al. 2009); forestry (Hanberry et al. 2012); agroforestry (Wunderle Jr. and Latta 1998; Sekercioglu 2012), industrial activity (Lenda et al. 2012) and human settlements (Fairbanks et al. 2002; Fairbanks 2004; Evans et al. 2006; Hugo and Van Rensburg 2009). Species may exhibit range-shifts contrary to those expected in response to climate change through their use of human-modified components of the landscape (Hockey et al. 2011). Movement across habitats is common in many species, and the spillover of organisms from natural habitats to agroecosystems is well documented in human-dominated landscapes (Thies and Tscharntke 1999; Bianchi et al. 2006).
Regional increases in avian species richness in human-modified landscapes can occur because generalist species from other ecological regions can be attracted to transformed areas, leading to increased species richness for the region as a whole (Fairbanks et al. 2002). Others have found that while habitat transformation may lead to an increase in local diversity at intermediate levels of development (resulting in a mixed land-use mosaic), there may be a decrease in overall regional diversity (Crooks 2004). The decrease in diversity may be due to the selective extirpation of ‘‘losers’’ and selective success of ‘‘winners’’, which act to enhance large-scale biotic homogenization and to accelerate biodiversity loss (McKinney and Lockwood 1999), resulting in an increase in invasive and exotic species in disturbed anthropogenic landscapes (Blair 1996). Thus, humans may increase habitat heterogeneity through habitat transformation, but only if the transformation results in the creation of a mosaic of natural and anthropogenic habitat types where various species may coexist (Fairbanks 2004).
Bird assemblage composition, diversity and abundance, including the number and diversity of niches, are affected by habitat structure, complexity and vertical organization (MacArthur et al. 1962; Seymour and Dean 2010). Species’ biological traits and habitat variables correlate because species have evolved within the limitations of their environments (Ricklefs 1991). By modifying the landscape and changing the architecture of vegetation, humans may select for different animal life-history, ecological and behavioral traits associated with specific vegetation characteristics (Seymour and Dean 2010). Knowledge of life-history traits and ecological attributes (functional traits) should enable an assessment of how human-mediated habitat changes influence species assemblages.
Agricultural landscapes in the developing world often retain large tracts of natural habitat, as is the case in our study area in the Kruger to Canyons Biosphere of Limpopo province, South Africa (Reyers et al. 2001). Mango Mangifera indica (Anacardiaceae) and citrus are the predominant crop types in the area (Department of Horticultural Sciences 2002), existing as agricultural islands in a sea of well-preserved natural habitat (Reyers 2004; Department of Environmental Affairs and Tourism 2006).
Our study area falls within the drier Zambezian woodland region, which includes bushveld, grassveld, marshland, forest and riparian habitats, within the Southern Savanna subregion. The Zambezian woodland region (species richness of 535 species, including vagrants, migrants and aerial foragers) comprises of 50 % of the species within the subregion (1057 species) (de Klerk et al. 2002). Where habitats such as our sampling area are surrounded by other habitats there is potential for contribution of additional species in a novel habitat. The provision of tall vertical structures and the availability of water in the mango habitat during the dry winter months could contribute as novel habitat for additional species.
We conducted 150 point counts each at both natural habitat and mango orchard locations and measured aspects of habitat structure. We tested the main hypotheses (1) That mango orchards would add habitat heterogeneity at a regional scale by adding habitat components to the landscape that are complementary to those already present, and therefore that (2) Bird assemblages in mango orchards would be complementary (i.e., would contain species from other regions) to those in natural habitat areas; and (3) that the representation of bird life history traits within these assemblages would reflect habitat structure.
Methods
Study area
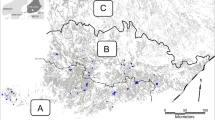
Our study site (Fig. 1) was situated within the Kruger to Canyons Biosphere near Hoedspruit, Limpopo province, South Africa (23°57′6″S, 30°51′5″E). The vegetation type is classified as Granite Lowveld (Mucina and Rutherford 2011). Granite Lowveld (hereafter referred to as “bushveld”) is characterized by dense thicket to open savanna with a woody layer comprised of Acacia nigrescens (Senegalia nigrescens), Sclerocarya birrea, Dichrostachys cinerea, Grewia bicolor, and a herbaceous layer dominated by Digitaria eriantha, Panicum maximum and Aristida congesta (Mucina and Rutherford 2011).
We conducted this research at the start of the dry season (March 2013–June 2013). During this transitional period, the natural deciduous trees have not yet lost their foliage, the mangos had already been harvested and the mango trees were in the process of being pruned. Surveying during this period allows us a snapshot of the year when natural resources are limited.
The use of agrochemicals during this time was minimal. The mango orchards featured various non-native mango cultivars planted in rows, with lines of non-native Casuarina sp. (family Casuarinaceae) acting as wind-breaks. The majority of the survey plots in mango orchards included some open ground resulting from the presence of fallen Casuarina twigs and scale leaves, with herbaceous cover dominated by non-native agricultural weeds such as Bidens pilosa, Tagetes minuta, and Tridax procumbens, which were regularly mowed to ease access between lanes (Manning 2003). Mango orchards also contained the greatest vertical structure, with the Casuarina windbreaks protruding over both the mango trees and the trees in the surrounding natural vegetation. The two habitat types are illustrated in Appendix S1. The Mean annual precipitation at Hoedspruit is 450 mm, and the winter months are classified as the dry season (Mucina and Rutherford 2011).
Study design
We identified suitable study sites from Google Earth Pro (Sullivan 2009). We identified eight study sites of differing sizes and the boundary areas were then digitized and imported into ArcGIS ArcMap v10. (Environmental Systems Research Institute 2011). All the selected mango sites were orchards with adjacent natural habitats left undisturbed or grazed by livestock, and included patches of natural vegetation unsuitable for cultivation interspersed within the orchards. We avoided riparian areas and each site was subdivided into natural (hereafter “bushveld”) and mango habitats, and overlaid a grid (200 × 200 m, Fig. 1) on each sample site in GIS to assign survey points at intersects to ensure an even distribution across habitat types and sample areas. The numbers of points per site were thus proportional to the overall size of the site (Bibby et al. 2000).
Survey methods
We surveyed birds using fixed-radius point counts (Gibbons and Gregory 2006), which allowed for comparison between open and low visibility habitats and enabled the detection of less mobile and cryptic birds, thus reducing detection differences between habitats of different densities (Bibby and Buckland 1987; Buckland et al. 1993). Additionally point counts are preferred to transects, if the identification of habitat determinants of bird communities present is an objective of the study (Bibby et al. 2000). The use of point sampling is standard for surveying habitats such as bushveld and mango orchards, where walking transects through dense vegetation and circumventing natural boundaries may not be possible as part of a survey and ensuring equal sampling effort for the same habitat area is difficult.
Each survey lasted 10 min and occurred within 3 h after sunrise on dry, low-wind (<4 km/h) days. Surveys were then repeated in the 3 h before sunset, resulting in 20 min of survey time per point. Surveys during these periods avoided disturbance by farm workers. All birds within a 100 m radius of the central point were counted either using visual or audio identification. If there was uncertainty as to whether an individual had already been included, it was ignored. Point counts are limited in their accuracy for surveying raptors and aerial foragers (swallows, swifts and bee-eaters; Bibby et al. 2000), so these were excluded from analysis.
Habitat measures
We recorded the habitat structure in a 10 m radius circle around each survey point to identify ecological associations of bird species with the various habitat components, (Bibby et al. 2000), including the average height of grass, herbaceous plants, scrub (woody plants) and trees using a graduated telescopic pole equipped with a rule. To create foliage profiles, a vertical section of the sampling area was broken into vegetation height bands and the percentage cover of each foliage type at each height-band was estimated. Scrub was classified as woody plants with an ellipsoid shape arising out of the ground and trees as ‘lollipop’ shaped (i.e. as ellipsoids on a ‘stick’) above 2 m tall.
Data analysis
Habitat heterogeneity and composition in bushveld and mango orchards
To examine habitat characteristics we converted the foliage profiles into foliage height diversity (FHD) values using the Shannon-Weiner formula,
where p i is the proportion of the total foliage which lies in the ith layer of the chosen horizontal layers (Bibby et al. 2000).
We tested for significant differences in FHD values between mango and bushveld habitats using a one-way analysis of variance (ANOVA), with ‘farm identity’ as a random effect. Additionally, we tested for significant differences between the means of percentage cover at various habitat layers using t-tests, correcting for false discovery rates using sequential Bonferroni tests. All of these analyses were conducted in the statistical interface ‘R’ (R Development Core Team 2008). We applied ANOSIM (Analysis of Similarities) using percentage cover at various habitat layers per survey point data within the program PAST (Harper and Ryan 2001). ANOSIM was used to test for similarities in layer composition between habitat types.
Species richness, abundance and composition
Mean relative bird abundance (number of individuals) and species richness (number of species) was calculated for each survey point. Average species richness and bird density per hectare, and percentage audio and visual observations was calculated for bushveld and mango habitats respectively. To evaluate the overall sampling effort, and to account for possible differences in sampling adequacy in the two structurally different habitats, expected species accumulation curves were generated using the EstimateS package(Colwell et al. 2004; Cowell 2006). Additionally, the observed species richness was compared with the Chao1 non-parametric richness estimator for the species richness and Cole’s rarefaction values, calculated in Estimates (Cowell 2006). The use of the Chao1 estimator requires information on the abundance of species and therefore in addition corrects for estimates in relative abundance (Chao 1984; Koh 2008). Generalized Linear Mixed Effects Modeling (GLMEM) was performed to assess how species richness and relative bird abundance varied across habitat types (Poisson error structure) with ‘farm identity’ as a random effect. The models were executed using the lme4 library in the statistical interface ‘R’ (R Development Core Team 2008).
In the field of landscape ecology scientists are increasingly aware of the importance of considering spatial scale effects in biodiversity studies, therefore we tested for spatial autocorrelation in the data using the Spatial Autocorrelation (Global Moran’s I) tool in ArcGIS ArcMap v10. (Environmental Systems Research Institute 2011). Where spatial autocorrelation was detected, we included a spatial correlation structure with and without a nugget, in a linear mixed-effects model fit by maximum likelihood (function lme) when fitting models to our data. Species richness…We used log (Species Richness) as the dependent variable, habitat type as the fixed effect and farm as the random effect. We included various spatial correlation structures (i.e., Gaussian, exponential, spherical and ratio) in different models and then compared model fit using AIC, with the model with the lowest AIC being chosen when ΔAIC was greater than 2. We also compared this with the AIC for the model that included a constant, only. The models were executed using the lme4 library in the statistical interface ‘R’ (R Development Core Team 2008).
ANOSIM, non-metric multidimensional scaling (MDS) and SIMPER (Similarity percentage) analyses were performed using species composition per survey point data within the program PAST (Harper and Ryan 2001). Bird species recorded in 5 % or less of the surveys were not included within these analyses as they were considered rare. ANOSIM was applied to test for similarities in species composition between bird assemblages using both abundance (Bray-Curtis method) and species presence and absence data (Jaccard method) between habitat types. Ordinations produced by MDS analysis were used to allow for a visual interpretation of species composition, and SIMPER analysis to determine which species were responsible for the patterns observed, and which species contributed the most to the dissimilarity in abundance and species richness between the habitat types, as found in ANOSIM. The SD/dissimilarity values calculated in the SIMPER analysis provide a measure of how consistently responsible species were for the clustering of habitat types. Values over 1 were considered as strong indications of consistency. Additionally, the more abundant a species was within a group, the more it contributed to the intragroup similarities, and thus characterized a group if it was found at a consistent abundance throughout (Clarke 1993).
Habitat structure and biological traits
RLQ analysis developed by Dolédec et al. (1996) is a three table ordination technique, (similar to other methods such as principal component analysis, correspondence analysis and non-metric multi-dimensional scaling) that is increasingly used in an ecological context to evaluate the link between species traits and environmental variables. Various methods have different strengths and weaknesses and RLQ analysis is suitable for our purposes (Cleary et al. 2007; Seymour and Dean 2010; Wesuls et al. 2012; Ikin et al. 2012; Kleyer et al. 2012). The analysis uses the species present and the sites at which they occur (Table L), the qualitative traits of these species (Table Q) and the habitat characteristics of each of the sites (Table R), and maximizes the covariance between them. RLQ ordination analysis was performed to assess how life-history traits and ecological attributes were represented within bird assemblages, and how their relative representation changed with different habitat features (i.e. the relationship between species traits and habitat characteristics according to habitat type; Dolédec et al. 1996). As with other ordination techniques, species that occur in sites that have similar vegetation features are located close together within the ordination space. Correspondingly, sites that contain species with similar traits are also closely located. The qualitative species data (Appendix S2 in Supporting Information) were obtained from Hockey et al. (2005).
RLQ analysis uses a principal components analysis of table R; correspondence analysis of table L and Hill-Smith principal components analysis of table Q. Thus, the total amount of variance explained by the RLQ analysis is limited by the variance explained by these individual ordinations. The product of the RLQ analyses was compared with the individual ordinations to see how the analysis explained the relationship between species traits and habitat characteristics. The significance of the relationship was tested using a Monte-Carlo permutation test based on 999 replications (Dolédec et al. 1996).
The analysis was performed using the ADE-4 package (Dray and Dufour 2007) and the ade4TkGUI graphical user interface (Thioulouse and Dray 2007) in the statistical interface ‘R’ (R Development Core Team 2008).
Results
Habitat heterogeneity and composition in bushveld and mango orchards
FHD differed significantly between bushveld and mango survey points (One-way ANOVA, F = 9.785, df = 1.7, p < 0.01). Bushveld survey points were structurally more diverse (mean FHD = 1.36, SD 0.41) than those in the mango (mean FHD = 1.15, SD 0.33; Appendix S3).
Mean percentage cover at various habitat layers differed significantly between bushveld and mango orchards (Fig. 2; all t-tests, p < 0.001). Mango orchards included the greatest vertical structure, because the mean height of the Casuarina windbreaks was 19.63 m (SD 2.45), compared to the mean height of trees above 10 m in the surrounding bushveld of 12.5 m (SD 2.53). With the exception of percentage canopy cover at heights greater than 10 m, mean percentage cover at each layer was greater in bushveld habitats.
Habitat structure differed significantly in composition between bushveld and mango orchards (ANOSIM: Global R = 0.284, p = 0.0001, Bray-Curtis method; Global R = 0.464, p = 0.0001, Jaccard method).
Bird species richness, abundance and composition
Across all 300 survey points, a total of 14,278 individuals representing 151 species were recorded (Appendix S4). The asymptotic accumulation curve indicated that sampling had been sufficient (Appendix S5).Compared to the Chao 1 estimator of ‘true’ species richness, sampling effort yielded 90 % of the ‘true’ species richness for birds in the mango orchards and 91.5 % within the bushveld habitats. For bushveld sites, the estimator stabilizes after about 110 samples have been pooled (with 155 species). When all 150 samples have been pooled (160 species detected), the mean proportion of new species detected at a survey point levelled off <0.12 species per additional sample sites (5 additional species per 40 additional survey sites). For mango sites the estimator stabilizes after about 117 samples have been pooled (with 108 species). When all 150 samples have been pooled (113 species detected), the mean proportion of new species detected at a survey point levelled off at 0.15 species per additional sample sites (5 species added over 33 survey sites; Appendix S6).Bushveld habitats had significantly more species (bushveld: 6.71, SD 0.97; mango: 3.93 SD 0.63; GLMEM, χ2 = 369.27, df = 1, p < 0.001) and greater bird abundance (bushveld 16.66, SD 2.39; mango: 13.63, SD.08; GLMEM, χ2 = 548.11, df = 1, p < 0.001) per hectare than the mango orchards (Table 1).
Species richness was spatially autocorrelated (Moran’s Index: 0.38; z-score: 10.2; p = 0), but not bird abundance (Moran’s Index: 0.03; z-score: 1.03; p = 0.30).
We found that both ratio and exponential error structures gave equally good model fits. After correcting for spatial autocorrelation, natural bushveld contained significantly more species than mango (Table 2).
After excluding the species that occurred at fewer than 5 % of survey points, the resultant data set comprised 73 species and 13,479 individual observations. Bird communities differed in composition between bushveld and mango orchards, whether or not abundance was considered (ANOSIM: Global R = 0.383, p = 0.001, Bray-Curtis abundance method; Global R = 0.461, p = 0.001, Jaccard presence/absence method).
Bushveld and mango habitats emerged as distinct in the MDS configuration (Fig. 3), but the species found in mango represented a subset of those found in the bushveld survey points. There were no species present in the mango survey points that were not present within bushveld. The relatively low stress values (0.41 and 0.43 for Bray-Curtis and Jaccard methods, respectively) for the MDS configuration indicated that the ordination was a good representation of the between-habitat similarities in two dimensions (Clarke 1993).
SIMPER analysis was used to determine which bird species made the largest contributions to the Bray-Curtis dissimilarity between mango and bushveld habitats, which equated to 75.5 % difference (Table 3). Yellow-fronted canaries (Crithagra mozambica) and dark-capped bulbuls (Pycnonotus tricolor) characterised the mango survey points, whereas white-bellied sunbirds (Cinnyris talatala) and white-browed scrub-robins (Erythropygia leucophrys) were responsible for the similarites in the bushveld survey points. Additionally, yellow-fronted canaries, dark-capped bulbuls, hadeda ibis (Bostrychia hagedash), red-eyed doves (Streptopelia semitorquata), and helmeted guineafowls (Numida meleagris) were the only species more abundant in mango than in bushveld.
Habitat structure and functional traits
There were significant relationships between species traits and habitat characteristics (RLQ analysis: Monte-Carlo permutation test, p = 0.001). A total of 81.5 % of the variance was explained by the first two axes of the RLQ analysis together. Axes one and two of the principle component analysis (R) signified 99.4 and 91.8 % of the correlation, whereas the correspondence analysis (L) represented 27.6 and 22.2 %. The Hill-Smith component analysis (Q) represented 88.5 and 85.2 % of the overall RLQ results (Table 4).
Similar positions of survey points (Fig. 4a), and habitat characteristics responsible for positioning of those survey points (Fig. 4b), species traits (Fig. 5a), and habitat characteristics responsible for species traits relative to the origin in the four plots (Fig. 5), indicated similar contributions to the RLQ axes.
a Results from correspondence analysis (L). Survey points according to species distribution. b Results from principle component analysis (R) highlighting the habitat characteristics responsible for the pattern of survey points as seen in (a). The direction and length of the arrows indicate the strength of the correlation between variables and principal components (Chessel et al. 2004)
There was distinct clustering of survey points based on species richness (correspondence analysis, Fig. 4a). Habitat characteristics (Fig. 4b) corresponded to the clustering (Fig. 4a), where percentage tree cover and percentage ground cover related to mango habitats, and percentage herbaceous cover, scrub height and percentage scrub cover related to bushveld habitats.
The habitat characteristic that most distinguished anthropogenic from natural survey points was the absence of a woody scrub (1–2 m height) layer from the habitat profile (Fig. 1). Thus, the habitat characteristics of most interest were scrub height and percentage scrub cover, which fell on the first axis (Fig. 5b). Gleaning, bark foraging and hawk-gleaning were foraging strategies positively correlated with an increase in scrub cover. Insectivores, nectarivores, nectarivores/insectivores and granivores/insectivores also increased in abundance as woody plants increased. Frugivores, frugivores/insectivores, granivores and hawk feeders were negatively associated with an increase in woody scrub (Fig. 5a).
Discussion
Habitat heterogeneity and composition in bushveld and mango orchards
Despite containing both the tallest trees in the region and significantly more habitat structure above 10 m (6 vs. 2.4 %) than natural vegetation, mango orchards were significantly less structurally diverse than the surrounding natural bushveld, with the scrub layer notably absent in mango. Mango orchards were also characterized by greater amounts of bare ground than natural vegetation. As the natural vegetation had greater FHD, and the two habitats were found to be significantly different based on vegetation structure within different layers (ANOSIM, t-tests), we accept our first hypothesis that mango orchards add habitat heterogeneity at a regional scale by adding components to the landscape that are complementary to those already present, because of the contribution to vertical habitat structure.
Bird species richness, abundance and composition
Bird species diversity and abundance were both greater in natural habitats than in mango orchards. Based on presence and absence data, species assemblages differed between bushveld and mango, and when relative abundance of bird species was considered, the differences were even more marked. The set of species present in mango were however not complementary to, but a subset of, those in natural habitats. Approximately a third of species recorded in bushveld points were unique to this habitat, but there were no species unique to mango orchards, despite the complementarity in habitat structure between mango and natural vegetation. This strongly indicates that the former does not enhance regional avian diversity, in contrast to previous comparisons of natural and anthropogenic habitats (Wunderle Jr. and Latta 1998; Fairbanks et al. 2002; Fairbanks 2004; Evans et al. 2006; Child et al. 2009; Hanberry et al. 2012). We therefore reject our second hypothesis that assemblages in the mango orchards would be complementary to those in natural habitats.
Habitat structure and functional traits
Vegetation structure is probably the most important feature influencing bird species assemblages and diversity in savanna habitats (Sirami et al. 2009; Joseph et al. 2011). Structurally, the mango orchards were missing the scrub and woody plant sub-strata present in bushveld, although they had a much taller tree layer associated with the Casuarina windbreaks (Appendix S1). The bird species associated with the scrub layer in the bushveld were those with gleaning, bark foraging and hawk-gleaning foraging strategies (57 % of foraging guilds), an association that has been found elsewhere (Seymour and Dean 2010). The absence of this layer could account for the absence of some 26 insectivorous and nectarivorous species from the mango orchards, similar to findings by Ndang’ang’a et al. (2013) in an agricultural setting in Kenya. The different structural characteristics in two habitat types were (environmental) filters of functional traits in bird assemblages. The results were consistent with our third hypothesis, that the representation of bird life history traits would correlate with habitat structure, and also indicated the dependence of functional traits on the presence of specific habitat characteristics. Cinnyris talatala and Erythropygia leucophrys were responsible for the similarities among the bushveld survey points, as these species were present at similar densities throughout and had a clear preference for bushveld habitats associated with denser undergrowth which characterized the natural habitat, but was missing from mango orchards.
Of the 102 species occurring in mango orchards, almost all were more abundant in the bushveld, with the exception of C. mozambica, Pycnonotus tricolor, B. hagedash, Streptopelia semitorquata and Numida meleagris (i.e. ~5 % of species). Three of these five species are generalists, and all but one (the granivorous C. mozambica) are considered commensal with humans, able to adapt well to alien plantations, intense agriculture and parks and gardens (Harrison et al. 1997).
An investigation of the effects of anthropogenic habitats on bird diversity found a positive correlation between anthropogenic habitat disturbance and bird diversity, but emphasized that rare or specialized species are less able to adapt to human activity (Hugo and van Rensburg 2009). Synanthropic (species associated with anthropogenic habitats) species are common, foraging generalists that are resilient to change and able to exploit commonly available resources (Tscharntke et al. 2005; Hugo and Van Rensburg 2009). Our results were consistent with other studies that indicate that generalist (and thus common) species expand their ranges by colonizing areas affected by land transformation (Fairbanks et al. 2002; Cerezo et al. 2011; Bonthoux et al. 2012). Mango orchards lacking vegetation structures (i.e., the low scrub layer) present in surrounding natural habitats thus appear detrimental to insectivorous and nectarivorous (and hence, pollinating) bird species, because of these species’ specialized niches that rely on the diversity of microhabitats within heterogeneous ecosystems (Child et al. 2009; Sekercioglu 2012).However, we cannot ignore seasonality and its effects on species richness and species assemblages across time. Nevertheless, our study was conducted when there were still a number of Palearctic migrants present, and yet we found no benefit of mango orchard structure to those species.
Conservation implications and implications for ecosystem services
Where natural heterogeneous habitats were replaced with comparatively homogenous anthropogenic habitats, the reduction in vegetation-structure, and hence niche availability, resulted in the reduction or loss of various foraging strategies, associated with 35 % of the species in this study, thus habitat heterogeneity at the landscape and habitat scale is crucial for facilitating species richness.
We highlight the importance of life-history traits/ecological attribute data in illustrating how anthropogenic habitat changes affect species assemblages. Our approach here demonstrates how assessing habitat structure, along with species’ functional traits, can allow predictions about the effects that anthropogenic habitats, specifically structural changes, may have on regional biodiversity, and can provide insights into ways in which negative effects can be reduced(Cleary et al. 2007; Ikin et al. 2012; Jamil et al. 2012).Structurally- and biologically-less diverse anthropogenic habitats and consequent declines in species functional richness has implications for the preservation and provision of ecosystem services, particularly pest control, seed dispersal and pollination (Sekercioğlu et al. 2004; Sekercioglu 2012). In our study area, patches of natural flowering species within orchards has been shown to increase invertebrate pollination of crops and fruit set (Carvalheiro et al. 2012). It is thus crucial for policy makers in countries such as South Africa, where natural habitats remain the predominant landscape features, to identify management strategies that provide the best synergy between crop productivity and biodiversity conservation in the context of sustainable agriculture (Fahrig et al. 2011).
We also highlight the potential for ecologically-sensitive management of agricultural practices to enhance biodiversity and support ecosystem services (Tscharntke et al. 2005; Child et al. 2009). Simply conserving biodiversity in the form of protected area networks, without taking other larger high-impact land uses and their potential for aiding biodiversity into consideration, will not be enough to halt biodiversity loss by 2020 (Tscharntke et al. 2005; Fahrig et al. 2011; Bertzky et al. 2012).
The consequences of farmland management will have critical effects on conservation planning for biodiversity, as well as implications for food security (Tscharntke et al. 2005). Therefore, and in conjunction with the conclusions of Carvalheiro et al. (2010 and 2012) and Ndang’ang’a et al. (2013), we recommend the inclusion of patches of natural vegetation surrounding (e.g. hedgerows; Doxa et al. 2010) and within agricultural landscapes to increase habitat heterogeneity and maintain natural levels of species diversity. Given that our results indicate the exclusion of the insectivorous guild of birds through the lack of a low scrub layer, and that studies elsewhere in the tropics have found a benefit of birds in pest control (Perfecto et al. 2004; Ndang’ang’a et al. 2013), future studies could investigate the economic value of including a native scrub layer, in conjunction with reduced use of pesticide in mango farms to increase the ecosystem service of pest control by birds.
References
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188. doi:10.1016/S0169-5347(03)00011-9
Bertzky B, Corrigan C, Kemsey J, Kenney S, Ravilious C, Besançon C, Burgess N (2012) Protected Planet Report 2012: tracking progress towards global targets for protected areas. IUCN & UNEP-WCMC, Gland, Switserland and Cambridge, UK
Bianchi FJJ, Booij CJ, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc R Soc B Biol Sci 273:1715–1727. doi:10.1098/rspb.2006.3530
Bibby C, Buckland S (1987) Bias of bird census results due to detectability varying with habitat. Acta Oecol 8:103–112
Bibby C, Burgess N, Hill D (2000) Bird census techniques. Academic Press, London
Blair R (1996) Land use and avian species diversity along an urban gradient. Ecol Appl 6:506–519
Bonthoux S, Barnagaud J-Y, Goulard M, Balent G (2012) Contrasting spatial and temporal responses of bird communities to landscape changes. Oecologia 172:563–574. doi:10.1007/s00442-012-2498-2
Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915. doi:10.1007/s10531-010-9936-4
Buckland S, Anderson D, Burnham K, Laake J (1993) Distance sampling: estimating abundance of biological populations. Chapman & Hall, London
Carvalheiro LG, Seymour CL, Veldtman R, Nicolson SW (2010) Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J Appl Ecol 47:810–820. doi:10.1111/j.1365-2664.2010.01829.x
Carvalheiro LG, Seymour CL, Nicolson SW, Veldtman R (2012) Creating patches of native flowers facilitates crop pollination in large agricultural fields: mango as a case study. J Appl Ecol 49:1373–1383. doi:10.1111/j.1365-2664.2012.02217.x
Cerezo A, Conde MC, Poggio SL (2011) Pasture area and landscape heterogeneity are key determinants of bird diversity in intensively managed farmland. Biodivers Conserv 20:2649–2667. doi:10.1007/s10531-011-0096-y
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chessel D, Dufour AB, Thioulouse J (2004) The ade4 package—1: one-table methods. R news 4:5–10
Child MF, Cumming GS, Amano T (2009) Assessing the broad-scale impact of agriculturally transformed and protected area landscapes on avian taxonomic and functional richness. Biol Conserv 142:2593–2601. doi:10.1016/j.biocon.2009.06.007
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Cleary DFR, Boyle TJB, Setyawati T, Anggraeni CD, Loon EE Van, Menken SBJ (2007) Bird species and traits associated with logged and unlogged forest in Borneo. Ecol Appl 17:1184–1197.
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Cowell RK (2006) EstimateS: Statistical estimation of species richness and shared species from samples
Crooks K (2004) Avian assemblages along a gradient of urbanization in a highly fragmented landscape. Biol Conserv 115:451–462. doi:10.1016/S0006-3207(03)00162-9
De Klerk HM, Crowe TM, Fjeldsa J, Burgess ND (2002) Biogeographical patterns of endemic terrestrial Afrotropical birds. Divers Distrib 8:147–162. doi:10.1046/j.1472-4642.2002.00142.x
Department of Environmental Affairs and Tourism (2006) Chapter 4: Land. South Africa Environ. outlook. A Rep. State Environ. DEAT, Pretoria, pp 87–173
Department of Horticultural Sciences (2002) Horticulture in the Limpopo Province. Abstr Agric Stat pp 1–23
Dolédec S, Chessel D, Braak CJF, Champely S (1996) Matching species traits to environmental variables: a new three-table ordination method. Environ Ecol Stat 3:143–166. doi:10.1007/BF02427859
Doxa A, Bas Y, Paracchini ML, Pointereau P, Terres J-M, Jiguet F (2010) Low-intensity agriculture increases farmland bird abundances in France. J Appl Ecol 47:1348–1356. doi:10.1111/j.1365-2664.2010.01869.x
Dray S, Dufour A-B (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
ESRI (Environmental Systems Research Institute) (2011) ArcGIS Desktop 10
Evans KL, van Rensburg BJ, Gaston KJ, Chown SL (2006) People, species richness and human population growth. Glob Ecol Biogeogr 14:625–636. doi:10.1111/j.1466-822X.2006.00253.x
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin J-L (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112. doi:10.1111/j.1461-0248.2010.01559.x
Fairbanks DHK (2004) Regional land-use impacts affecting avian richness patterns in Southern Africa-insights from historical avian atlas data. Agric Ecosyst Environ 101:269–288. doi:10.1016/j.agee.2003.09.009
Fairbanks DHK, Kshatriya M, Jaarsveld AS, Underhill LG (2002) Scales and consequences of human land transformation on South African avian diversity and structure. Anim Conserv 5:61–73. doi:10.1017/S1367943002001087
Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, Mueller ND, O’Connell C, Ray DK, West PC, Balzer C, Bennett EM, Carpenter SR, Hill J, Monfreda C, Polasky S, Rockström J, Sheehan J, Siebert S, Tilman D, Zaks DPM (2011) Solutions for a cultivated planet. Nature 478:337–342. doi:10.1038/nature10452
Gibbons DW, Gregory RD (2006) Birds. In: Sutherland WJ (ed) Ecological census techniques: a handbook. Cambridge University Press, Cambridge, pp 308–350
Hanberry BB, Hanberry P, Riffell SK, Demarais S, Jones JC (2012) Bird assemblages of intensively established pine plantations in Coastal Plain Mississippi. J Wildl Manag 76:1205–1214. doi:10.1002/jwmg.361
Harper D, Ryan P (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Harrison JA, Allan DG, Underhill LG, Herremans M, Tree AJ, Parker V, Brown CJ (1997) The atlas of southern African birds. Vol. 2: Passerines. Birdlife South Africa, Johannesburg
Hockey PAR, Dean WRJ, Ryan P (2005) Birds of Southern Africa, 7th edn. John Voelcker Bird Book Fund, Cape Town
Hockey PAR, Sirami C, Ridley AR, Midgley GF, Babiker HA (2011) Interrogating recent range changes in South African birds: confounding signals from land use and climate change present a challenge for attribution. Divers Distrib 17:254–261. doi:10.1111/j.1472-4642.2010.00741.x
Hugo S, Van Rensburg BJ (2009) Alien and native birds in South Africa: patterns, processes and conservation. Biol Invasions 11:2291–2302. doi:10.1007/s10530-008-9416-x
Ikin K, Knight E, Lindenmayer DB, Fischer J, Manning AD (2012) Linking bird species traits to vegetation characteristics in a future urban development zone: implications for urban planning. Urban Ecosyst 15:961–977. doi:10.1007/s11252-012-0247-2
Jamil T, Ozinga WA, Kleyer M, ter Braak CJF (2012) Selecting traits that explain species-environment relationships: a generalized linear mixed model approach. J Veg Sci 24:988–1000. doi:10.1111/j.1654-1103.2012.12036.x
Jones JE, Kroll AJ, Giovanini J, Duke SD, Ellis TM, Betts MG (2012) Avian species richness in relation to intensive forest management practices in early seral tree plantations. PLoS ONE 7:1–10. doi:10.1371/journal.pone.0043290
Joseph GS, Cumming GS, Cumming DHM, Mahlangu Z, Altwegg R, Seymour CL (2011) Large termitaria act as refugia for tall trees, deadwood and cavity-using birds in a miombo woodland. Landsc Ecol 26:439–448. doi:10.1007/s10980-011-9572-8
Kleyer M, Dray S, Bello F, Lepš J, Pakeman RJ, Strauss B, Thuiller W, Lavorel S (2012) Assessing species and community functional responses to environmental gradients: which multivariate methods? J Veg Sci 23:805–821. doi:10.1111/j.1654-1103.2012.01402.x
Koh L (2008) Can oil palm plantations be made more hospitable for forest butterflies and birds? J Appl Ecol 45:1002–1009. doi:10.1111/j.1365-2664.2007.0
Lenda M, Skórka P, Moroń D, Rosin ZM, Tryjanowski P (2012) The importance of the gravel excavation industry for the conservation of grassland butterflies. Biol Conserv 148:180–190. doi:10.1016/j.biocon.2012.01.014
MacArthur RH, MacArthur JW, Preer J (1962) On bird species diversity II Prediction of bird census from habitat measurements. Am Nat 96:167–174
Manning JC (2003) Photographic guide to the wildflowers of South Africa. Briza, Pretoria
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453. doi:10.1016/S0169-5347(99)01679-1
Mucina L, Rutherford MC (2011) The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. South African National Biodiversity Institute, Pretoria
Ndang’ang’a PK, Njoroge JBM, Vickery J (2013a) Quantifying the contribution of birds to the control of arthropod pests on kale, Brassica oleracea acephala, a key crop in East African highland farmland. Int J Pest Manag 59:211–216. doi:10.1080/09670874.2013.820005
Ndang’ang’a PK, Njoroge JB, Githiru M (2013b) Vegetation composition and structure influences bird species community assemblages in the highland agricultural landscape of Nyandarua, Kenya. Ostrich 84:171–179. doi:10.2989/00306525.2013.860929
Okes NC, Hockey PAR, Cumming GS (2008) Habitat use and life history as predictors of bird responses to habitat change. Conserv Biol 22:151–162. doi:10.1111/j.1523-1739.2007.00862.x
Perfecto I, Vandermeer J, Bautista G (2004) Greater predation in shaded coffee farms: the role of resident neotropical birds. Ecology 85:2677–2681
Pickett ST, Cadenasso M (1995) Landscape ecology: spatial heterogeneity in ecological systems. Science 269:331–334
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reyers B (2004) Incorporating anthropogenic threats into evaluations of regional biodiversity and prioritisation of conservation areas in the Limpopo Province, South Africa. Biol Conserv 118:521–531. doi:10.1016/j.biocon.2003.09.027
Reyers B, Fairbanks DHK, Van Jaarsveld AS, Thompson M (2001) Priority areas for the conservation of South African vegetation: a coarse-filter approach. Divers Distrib 7:79–95. doi:10.1046/j.1472-4642.2001.00098.x
Ricklefs RE (1991) Structures and transformations of life histories. Funct Ecol 5:174–183
Secretariat of the Convention on Biological Diversity (2010) Global Biodiversity. Outlook 3(104):1–94
Sekercioglu CH (2012) Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J Ornithol 153:153–161. doi:10.1007/s10336-012-0869-4
Sekercioğlu CH, Daily GC, Ehrlich PR (2004) Ecosystem consequences of bird declines. Proc Natl Acad Sci U S A 101:18042–18047. doi:10.1073/pnas.0408049101
Seymour CL, Dean WRJ (2010) The influence of changes in habitat structure on the species composition of bird assemblages in the southern Kalahari. Austral Ecol 35:581–592. doi:10.1111/j.1442-9993.2009.02069.x
Sirami C, Seymour C, Midgley G, Barnard P (2009) The impact of shrub encroachment on savanna bird diversity from local to regional scale. Divers Distrib 15:948–957. doi:10.1111/j.1472-4642.2009.00612.x
Skórka P, Lenda M, Moroń D, Tryjanowski P (2013) New methods of crop production and farmland birds: effects of plastic mulches on species richness and abundance. J Appl Ecol 50:1387–1396. doi:10.1111/1365-2664.12148
Sullivan D (2009) Google Earth Pro. 16–18
Thies C, Tscharntke T (1999) Landscape structure and biological control in agroecosystems. Science 285:893–895
Thioulouse J, Dray S (2007) Interactive multivariate data analysis in R with the ade4 and ade4tkgui packages. J Stat Softw 22:1–14
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity and ecosystem service management. Ecol Lett 8:857–874. doi:10.1111/j.1461-0248.2005.00782.x
Wesuls D, Oldeland J, Dray S (2012) Disentangling plant trait responses to livestock grazing from spatio-temporal variation: the partial RLQ approach. J Veg Sci 23:98–113. doi:10.1111/j.1654-1103.2011.01342.x
Weyland F, Baudry J, Ghersa CM (2012) A fuzzy logic method to assess the relationship between landscape patterns and bird richness of the Rolling Pampas. Landsc Ecol 27:869–885. doi:10.1007/s10980-012-9735-2
Wunderle JM Jr, Latta SC (1998) Avian resource use in Dominican shade coffee plantations. Wilson Bull 110:271–281. doi:10.2307/4163936
Acknowledgments
The research was conducted with financial support to the ‘NETWORK’ project from the European Commission Marie Curie International Research Staff Exchange Scheme (IRSES) (Grant agreement: PIRSES-GA-2012-318929). CLS was also financially assisted by DST Financial Assistance agreement DST/CON0054/2013 and NRF Grant 91039. We thank all the farmers involved for providing us access to the sites and D. Henri for statistical guidance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ehlers Smith, Y.C., Ehlers Smith, D.A., Seymour, C.L. et al. Response of avian diversity to habitat modification can be predicted from life-history traits and ecological attributes. Landscape Ecol 30, 1225–1239 (2015). https://doi.org/10.1007/s10980-015-0172-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-015-0172-x