Abstract
Previous studies have suggested that l-cysteine regulates gut motility through hydrogen sulfide. However, the mechanisms involved in the l-cysteine-induced response have not been extensively studied. This study aimed to investigate the underlying mechanisms of action of l-cysteine on spontaneous contraction of rat colon. Longitudinal and circular muscle strips from rat middle colon were prepared to measure the spontaneous contractile activities of colon in an organ bath system. Whole-cell voltage-clamp techniques were applied to record the currents of L-type voltage-dependent Ca2+ channels (VDCCs) and voltage-gated K+ channels (Kv) in isolated smooth muscle cells (SMCs) from colon. l-cysteine inhibited the spontaneous contraction of longitudinal and circular muscle strips from the rat colon in a concentration-dependent manner. The inhibition induced by l-cysteine was significantly decreased by inhibitors of H2S synthesis (p < 0.05). Furthermore, the suppression induced by l-cysteine was partially attenuated by tetrodotoxin, L-NNA and glibenclamide (p < 0.05). Whole‐cell voltage‐clamp recordings showed that l-cysteine caused a remarkable reduction in the peak currents of VDCCs and significantly increased the membrane currents of Kv channels in isolated SMCs (p < 0.05). We concluded that l-cysteine inhibits the contractile activities of smooth muscle strips from the rat colon. The relaxation in response to l-cysteine may be in part mediated by a nitrergic pathway and by inhibiting the VDCCs in combination with a direct activation of the KV channels and KATP channels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional amino acids (FAAs), including cysteine, regulate not only signalling pathways in cells but also gene expression and enzyme activity (Wu 2009). l-cysteine, an endogenous thiol-containing FAA, has been reported to modulate the disulfide linkage and transport of sulfur in proteins (Wu 2009). Moreover, the biological functions of l-cysteine can also be achieved through its metabolites, including hydrogen sulfide (H2S) (Wu 2009; Ingenbleek and Kimura 2013). Emerging evidence indicates that l-cysteine regulates gastrointestinal (GI) motility. In the rat jejunum, l-cysteine caused a consistent, concentration-dependent inhibition of circular muscle contractile activity (Nagao et al. 2012). Nalli et al. demonstrated an inhibitory action of l-cysteine on colon contraction, which was mediated by the cGMP/PKG pathway (Nalli et al. 2017). In contrast, l-cysteine caused excitation in isolated duodenal muscle strips and enhanced motility (Lu et al. 2014). These results imply that l-cysteine may be involved in regulating the GI motility.
l-cysteine is commonly used as a precursor of H2S in experiments. The underlying mechanisms of either excitatory or inhibitory action of l-cysteine on GI motility are thought to be due to endogenous H2S (Nagao et al. 2012; Nalli et al. 2017; Lu et al. 2014). The attenuated effects of l-cysteine on GI motility in response to inhibition of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), the critical enzymes generating H2S, support the hypothesis that H2S has an important role in the effect of l-cysteine on GI motility (Lu et al. 2014; Jimenez et al. 2017). However, this mechanism would exclude any direct participation of l-cysteine in the regulatory process of GI motility. l-cysteine might have more potent pharmacological properties that are irrelevant to H2S production. For example, it has been reported that l-cysteine directly inhibits stretch-dependent K+ channels in murine colonic smooth muscle cells (SMCs) (Park et al. 2005). Furthermore, l-cysteine causes an increase in currents of T-type Ca2+ channels located in dorsal root ganglion cells of rats (Nelson et al. 2010). Therefore, it is possible that changes in GI motility in response to l-cysteine might be in part ascribed to a direct effect of l-cysteine on ion channels.
In the GI tract, the properties of L-type voltage-dependent Ca2+ channels (VDCCs) and voltage-gated K+ channels (Kv) are closely related to excitation-contraction coupling in SMCs (Yan et al. 2019; Bolton et al. 1999). Moreover, ATP-sensitive K+ channels (KATP) and small conductance Ca2+-activated K+ channels (SKCa) also contribute to the electrical activities of the smooth muscle of the GI tract (Kuo IY, et al. 2015). However, the role of these ion channels in the regulation of contractility by l-cysteine in the GI tract has not been elucidated in detail. Given that l-cysteine regulates GI motility, we hypothesized that l-cysteine might affect colonic contraction in part by directly regulating the ion channels of SMCs. Here, we investigated the effects of l-cysteine on colonic longitudinal and circular muscle strips and explored whether ion channels on SMCs contribute to the effect of l-cysteine on colonic motility.
Materials and methods
Animals
Forty male Wistar rats (weight, 180–220 g; age, 10–12 weeks) were purchased from the Experimental Animal Center of Xi’an Jiaotong University (Xi’an, China). The housing and handling of the rats were reviewed and approved by the Ethics Committee for Research on Animals, Xi’an Jiaotong University (Approval ID: 2,021,866).
Chemicals
l-cysteine, tetrodotoxin (TTX), L-propargylglycine (PAG), amino-oxyacetic acid (AOAA), Nω-nitro‐L‐arginine (L‐NNA), glibenclamide (Gliben) were purchased from Sigma‐Aldrich (Sigma‐Aldrich Co., St. Louis, MO, USA). Apamin was purchased from Tocris Bioscience (Tocris Bioscience, Bristol, UK). l-cysteine, TTX, PAG, AOAA, L-NNA and apamin were dissolved in purified water. Gliben was dissolved in dimethylsulfoxide.
Solutions
Ca2+-free, phosphate-buffered saline (PBS) contained (mM) NaCl 135, KCl 5.0, NaOH 15.5, MgCl2 1.2, glucose 10, and N-[2-hydroxyethyl] piperazine- N0- [2-ethanesulfonic acid] (HEPES) 10, adjusted to pH 7.4 with NaOH. Tyrode’s buffer contained (mM) 147.0 NaCl, 4.0 KCl, 2.0 CaCl2, 0.42 NaH2PO4, 2.0 Na2HPO4, 1.05 MgCl2, and 5.5 glucose, adjusted to pH 7.4 with NaOH. The pipette solution for VDCC contained (mM) CsCl 135, MgCl2 4, HEPES 10, Na2ATP 2, EGTA 10, TEA 20, adjusted to pH 7.3 with CsOH. The pipette solution for KCa contained (mM) KCl 125, MgCl2 4, HEPES 10, EGTA 10, Na2ATP 5, adjusted pH to 7.3 with KOH. The pipette solution for Kv contained (mM) potassium-aspartic acid 110, Mg-ATP 5, HEPES 5, MgCl2 1, KCl 20, EGTA 10, CdCl2 1, di-tris-creatine phosphate 2.5, and di-sodium-creatine phosphate 2.5, adjusted to pH 7.3 with KOH.
Mechanical responses
Rats were killed by cervical dislocation, and segments of the middle colon were quickly removed and put into a dish containing 4 °C Tyrode’s buffer. The mesenteric fat was removed, and then the colon segments were opened along the mesenteric border. Longitudinal muscle (LM) strips and circular muscle (CM) strips (3 mm wide, 10 mm long) were prepared by cutting parallel to the longer and circular axes of the colon segments, respectively.
LM and CM strips were mounted in an 8 mL organ bath containing Tyrode’s buffer maintained at 37.2 °C and bubbled with 5% CO2/95% O2. Contractile activities were measured using an isometric force transducer (JZJOIH, Chengdu Instrument Factory, Chengdu, China) connected to a computer through an amplifier. An RM6240 multichannel physiological signal system (Chengdu, China) was applied to record the mechanical activities. Each muscle strip was incubated with a 1 g preload and was allowed to equilibrate for 1 h. After the equilibration period, spontaneous contractions began. Then, the effect of l-cysteine on the contractions was detected. Each muscle strip was exposed to l-cysteine only once. In other experiments, the muscle strips were pretreated individually with TTX (1 µM), AOAA (0.1 mM) + PAG (0.5 mM), L-NNA (1 mM), Gliben (10 µM) and apamin (1 µM) for 6 min before l-cysteine application. The concentrations of l-cysteine and different blockers applied in the present study have been used in previous studies (Yan et al. 2019).
Preparation of isolated myocytes
Dissociated SMCs were prepared from adolescent Wistar rats (180–220 g) and used within 8 h for whole-cell patch-clamp recordings as described previously (Yan et al. 2019). In brief, the middle colon tissues were placed in Ca2+-free PBS and opened along the mesenteric border, and the mucosa and submucosa were peeled. Then, muscle strips were cut into small pieces and incubated in Ca2+-free PBS containing 1.2 mg ml−1 collagenase II, 2 mg ml−1 trypsin inhibitor and 2 mg ml−1 bovine serum albumin for 15–20 min at 36.5 °C. After enzymatic incubation, the tissue pieces were washed three times with Ca2+-free PBS at room temperature and stored in Ca2+-free PBS at 4 °C.
Electrophysiology
Single SMCs were obtained at room temperature by a series of triturations through a fire-polished glass pipette. For recordings, drops of the cell suspensions were plated on an uncoated glass coverslip, formed in a culture dish, and perfused with external solution from gravity-fed perfusion pipes at a flow rate of 1 ml/min. The whole-cell voltage-clamp technique was applied to record membrane currents from dissociated SMCs. Membrane currents were amplified by an Axon patch 700B Amplifier (Axon Instruments, Burlingham, CA, USA) and digitized with an A/D converter (Digidata 1322 A, Axon Instruments). Pipette resistances were 5–7 MΩ. Data were collected at 1 kHz and filtered at 800 Hz via a Bessel filter and digitized using pClamp software (version 10.2, Axon Instruments). All experiments were conducted at room temperature.
Statistical analysis
Each experiment for the measurement of colonic muscle contraction was conducted using different animals. All data were expressed as the mean ± SD. The mean amplitude before drug application was considered baseline, and the values after drug treatment were normalized to R by dividing by the baseline. The R value before drug application was equal to 1. The frequency was calculated as the number of spontaneous contractions per minute and expressed as cycles per min (cpm). The significance of the differences between two groups was assessed using two-tailed Student’s t-tests, and multiple comparisons were compared by one-way ANOVA followed by Dunnett’s test. p values of less than 0.05 were considered to be significant. N values reported in the text refer to the number of animals, and n values refer to the number of single SMCs.
Results
Effect of l-cysteine on spontaneous contraction of colonic muscle strips
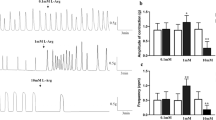
The effect of l-cysteine on spontaneous contraction of colonic muscle strips was presented in Fig. 1a. As shown above, l-cysteine induced a remarkable inhibition on the spontaneous contractions of both LM and CM strips in a concentration-dependent manner. Before the addition of l-cysteine, the mean amplitude of contraction in LM strips was 1.21 ± 0.31 g (N = 8). After the addition of l-cysteine at concentrations of 0.01, 0.1, 0.5, 1 and 5 mM, the amplitude was 1.22 ± 0.31 g (p > 0.05 vs. normal), 1.20 ± 0.32 g (p > 0.05 vs. normal), 1.13 ± 0.33 g (p > 0.05 vs. normal), 0.69 ± 0.22 g (p = 0.0005 vs. normal) and 0.10 ± 0.11 g (p = 0.0001 vs. normal), respectively (Fig. 1b). As for CM strips, before the addition of l-cysteine, the mean amplitude of contraction was 0.76 ± 0.26 g (N = 8). After the addition of l-cysteine at concentrations of 0.01, 0.1, 0.5, 1 and 5 mM, the amplitude was 0.72 ± 0.20 g (p > 0.05 vs. normal), 0.72 ± 0.20 g (p > 0.05 vs. normal), 0.68 ± 0.17 g (p > 0.05 vs. normal), 0.46 ± 0.14 g (p = 0.0073 vs. normal) and 0.07 ± 0.09 g (p = 0.0005 vs. normal), respectively (Fig. 1b).
Effect of l-cysteine on the spontaneous contractile activities of colonic muscle strips. a Representative recordings of the effects of l-cysteine (0.01-5 mM) on the contraction of longitudinal muscle (LM) and circular muscle (CM) strips from rat colon. b and c Summarized data of the changes in amplitude and contraction frequency after l-cysteine treatment. (**p < 0.01, ***p < 0.001 vs. normal, N = 8)
As shown in Fig. 1c, l-cysteine (0.01-1 mM) had no significant effect on contraction frequency of both LM and CM strips. l-cysteine (5 mM) reduced the frequency of LM strips from 0.50 ± 0.15 cpm to 0.11 ± 0.12 cpm (p = 0.0002 vs. normal), and reduced the frequency of CM strips from 0.98 ± 0.20 cpm to 0.19 ± 0.26 cpm (p = 0.0001 vs. normal).
Effect ofl-cysteine on spontaneous contraction of colonic muscle strips in the presence of AOAA and PAG
LM and CM strips were incubated with AOAA (0.1 mM) combined with PAG (0.5 mM) to investigate the possible involvement of endogenous H2S in l-cysteine-induced suppression of colonic muscle strips. In the studies on mechanism of action, we used 1 mM l-cysteine, which had a submaximal effect on the contraction amplitude. The contraction amplitude of LM and CM decreased remarkably after application of 1 mM l-cysteine, while there was no significant change in contraction frequency (Fig. 2a, c and d). As shown in Fig. 2b, AOAA combined with PAG had no significant effect on the contraction of the muscle strips (p > 0.05, vs. baseline).The inhibitory effects of l-cysteine on both the LM and CM strips were partially attenuated by pretreatment with AOAA and PAG. The R values (normalized amplitude) for LM and CM in the presence of l-cysteine (1 mM) were 0.40 ± 0.15 and 0.49 ± 0.16, respectively (p < 0.05 vs. l-cysteine alone, N = 8) (Fig. 2c). In the presence of AOAA and PAG, no significant differences in the frequency of spontaneous contraction were observed before and after l-cysteine treatment (p > 0.05) (Fig. 2d).
Inhibition ofl-cysteine on the spontaneous contractile activities of colonic muscle strips in the presence of AOAA and PAG. a and b The effects of l-cysteine (1 mM) on both longitudinal muscle (LM) and circular muscle (CM) strips in the absence and presence of AOAA combined with PAG. c and d Summarized results of amplitudes and contraction frequency for LM and CM before and after l-cysteine treatment in the presence of AOAA and PAG (#p < 0.05, ##p < 0.01 vs. l-cysteine alone, N = 8)
Effect of NO on l-cysteine-induced inhibition of colonic muscle strips
To investigate the possible involvement of NO in the inhibition caused by l-cysteine (1mM), the muscle strips were first incubated with TTX (1 µM). As shown in Fig. 3a and c, the normalized amplitudes of both LM and CM were slightly enhanced after TTX pretreatment (p < 0.05, N = 8). The suppression of l-cysteine on LM and CM strips was significantly attenuated by TTX. In the presence of TTX, the R values for LM and CM after l-cysteine treatment were 0.39 ± 0.09 and 0.47 ± 0.10, respectively (p < 0.05 vs. l-cysteine alone, N = 8). In the presence of TTX, no significant differences in the frequency of spontaneous contraction were found before and after l-cysteine application (p > 0.05) (Fig. 3d).
The role of nitric oxide (NO) inl-cysteine-induced inhibition of the spontaneous contractile activities of colonic muscle strips. a and b The effects of l-cysteine (1 mM) on both longitudinal muscle (LM) and circular muscle (CM) strips in the presence of TTX and L-NNA. c and d Summarized results of amplitudes and contraction frequency for LM and CM before and after l-cysteine treatment in the presence of TTX and L-NNA. (#p < 0.05, ##p < 0.01 vs. l-cysteine alone, N = 8)
L-NNA, an inhibitor of NO synthesis, significantly increased the contraction of both LM and CM strips (p < 0.05, N = 8). In the presence of L-NNA, l-cysteine-induced inhibition on both LM and CM strips was significantly attenuated, and the R values of LM in l-cysteine alone and L-NNA combined with l-cysteine were 0.24 ± 0.11 and 0.42 ± 0.14, respectively (p < 0.05, N = 8). The R values of CM with l-cysteine alone and L-NNA combined with l-cysteine were 0.29 ± 0.16 and 0.50 ± 0.12, respectively (p < 0.01, N = 8) (Fig. 3b and c). In the presence of L-NNA, no significant differences in the frequency of spontaneous contraction were found before and after l-cysteine treatment (p > 0.05) (Fig. 3d).
Role of KATP and SK channels in l-cysteine-induced inhibition
Gliben, an inhibitor of KATP, had no effect on the contraction of the colonic muscle strips. However, glibenclamide pretreatment reduced the l-cysteine-induced relaxation of both LM and CM. As shown in Fig. 4a and c, the R values for LM and CM after l-cysteine treatment were 0.45 ± 0.18 and 0.47 ± 0.13, respectively (p < 0.01 for LM and p < 0.05 for CM vs. l-cysteine alone, N = 8). In the presence of glibenclamide, no significant differences in the frequency of spontaneous contraction were found before and after l-cysteine treatment (p > 0.05) (Fig. 4d).
Role of KATPand SK channels inl-cysteine-induced inhibition of colonic muscle strips. a and b The effects of l-cysteine (1 mM) on both longitudinal muscle (LM) and circular muscle (CM) strips in the presence of glibenclamide and apamin. c and d Summarized results of amplitudes and contraction frequency for LM and CM before and after l-cysteine treatment in the presence of glibenclamide and apamin (#p < 0.05, ##p < 0.01 vs. l-cysteine alone, N = 8)
Apamin, an inhibitor of SK channels, had no effect on the contraction of the colonic muscle strips. Apamin was also unable to block or reduce the l-cysteine-induced inhibition on amplitude and frequency of spontaneous contraction (p > 0.05) (Fig. 4b, c and d).
Involvement of VDCCs in l-cysteine-induced inhibition of the contraction of the muscle strips
The representative traces recorded before and after l-cysteine application are shown in Fig. 5a. l-cysteine (1 and 5 mM) exerted an inhibitory effect on the currents of VDCCs and had no effect on the shape of the current-voltage (I–V) curves (Fig. 5b). The peak current density of VDCCs at 0 mV after l-cysteine application decreased from − 4.36 ± 0.55 pA/pF to -3.42 ± 0.85 pA/pF (p < 0.01 vs. Control) and − 2.69 ± 1.34 pA/pF, respectively (p < 0.01 vs. Control) (N = 8, n = 10) (Fig. 5c).
Involvement of VDCCs inl-cysteine-induced inhibition of the contraction of muscle strips. a The raw traces of whole-cell recordings in response to a series of depolarizing voltage pulses from a holding potential of − 50 mV to + 40 mV in 10 mV steps before (Control) and after the addition l-cysteine (1 and 5 mM). b The representative effects of l-cysteine on the I–V relationship of VDCCs. c Effect of l-cysteine on the peak currents of VDCCs at 0 mV. d Original traces of the steady-state activation protocol recording of VDCCs. e Effects of l-cysteine on the stead-state activation curve of VDCCs. f Original traces of the steady-state inactivation protocol recording of VDCCs. g The stead-state inactivation curve of VDCCs before and after l-cysteine treatment (**p < 0.01 vs. control, N = 8, n = 10)
The influence of l-cysteine on the steady-state activation of VDCCs was investigated in this study. The curve fit well with the Boltzmann equation as G/Gmax = 1/[1 + exp(VT-V1/2/κ)], in which I/Imax is used instead of G/Gmax, VT and V1/2 are the magnitude of the depolarizing pulses and the half-maximum activation membrane potential, respectively. Bath application of l-cysteine had no effect on the steady-state activation curve of VDCCs (Fig. 5d and e). The V1/2 values were − 21.4 ± 1.1 mV in the control and − 22.6 ± 1.8 mV in the l-cysteine-treated groups (p > 0.05, N = 8, n = 10). The values of κ were − 4.0 ± 1.1 and 3.8 ± 1.5 in the control and l-cysteine-treated groups, respectively (p > 0.05).
Figure 5f and g illustrated the significance of l-cysteine on the steady-state inactivation curve of VDCCs which was fitted by the Boltzmann equation: I/Imax = 1/[1 + exp(VT -V1/2/κ)], with VT representing the values of the depolarizing potential of the conditioning pulse, and V1/2 representing a half-maximum inactivation voltage. The V1/2 values were − 20.4 ± 4.7 mV and − 15.1 ± 2.9 mV in the control and l-cysteine-treated groups, respectively (p > 0.05 vs. control, N = 8, n = 10). κ values were approximately identical for both groups (p > 0.05).
Involvement of KV channels in l-cysteine-induced inhibition of the contraction of muscle strips
The currents of the KV channels were evoked by a series of depolarizing voltage pulses from a holding potential of − 80 mV to + 60 mV in 20 mV increments by the perforated path-clamp. Figure 6a shows the representative traces of the currents of the KV channels before and after l-cysteine application. l-cysteine (1 and 5 mM) had no effect on the voltage-dependent properties of the KV channels (Fig. 6b). l-cysteine concentration-dependently increased the currents of the KV channels. The current density of the KV channels at 60 mV increased from 15.40 ± 2. 39 pA/pF to 19.27 ± 3.38 pA/pF and 23.05 ± 4.48 pA/pF, respectively (p < 0.01, vs. Control, N = 8, n = 8) (Fig. 6c).
Involvement of Kv channels in l-cysteine-induced inhibition of the contraction of muscle strips. a The raw traces of whole-cell recordings in response to a series of depolarizing voltage pulses from a holding potential of − 80 mV to + 60 mV in 20 mV steps before (Control) and after the addition of l-cysteine (1 and 5 mM). b The representative effects of l-cysteine on the I–V relationship of the KV channels. c Effect of l-cysteine on the currents of the KV channels at 60 mV (**p < 0.01 vs. Control, N = 8, n = 8)
Discussion
The current study was conducted to determine the effect and mechanisms of action of l-cysteine on spontaneous contractile activity in rat colonic LM and CM. Using targeted inhibitors of specific signaling pathways, we aimed to explore potential pathways that might mediate the effects of l-cysteine, including endogenous H2S, nitrergic pathways and ion channels in SMCs. In summary, l-cysteine inhibited colonic contraction, and its inhibitory action appeared to be mediated by a nitrergic pathway and H2S production. In addition, VDCC, KATP and KV channels appeared to be involved, at least in part, in l-cysteine-induced suppression of rat colonic motility.
Similar to the findings of previous studies observed in rats, mice and rabbits, (Yamane et al. 2014) the present study showed that l-cysteine concentration-dependently inhibited the spontaneous contractile activities of the rat colon. In contrast, l-cysteine caused contractions in isolated duodenal muscle strips, consistent with the effect of exogenous H2S (Lu et al. 2014). This discrepancy from our study might be ascribed to the different tissues and preparations used for the tissue. l-cysteine is the precursor of H2S synthesis and has been applied previously to stimulate endogenous H2S production (Yamane et al. 2014). The blockade of the enzymes responsible for H2S generation provides information about the role of H2S in l-cysteine-induced suppression. Our results showed that a combination of PAG (a CSE inhibitor) and AOAA (a CBS inhibitor) attenuated the relaxation response to l-cysteine but it was not completely abolished, which implies that endogenous H2S is responsible for the action of l-cysteine. However, it should be noted that the observed effects of l-cysteine in some studies could be unrelated to H2S (Gallego et al. 2008; Kendig et al. 2014). For example, it has been reported that intraluminal perfusion of l-cysteine initiates the peristaltic reflex and pellet propulsion through activation of umami taste receptors in the distal colon of rats (Kendig et al. 2014). Taken together, these results provide further support for our hypothesis that in addition to endogenous H2S, many receptors and channels might contribute to the effect of l-cysteine.
Given that NO is an important neurotransmitter that inhibits GI motility, (Savidge 2011) its role in the suppression of smooth muscle contraction caused by l-cysteine was also investigated. We first blocked the neuronally mediated inhibition of l-cysteine with TTX, a blocker of voltage-sensitive sodium channels on nerve fibres. The inhibitory response to l-cysteine was significantly decreased, suggesting that enteric neurons are responsible for the action of l-cysteine. Moreover, L-NNA, an inhibitor of NO synthesis, reduced the inhibitory effect of l-cysteine but it was not completely abolished. A recent study reported that l-cysteine inhibited phosphodiesterase 5 activity and enhanced the increase in cGMP levels in response to an NO donor (Nalli et al. 2017). These results suggested a possible involvement of the nitrergic pathway in l-cysteine-induced suppression. Thus, the mechanisms of l-cysteine may be complex and multiple.
Ion channels play an important role in regulating the contraction of SMCs and are potential targets for pharmacological treatment of GI dysmotility (Rychter et al. 2014; Currò 2016). To the best of our knowledge, the effects of l-cysteine on ion channels in rat colon has not been previously investigated. Consistent with a previous study in the mouse ileum, (Yamane et al. 2014) our observations showed that glibenclamide, a KATP blocker, attenuated the l-cysteine-induced inhibition of colonic muscle contractions. These results raised the possibility that l-cysteine might act in part via KATP. Apamin, an inhibitor of small-conductance Ca2+-activated K+ (SK) channels, has been reported to participate in the inhibitory action induced by exogenous H2S (Gallego et al. 2008). However, the present study demonstrated that the inhibitory effect of l-cysteine was not affected by apamin, suggesting that l-cysteine-induced relaxation is independent of SK channels.
Further investigation of the mechanisms of suppression induced by l-cysteine was performed on SMCs of the colon with the whole-cell voltage-clamp technique. VDCCs play a critical role in regulating the contraction of SMCs by controlling Ca2+ influx.10,17 In the present study, we examined the effect of l-cysteine on VDCCs and found that the currents of VDCCs were significantly inhibited by l-cysteine without affecting the shape of the current-voltage curves, suggesting that VDCCs may contribute to the suppression induced by l-cysteine. Inhibition of l-cysteine on VDCCs reduced the Ca2+ influx with a subsequent decrease in the cytoplasmic Ca2+ concentration and relaxation of the SMCs.
Activation of K+ channels leads to hyperpolarization of the cell membrane with a subsequent reduction in cell excitability and contractility (Currò 2016). In the present study, l-cysteine concentration-dependently increased the currents of the KV channels, which provides convincing evidence that suppression induced by l-cysteine may be in part ascribed to the KV channels in SMCs. It has been reported that KV channels exert a dominant role in regulating the resting tension of oesophageal and gastric muscle (Wade et al. 1999; Zhao et al. 2009). However, l-cysteine had no effect on the basal tension of colonic muscle contraction in the present study. The exact reason for this result is unclear. It has been reported that H2S at low concentrations can enhance the tonic contraction of gastric smooth muscle via KV channels (Zhao et al. 2009). The concentrations of l-cysteine applied in the present study are capable of stimulating H2S synthesis (Linden et al. 2008). Thus, it is possible that the inhibitory effect of l-cysteine on the basal tension of colonic muscle might be offset by endogenous H2S.
In the GI tract, it has been established that VDCCs and KATP can be modified through S-sulfhydration with subsequent dysfunction of ion channels (Tang et al. 2018; Gade et al. 2013). l-cysteine, as an endogenous reducing agent, plays an important role in the maintenance of dynamic sulfhydryl/disulfide bridge homeostasis (Wu 2009; Ingenbleek and Kimura 2013). Thus, it is possible that l-cysteine may modify these ion channels through S-sulfhydration. The precise mechanisms underlying the modifications of ion channels by L-cysteine need to be elucidated in the future.
The digestive tract can sense various nutrients through receptors present on the gut lumen (Efeyan et al. 2015). Amino acids, including l-cysteine, which are commonly taken as important nutrients, often regulate gut motility and affect appetite (Nakato et al. 2017). And increasing number of studies suggest that dietary amino acids may play an important role in alleviating intestinal inflammation (Nakato et al. 2017). While the colon is often in a state of inflammation, which may be associated with certain intestinal diseases, including diarrhea, inflammatory bowel disease (IBD), and irritable bowel syndrome (IBS) (Camilleri et al. 2012). Hence, amino acid supplementation may have therapeutic potential in these diseases. In the current study, we confirmed that L-cysteine exerts an inhibitory effect on the colonic muscle contraction, which makes it possible that dietary supplementation with l-cysteine may be a simple treatment to improve colonic motility and gut discomfort in patients with diarrhea-predominant IBS and diarrhea.
Conclusion
In summary, l-cysteine inhibits the contractile activities of smooth muscle strips from the rat colon. The relaxation in response to l-cysteine may, in part, be mediated by a nitrergic pathway through NO and by inhibiting VDCCs in combination with the direct activation of KV channels and KATP channels on SMCs.
Abbreviations
- TTX:
-
Tetrodotoxin
- PAG:
-
L-propargylglycine
- AOAA:
-
Amino-oxyacetic acid
- L-NNA:
-
Nω-nitro-L-arginine
- Gliben:
-
Glibenclamide
- LM:
-
Longitudinal muscle
- CM:
-
Strips and circular muscle
- VDCCs:
-
L-type voltage-dependent Ca2+ channels
- Kv:
-
Voltage-gated K+ channels
- KATP:
-
ATP-sensitive K+ channels
- SKCa:
-
Small conductance Ca2+-activated K+ channels
References
Bolton TB, Prestwich SA, Zholos AV, Gordienko DV (1999) Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol 61:85–115
Camilleri M, Madsen K, Spiller R et al (2012) Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24(6):503–512
Currò D (2016) The modulation of potassium channels in the smooth muscle as a therapeutic strategy for disorders of the gastrointestinal tract. Adv Protein Chem Struct Biol 104:263–305
Efeyan A, Comb WC, Sabatini DM (2015) Nutrient-sensing mechanisms and pathways. Nature 517(7534):302–310
Gade AR, Kang M, Akbarali HI (2013) Hydrogen sulfide as an allosteric modulator of ATP-sensitive potassium channels in colonic inflammation. Mol Pharmacol 83(1):294–306
Gallego D, Clavé P, Donovan J et al (2008) The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil 20(12):1306–1316
Ingenbleek Y, Kimura H (2013) Nutritional essentiality of sulfur in health and disease. Nutr Rev 71(7):413–432
Jimenez M, Gil V, Martinez-Cutillas M et al (2017) Hydrogen sulphide as a signalling molecule regulating physiopathological processes in gastrointestinal motility. Br J Pharmacol 174(17):2805–2817
Kendig DM, Hurst NR, Bradley ZL et al (2014) Activation of the umami taste receptor (T1R1/T1R3) initiates the peristaltic reflex and pellet propulsion in the distal colon. Am J Physiol Gastrointest Liver Physiol 307(11):G1100–G1107
Kuo IY, Ehrlich BE (2015) Signaling in muscle contraction. Cold Spring Harb Perspect Biol 7(2):a006023
Linden DR, Sha L, Mazzone A et al (2008) Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem 106(4):1577–1585
Lu W, Li J, Gong L et al (2014) H2S modulates duodenal motility in male rats via activating TRPV1 and K(ATP) channels. Br J Pharmacol 171(6):1534–1550
Nagao M, Duenes JA, Sarr MG (2012) Role of hydrogen sulfide as a gasotransmitter in modulating contractile activity of circular muscle of rat jejunum. J Gastrointest Surg 16(2):334–343
Nakato J, Ho YY, Omae R et al (2017) l-Ornithine and l-lysine stimulate gastrointestinal motility via transient receptor potential vanilloid 1. Mol Nutr Food Res 61(11):1700230
Nalli AD, Bhattacharya S, Wang H et al (2017) Augmentation of cGMP/PKG pathway and colonic motility by hydrogen sulfide. Am J Physiol Gastrointest Liver Physiol 313(4):G330–G341
Nelson MT, Milescu LS, Todorovic SM, Scroggs RS (2010) A modeling study of T-type Ca2+channel gating and modulation by l-cysteine in rat nociceptors. Biophys J 98(2):197–206
Park KJ, Baker SA, Cho SY et al (2005) Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol 144(8):1126–1137
Rychter J, Espín F, Gallego D et al (2014) Colonic smooth muscle cells and colonic motility patterns as a target for irritable bowel syndrome therapy: mechanisms of action of otilonium bromide. Therapeut Adv Gastroenterol 7(4):156–166
Savidge TC (2011) S-nitrosothiol signals in the enteric nervous system: lessons learnt from big brother. Front Neurosci 5:31
Tang Q, Quan X, Yan L et al (2018) Mechanism of sodium hydrosulfide modulation of L-type calcium channels in rat colonic smooth muscle cells. Eur J Pharmacol 818:356–363
Wade GR, Laurier LG, Preiksaitis HG, Sims SM (1999) Delayed rectifier and ca(2+)-dependent K(+) currents in human esophagus: roles in regulating muscle contraction. Am J Physiol 277(4 Pt 1):G885–G895
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37(1):1–17
Yamane S, Kanno T, Nakamura H et al (2014) Hydrogen sulfide-mediated regulation of contractility in the mouse ileum with electrical stimulation: roles of l-cysteine, cystathionine β-synthase, and K+ channels. Eur J Pharmacol 740:112–120
Yan L, Tang Q, Quan X et al (2019) Effects of exendin-4 on colonic motility in rats and its underlying mechanism. Neurogastroenterol Motil 31(2):e13482
Zhao P, Huang X, Wang ZY et al (2009) Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur J Pharmacol 616(1–3):223–228
Acknowledgements
This work was supported by the Natural Science Foundation of Shaanxi Province [Grant number 2021JQ-411] and the National Natural Science Foundation of China [Grant number 82100567].
Author information
Authors and Affiliations
Contributions
QXJ performed research and drafted the paper. ZM, QZJ, KX and XQ collected and analyzed the data. WJH revised the manuscript for important intellectual content. LL designed the study and approved the final version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quan, X., Zhang, M., Qiao, Z. et al. Nitric oxide and ion channels mediate l-cysteine-induced inhibition of colonic smooth muscle contraction. J Muscle Res Cell Motil 45, 11–20 (2024). https://doi.org/10.1007/s10974-023-09664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-023-09664-2