Abstract
Phosphorylation of α-tropomyosin (Tpm1.1), a predominant Tpm isoform in the myocardium, is one of the regulatory mechanisms of the heart contractility. The Tpm 1.1 molecule has one site of phosphorylation, Ser283. The degree of the Tpm phosphorylation decreases with age and also changes in heart pathologies. Myocardial pathologies, in particular ischemia, are usually accompanied by pH lowering in the cardiomyocyte cytosol. We studied the effects of acidosis on the structural and functional properties of the pseudo-phosphorylated form of Tpm1.1 with the S283D substitution. We found that in acidosis, the interaction of the N- and C-ends of the S283D Tpm molecules decreases, whereas that of WT Tpm does not change. The pH lowering increased thermostability of the complex of F-actin with S283D Tpm to a greater extent than with WT Tpm. Using an in vitro motility assay with NEM- modified myosin as a load, we assessed the effect of the Tpm pseudo-phosphorylation on the force of the actin-myosin interaction. In acidosis, the force generated by myosin in the interaction with thin filaments containing S283D Tpm was higher than with those containing WT Tpm. Also, the pseudo-phosphorylation increased the myosin ability to resist a load. We conclude that ischemia changes the effect of the phosphorylated Tpm on the contractile function of the myocardium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorylation/dephosphorylation of sarcomere proteins of cardiomyocytes is one of the important mechanisms for regulating myocardial contraction. Tropomyosin (Tpm) belongs to the phosphoproteins of the sarcomere. Tpm is an α-helical coiled-coil protein that plays an essential role in Ca2+ regulation of striated muscle contractility (McKillop and Geeves 1993). The product of TPM1 gene α-Tpm (or Tpm 1.1) is a predominant Tpm isoform in cardiac and fast skeletal muscles (Perry 2001). The Tpm 1.1 molecule has one site of phosphorylation at near the C-terminus at Ser283 (Mak 1978). Phosphorylation of Tpm increases the interaction of the head-to-tail of its neighboring molecules (Heeley 1994; Heeley et al. 1989; Sano et al. 2000; Lehman et al. 2015) that may influence the cooperative activation of the thin filament (Rao et al. 2009). Additionally, the Tpm phosphorylation reduces Tpm’s affinity for the troponin T1 fragment (TnT) and increases the affinity for the troponin T2 fragment (Heeley 1994). In solution studies, it was found that the Tpm phosphorylation activates ATPase of myosin but does not affect Tpm’s affinity for actin ( Heeley et al. 1989).
In ontogenesis, the status of the Tpm phosphorylation varies considerably, and the degree of its phosphorylation decreases with age (Heeley et al. 1982; Schulz et al. 2013a). In the embryonic period, tropomyosin in the myocardium is phosphorylated up to 80%, whereas after birth, the degree of phosphorylation drops to 30–40% (Heeley et al. 1982). However, the physiological significance of the Tpm phosphorylation is still poorly understood.
On the mouse model, it was found that in nonischemic (remote) regions in the myocardial infarction, the Tpm phosphorylation, as well as the sliding velocity of native thin filaments from this area in an in vitro motility assay, increased (Rao et al. 2007). On the swine model of cardiac arrest followed by reperfusion, an increase in Tpm and troponin (Tn) phosphorylation was found (Woodward et al. 2015). Authors of that work also observed a rise in the maximal sliding velocity and calcium sensitivity of the sliding velocity of native thin filaments in the motility assay (Woodward et al. 2015).
In the transgenic mouse model, a high expression level of pseudo-phosphorylated Tpm (S283D substitution) led to dilated cardiomyopathy and animal death. In contrast, moderate Tpm S283D expression caused myocyte hypertrophy with fibrosis but did not affect lifespan (Rajan et al. 2019). Any expression level did not affect the calcium sensitivity of the myofibers (Rajan et al. 2019). Schulz et al. (2013a, b) did not detect the effect of Tpm dephosphorylation on the essential characteristics of the heart muscle of transgenic mice expressed Tpm when Ser283 was replaced by alanine (S283A substitution). However, in hypertrophic and dilated cardiomyopathy, the degree of the Tpm phosphorylation may affect the severity of hypertrophy (Warren et al. 2008; Schulz et al. 2012; Schulz et al. 2013a). In pressure overload of the heart, a significant decrease in ejection fraction in transgenic animals with S283A was observed, and those data suggest that the reduction in the Tpm phosphorylation impairs the ability of the myocardium to resist mechanical stress (Schulz et al. 2013b). The difference in the results of studies of the physiological role of the Tpm phosphorylation can be explained by the use of experimental conditions, including the duration of the development of the pathological state.
In increased load on the heart and myocardial pathologies, the pH of the cardiomyocyte cytosol decreases. We hypothesize that lowering pH changes the effect of phosphorylated Tpm on actin-myosin interaction in the myocardium. In this work, we studied changes in the structural and functional properties of the pseudo-phosphorylated form of cardiac α-Tpm induced by acidosis and how these changes affect the actin-myosin interaction.
Materials and methods
All procedures involving animal care and handling were performed according to institutional guidelines set forth by Animal Care and Use Committee at the Institute of Immunology and Physiology of RAS and Directive 2010/63/EU of the European Parliament.
Protein preparation
The Tpm and TnT1 (residues 1-158) fragments used in this work were recombinant proteins. The Tpm had Ala-Ser N-terminal extension to mimic the N-terminal acetylation of native Tpm (Monteiro et al. 1994). Pseudo-phosphorylated Tpm with S283D substitution was used as the phosphorylated form of Tpm (Sano et al. 2000). The human Tpm1.1 S283D mutant and WT Tpm were prepared in the bacterial expression plasmid pMW172 by PCR-mediated site-directed mutagenesis using Pfu DNA Polymerase (SibEnzyme, Novosibirsk, Russia) (Matyushenko et al. 2017). For S283D mutagenesis, the 5′-ATATATGAATTCTTATATGTCAGTCATATCGTTGAGAG 3′ oligonucleotide was used (mutant codon is underlined). TnT1 fragment was cloned into pet23a+ plasmid using specific primers: 5′-ATA TAT CAT ATG CAT CAC CAT CAC CAT CAC CTG GAA GTG CTG TTT CAG GGC CCG ATG AGC GAC ATT GAA GAA GTG GTG GAA G 3′ as a forward and 5′-ATA TAT GAA TTC TCA ACG GCG CGC ACG TTC TTC 3′ as a reverse. All constructs were sequenced to verify the correctness.
Tpm expression and purification were performed as described previously (Matyushenko et al. 2017). TnT1 fragment was expressed overnight in C41(dE3) E. coli bacterial cell cultures. Expression was induced by adding 1 mM IPTG until optical density reached 0.6 a.u. Then the cells were pelleted by centrifugation and resuspended in a buffer (50 mM Tris-HCl, 300 mM NaCl, 15 mM imidazole, pH 8.0) after that sonicated and spun down. The supernatant was loaded on HisTrap HP column, and the TnT1 fragment was purified with standard metal-affinity chromatography with 15 mM–500 mM imidazole gradient. Fractions containing TnT1 fragment were collected and treated by 3C protease at 4 °C overnight to remove His-tag. Finally, the protein was dialyzed against 30 mM Hepes-Na buffer with 100 mM NaCl at pH 7.3 for further experimenting.
Porcine cardiac myosin was extracted from the left ventricle by the standard method (Margossian and Lowey 1982). Isoform composition of myosin heavy chains (MHC) was determined using SDS-PAGE (Reiser and Kline 1998). Myosin contained 20% α- and 80% β-MHC and ventricular light chains. Rabbit skeletal actin and pig cardiac troponin were prepared by standard methods (Pardee and Spudich 1982; Potter 1982). For the in vitro motility assay experiments, F-actin was labeled with a 2-fold molar excess of TRITC-phalloidin (Sigma Chemical Co., St Louis, MO, USA).
Differential scanning calorimetry (DSC)
DSC experiments were performed as describer earlier (Matyushenko et al. 2015; Matyushenko et al. 2018) on a MicroCal VP-Capillary DSC differential scanning calorimeter (Malvern Instruments, Northampton, MA 01060, USA) at a heating rate of 1 °C/min in 30 mM Hepes-Na buffer (pH 7.3) containing 100 mM NaCl. The protein concentration was 2 mg/ml. The reversibility of the heat sorption curves was assessed by reheating the sample immediately after it had cooled from the previous scan. The temperature dependence of the excess heat capacity was further analyzed and plotted using Origin software (MicroCal Inc., Northampton, MA, USA). The thermal stability of Tpm species was assesed by the maximum temperature of their thermal transition (Tm). Calorimetric enthalpy (ΔHcal) was calculated as the area under the excess heat capacity function. Deconvolution analysis of the heat sorption curves, i.e. decomposition into separate thermal transitions (calorimetric domains) was performed as described earlier (Matyushenko et al. 2015).
Light scattering
Thermally-induced dissociation of the Tpm-F-actin complex was detected by changes in the light scattering at 90°, as described earlier (Matyushenko et al. 2015, 2017). The measurements were performed at 350 nm on a Cary Eclipse fluorescence spectrophotometer (Varian Australia Pty Ltd, Mulgrave, Victoria, Australia) equipped with a temperature controller and a thermoprobe. All measurements were carried out at a constant heating rate of 1 °C/min. The light scattering of F-actin solutions containing the same concentration of actin (20 μM) as that in the Tpm−F-actin samples was measured before the experiments. The dissociation curves with the temperature dependence of the light scattering for F-actin alone deducted were analyzed by fitting to a sigmoidal decay function (Boltzman). The primary parameter extracted from this analysis is Tdiss, i.e., the temperature at which a 50% decrease in the light scattering occurred.
Viscosimetry
The viscosity measurements were performed on a falling ball micro viscometer Anton Paar AMVn (VA, USA) in 0.5 ml capillary at 25 °C. The specific density of the Tpm solutions was measured with an Anton Paar DMA 4500 (VA USA) and taken into account for accurate viscosity calculation. All measurements were performed at a Tpm concentration of 0.5 mg/ml in a 30 mM Hepes-NaOH, 30 mM NaCl buffer at pH 6.5, 6.8, 7.0, and 7.3. The measurements for each Tpm sample were repeated three times and averaged.2.5.
In vitro motility assay
The protocol of the in vitro motility assay experiments and the composition of buffers used were as described previously (Matyushenko et al. 2015, 2017). All measurements were done at 30 °C. In brief, myosin (300 µg/ml) in AB buffer (25 mM KCl, 25 mM imidazole, 4 mM MgCl2, 1 mM EGTA, and 20 mM DTT, pH 7.5) containing 0.5 M KCl was loaded into the experimental flow cell. After two minutes, 0.5 mg/ml BSA was added for 1 min. Further, 50 µg/ml of non-labeled F-actin in AB buffer with 2 mM ATP was added for 5 min to block nonfunctional myosin heads. Then 10 nM TRITC-phalloidin labeled F-actin was added for five minutes. Unbound F-actin was washed out with AB buffer. Finally, the cell was washed with AB buffer containing 0.5 mg/ml BSA, oxygen scavenger system, 20 mM DTT, 2 mM ATP, 0.5% methylcellulose, 100 nM Tpm/Tn, and appropriate Ca2+/EGTA in proportions calculated with the Maxchelator program (http://www.stanford.edu/~cpatton/webmaxc/webmaxcS.htm).
Fluorescently labeled thin filaments were visualized with Axiovert 200 (Carl Zeiss) inverted epifluorescence microscope equipped with a 100x/1.45 oil-immersion alpha Plan-Fluar objective and an EMCCD iXon-897BV (Andor Technology) videocamera. For each flow cell, ten 30-s image sequences were recorded from different fields of view at 2 frames/s rate.
The filament sliding velocity was measured using the GMimPro software (Mashanov and Molloy 2007) as described (Matyushenko et al. 2017). In every flow cell, the sliding velocities of ~ 50–100 filaments were averaged to get the mean and standard deviation (SD) of the velocity. Experiments were repeated three times, and the means of individual experiments were fit with the Hill equation: V = Vmax × (1+10h(pCa − pCa50))−1, where V and Vmax are current velocity and the maximal velocity at saturating calcium concentration, respectively; pCa50 (i.e., calcium sensitivity) is pCa at which half-maximal velocity was achieved, and h is the Hill coefficient. All values are expressed as mean ± SD. All comparisons were performed by paired t test or Mann–Whitey U est (p < 0.05).
To study the effect of the Tpm pseudo-phosphorylation on its interaction with TnT1, we analyzed the dependence of the sliding velocity of Tpm–F-actin filaments over myosin in the in vitro motility assay on the TnT1 concentration.
The effect of the Tpm pseudo-phosphorylation on the force generation was assessed using NEM-modified myosin as an external load (Haeberle et al. 1992). The experimental protocol was like that described above, except the blocking of nonfunctional myosin heads with non-labeled F-actin was omitted. A mixture of native and NEM-myosin in the total concentration of 100 µg/ml was used in the motility assay at saturating Ca2+ concentration. We analyzed the dependence of the sliding velocity of thin filaments on the percent ratio of NEM-myosin in the mixture added to the flow cell (Haeberle et al. 1992; Shchepkin et al. 2017). The relative force was expressed as a percentage of NEM-myosin, linearly extrapolated to zero velocity, that is the required to stop the filament movement.
Results
Effect of Tpm pseudo-phosphorylation on the thermal stability of its molecule
Studies carried out with differential scanning calorimetry (DSC) showed that the S283D mutation had no significant effect on the thermal denaturation and domain structure of Tpm (Fig. 1a,b; Table 1).
(a, b)Differential scanning calorimeter (DSC) curves for WT Tpm (a) and Tpm S283D (b) preparations and their decomposition into separate thermal transitions (calorimetric domains). The experiments were carried out three times. The maximum temperature and calorimetric enthalpy for domains are given in Table 1. c The pH dependencies of the viscosity of Tpm solutions for WT Tpm and S283D Tpm. Each data point represents the mean ± SD of three experiments. The error of the viscosity values did not exceed ± 4%
The pH dependence of the interaction of neighboring Tpm molecules
Neighboring Tpm molecules make head-to-tail interactions, forming a continuous strand on the surface of the actin filament. The extent of interaction between the ends of neighboring molecules makes it possible to evaluate the measurement of the viscosity of the Tpm solution: the better the interaction between the N- and C-ends of the molecules, the higher the ability to form long strands and, accordingly, the higher the viscosity of the solution. We have measured the viscosity of WT and S283D Tpm solutions and their dependence on pH. The results are shown in Fig. 1c. It is seen that in pH range from 6.5 to 7.3, the viscosity of S283D Tpm increased by more than 50%, while that of WT Tpm virtually did not change.
Thermally induced dissociation of the Tpm-F-actin complex
To test the stability of the Tpm–F-actin complex, we measured temperature dependence of the light scattering of the Tpm–F-actin solutions containing WT or S283D Tpm in the pH range from 6.5 to 7.3. The curves fitted to the normalized changes in the light scattering accompanying Tpm dissociation from F-actin are shown in Fig. 2 and S1. As seen in Table 2 by Tdiss data, the thermostability decreased with pH rise. At pH from 6.8 to 7.3, the thermostability of the complex of F-actin with S283D Tpm was less than that with WT Tpm.
Normalized temperature dependencies of thermally-induced dissociation of the Tpm−F-actin complexes with WT and S283D Tpm. A value of 100% corresponds to the difference in the light scattering of Tpm–F-actin complexes measured at 25 °C and that of pure phalloidin-stabilized F-actin, which was temperature-independent within temperature range used. A decrease in the light-scattering intensity reflects the dissociation of the Tpm–F-actin complex. Samples contained 20 μM F-actin stabilized by 30 μM phalloidin and 10 μM Tpm in 30 mM HEPES, pH 7.3, 100 mM NaCl, 1 mM MgCl2, and 1 mM DTT. The heating rate was 1°C·min−1. The experiments were carried out three times. Tdiss values for WT Tpm and S283D Tpm are presented in Table 2
The effect of pseudo-phosphorylation on the Tpm affinity for TnT1
To estimate the effect of pseudo-phosphorylation on the Tpm affinity for TnT, we analyzed the dependence of the sliding velocity of the Tpm–F-actin filaments over myosin in the in vitro motility assay on the concentration of TnT1 (Fig. 3a–c). Adding TnT1 decreased the sliding velocity of the Tpm–F-actin filaments. To stop the movement of Tpm-F-actin filaments containing WT Tpm, it required less TnT1 than that of the filaments with S283D Tpm.
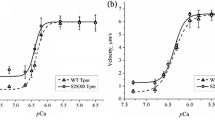
(a–c)The dependence of the sliding velocity of F-actin and the Tpm–F-actin filaments over myosin in the in vitro motility assay on the TnT1 concentration. The concentration of Tpm in AB buffer with ATP was 100 nM. To change the molar TnT1/Tpm ratio, we changed the TnT1 concentration. AB buffer with ATP did not contain calcium ions. Each data point represents mean ± SD of three experiments. (d) The Ca2+ dependence of the sliding velocity of thin filaments containing S283D Tpm and WT Tpm over myosin in the in vitro motility assay. Each data point represents the mean ± SD of three experiments. The data are fit to the Hill equation. Parameters of pCa–velocity relationships are presented in Table 3
The pCa–velocity relationship
To study the effect of the Tpm pseudo-phosphorylation on the calcium regulation of the actin-myosin interaction, we analyzed the Ca2+ dependence of the sliding velocity of thin filaments reconstructed from F-actin, Tn, and WT Tpm or S283D Tpm over cardiac myosin in the motility assay. Lowering pH reduced both the Ca2+ sensitivity of the sliding velocity and the maximal velocity of the filaments (Fig. 4d and Table 3). The Tpm pseudo-phosphorylation did not affect the pCa–velocity relationship (Fig. 4 and Table 3).
(a-c)The dependence of the sliding velocity of thin filaments containing S283D Tpm and WT Tpm on the load, i.e., on percent ratio of NEM-myosin in the mixture with unmodified myosin added to the flow cell at pCa 4. The fraction of NEM-myosin required to stop the filament movement is a measure of relative force, which myosin can develop. Each data point represents mean ± SD of three experiments. The data are shown in Table 3. (d) The effect of Tpm pseudo-phosphorylation on the filament sliding velocity in the presence of 20% NEM-myosin as a load. Each data point represents mean ± SD of three experiments
The effect of Tpm pseudo-phosphorylation on the force-generating ability of myosin
We found that Tpm pseudo-phosphorylation almost did not affect the force-generating ability of myosin at pH 7.5. However, at lowered pH, the force developed by myosin in the interaction with thin filaments containing S283D Tpm was higher than with those containing WT Tpm. Relative force assessed by a fraction of NEM-modified myosin required to stop the filament movement in vitro is shown in Table 3 and Fig. 4a–c.
The effect of the Tpm pseudo-phosphorylation on the filament sliding velocity under a load was estimated by adding in the flow cell a mixture of 20% NEM-myosin with native myosin (Fig. 4d). Under load, lowering pH slowed down the movement of the filaments, and this effect was more pronounced for the filaments with WT Tpm compared to those with S283D Tpm.
Discussion
Normally, the intracellular pH of cardiomyocytes is approximately 7.2 (Garciarena et al. 2013; Swietach et al. 2015; Ford et al. 2017). However, in the heart overload, it can drop by 0.15-0.18 units, and by 0.7-0.8 units in pathology (Garciarena et al. 2013; Swietach et al. 2015; Ford et al. 2017). It was found that low pH leads to a decrease in heart contractility, rhythm disturbance, and cardiomyocyte calcium overload (Fabiato and Fabiato 1978; Gulati and Babu 1989; Harrison et al. 1992; Kohmoto et al. 1990; Garciarena et al. 2013; Swietach et al. 2015; Ford et al. 2017). In these pathologies, the degree of phosphorylation of sarcomere proteins, including Tpm, can either increase or decrease depending on the pathology type. We have studied the influence of acidosis on the properties of pseudo-phosphorylated Tpm at the molecular level. The results of the study demonstrate that the effect of the Tpm pseudo-phosphorylation on its structural and functional properties depends on pH, which can be essential for myocardial function both in the normal and pathological states.
With differential scanning calorimetry (DSC), we ascertained that the S283D mutation has no significant effect on the thermal denaturation and domain structure of Tpm (Fig. 1, Table 1).
We found that at pH 7.3, the viscosity of the S283D Tpm solution is significantly higher than that of WT Tpm in a good agreement with the data of Heely et al. (1989). This result means that the interaction of N- and C-termini in S283D Tpm is stronger than in WT Tpm. Lowering pH from 7.3 to 6.5 led to a two-fold decrease in the viscosity of the S283D Tpm solution, while the viscosity of the WT Tpm solution did not change (Fig. 1c). This result can be explained by the weakening of electrostatic interaction between the N- and C-termini of neighboring Tpm molecules due to H+ binding to the negative charges.
At pH 7.3, pseudo-phosphorylated Tpm reduced the thermal stability of the Tpm–F-actin complex (Fig. 2). Lowering pH increased the stability of the complex of F-actin with pseudo-phosphorylated Tpm to a higher degree than that with WT Tpm. At pH 6.5, the stability of the Tpm–F-actin complex for both Tpm did not differ. Thus the Tpm pseudo-phosphorylation affected the interaction of Tpm with F-actin, and this effect depends on pH. The decrease in the stability of the Tpm–F-actin complex, together with a change in the Tpm-TnT interaction (Heeley 1994), can appreciably affect the thin filament assembly, especially in embryogenesis, when myofibrillogenesis is accompanied by an intensive Tpm phosphorylation (Heeley et al. 1982; Solaro 2011).
Using the in vitro motility assay data, we have confirmed the effect of the Tpm phosphorylation on the Tpm-TnT1 interaction (Fig.3a-c). To stop the movement of Tpm–F-actin filaments containing S283D Tpm, it required more TnT1 than that of the filaments with WT Tpm (Fig. 3a-c), indicating that the pseudo-phosphorylation weakened Tpm’s interaction with TnT1. Earlier, Heeley (Heeley 1994) found that fast skeletal TnT1 is less effective in inhibiting the ATPase activity of myosin when Tpm is phosphorylated.
At lowered pH, the sliding velocity of F-actin, the maximal sliding velocity of thin filaments with WT Tpm, and the calcium sensitivity of the pCa-velocity relationship were reduced (Fig. 3d) that agrees with the results obtained earlier (Sata et al. 1995; Debold et al. 2008). The pseudo-phosphorylated Tpm did not significantly affect the characteristics of the pCa-velocity relationship obtained in the in vitro motility assay. In different experimental models of heart pathologies, it was shown that the extent of the Tpm phosphorylation either increase (Rao et al. 2007; Woodward et al. 2015) or does not change (Avner et al. 2012). On the porcine model of the ischemia and subsequent reperfusion, Woodward et al. (2015) found an increase in phosphorylation of Tpm and troponin I, which enhanced the sliding velocity and calcium sensitivity of native thin filaments. This discrepancy in our and Woodward et al. (2015) results may be because the authors of that work used native thin filaments extracted from the myocardium and regulatory proteins had various post-translational modifications that could affect the calcium regulation of the actin-myosin interaction.
The data on the effect of the Tpm phosphorylation on the Ca2+ activation of the thin filament obtained by different researchers are controversial. Heeley (1994), who used naturally fully phosphorylated and non-phosphorylated Tpm, showed that the Tpm phosphorylation does not affect the Ca2+ sensitivity of the ATPase activity. On the other hand, Schulz et al. (2013b) have not found the effects of the Tpm phosphorylation on the Ca2+ sensitivity of tension of myocardium preparations of transgenic mice. Our results obtained in the in vitro motility assay entirely correspond to those observations, and besides, we found that at pH 7.5, the filament movement at low Ca2+ concentration inhibited incompletely. The only study where an increase in the calcium sensitivity of tension due to the Tpm phosphorylation was observed is the work of Lu et al. (2010). They used a reconstitution of thin filaments in myocardium preparations. The difference in experimental methods may account for this contradiction.
We found that as the pH was lowered from 7.5 to 6.5, pseudo-phosphorylated Tpm increased the force generation (Fig. 4a-c) and the ability of myosin to resist a load (Fig. 4d). Higher force generation in acidosis can be explained by an increase in the duration of the strongly-bound state of myosin on F-actin (Debold et al. 2008). As known, such change in the myosin kinetics may result in increased force generation (VanBuren et al. 1995). The kinetics of myosin cross-bridges is also affected by regulatory proteins (Gordon et al. 1998; VanBuren et al. 1999; Fujita et al. 2002; Homsher et al. 2003; Shchepkin et al. 2017; Kopylova et al. 2019), and mutations in these proteins may influence force generation via cooperative and allosteric mechanisms (Shchepkin et al. 2017; Kopylova et al. 2019).
The rise in force derived from our experiments on the isolated proteins contradicts the results obtained on myocardial preparations. In acidosis, the force developed by myocardial preparations decreases (Kohmoto et al. 1990; Harrison et al. 1992), unlike its rise in the isolated proteins.
The difference between the in vitro and muscle fiber results can be accounted for by several reasons. Solution studies have been carried out at a lower ionic strength (~ 60 mM) conditions with a very low concentration of protein molecules. In contrast, studies on permeabilized fibers were done at physiological ionic strength (~ 200 mM) and physiological concentrations of proteins, which were well-ordered, unlike in vitro studies. And at last, one should consider that in the myocardial pathology, many sarcomere proteins are subjected to post-translational modifications, which can modify the contractile characteristics (Avner et al. 2012; Rao et al. 2007; Woodward et al. 2015). For this reason, the effect(s) of any particular protein is not apparent, while studies on isolated proteins allow one to reveal its role and contribution to pathology development.
These results at the molecular level explain the effect previously obtained by Schulz et al. (2013a, b). The authors did not detect the impact of Tpm dephosphorylation on the basic characteristics of the heart muscle of transgenic mice that express Tpm with S283A substitution (Schulz et al. 2013a, b). However, in pressure overload in transgenic animals, a significant decrease in ejection fraction was observed. Based on these data, the authors concluded that a reduction of the Tpm phosphorylation impairs the ability of the myocardium to resist mechanical stress (Schulz et al. 2013b).
The results of our work demonstrate the effects of the Tpm pseudo-phosphorylation on its structural and functional properties. The Tpm pseudo-phosphorylation affected the interaction of Tpm with F-actin and TnT1 in a pH-dependent manner. At lowering pH, the Tpm pseudo-phosphorylation increased the force-generation ability of myosin and its resistance to a load.
Thus, we can conclude that acidosis affects the characteristics of phosphorylated Tpm, and its influence on the actin-myosin interaction in the heart pronounced to a greater extent than those of non-phosphorylated Tpm. To access the significance of the Tpm phosphorylation in acidosis for the contractile function of the myocardium, in vivo studies are needed.
References
Avner BS, Shioura KM, Scruggs SB et al (2012) Myocardial infarction in mice alters sarcomeric function via post-translational protein modification. Mol Cell Biochem 363(1–2):203–215. https://doi.org/10.1007/s11010-011-1172-z
Debold EP, Beck SE, Warshaw DM (2008) Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol Cell Physiol 295(1):C173–C179. https://doi.org/10.1152/ajpcell.00172.2008
Fabiato A, Fabiato F (1978) Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol 276:233–255. https://doi.org/10.1113/jphysiol.1978.sp012231
Ford KL, Moorhouse EL, Bortolozzi M, Richards MA, Swietach P, Vaughan-Jones RD (2017) Regional acidosis locally inhibits but remotely stimulates Ca2+ waves in ventricular myocytes. Cardiovasc Res 113(8):984–995. https://doi.org/10.1093/cvr/cvx033
Fujita H, Sasaki D, Ishiwata S, Kawai M (2002) Elementary steps of the cross-bridge cycle in bovine myocardium with and without regulatory proteins. Biophys J 82:915–928. https://doi.org/10.1016/S0006-3495(02)75453-2
Garciarena CD, Ma YL, Swietach P, Huc L, Vaughan-Jones RD (2013) Sarcolemmal localisation of Na+/H+ exchange and Na+-HCO3- co-transport influences the spatial regulation of intracellular pH in rat ventricular myocytes. J Physiol 591(9):2287–2306. https://doi.org/10.1113/jphysiol.2012.249664
Gordon AM, Chen Y, Liang B, LaMadrid M, Luo Z, Chase PB (1998) Skeletal muscle regulatory proteins enhance F-actin in vitro motility. Adv Exp Med Biol 453:187–196
Gulati J, Babu A (1989) Effect of acidosis on Ca2+ sensitivity of skinned cardiac muscle with troponin C exchange Implications for myocardial ischemia. FEBS Lett 245(1–2):279–282. https://doi.org/10.1016/0014-5793(89)80237-6
Haeberle JR, Trybus KM, Hemric ME, Warshaw DM (1992) The effects of smooth muscle caldesmon on actin filament motility. J Biol Chem 267(32):23001–23006
Harrison SM, Frampton JE, McCall E, Boyett MR, Orchard CH (1992) Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. Am J Physiol 262(2 Pt 1):C348–C357. https://doi.org/10.1152/ajpcell.1992.262.2.C348
Heeley DH (1994) Investigation of the effects of phosphorylation of rabbit striated muscle alpha alpha-tropomyosin and rabbit skeletal muscle troponin-T. Eur J Biochem 221:129–137
Heeley DH, Moir AJ, Perry SV (1982) Phosphorylation of tropomyosin during development in mammalian striated muscle. FEBS Lett 146(1):115–118. https://doi.org/10.1016/0014-5793(82)80716-3
Heeley DH, Watson MH, Mak AS, Dubord P, Smillie LB (1989) Effect of phosphorylation on the interaction and functional properties of rabbit striated muscle alpha alpha-tropomyosin. J Biol Chem 264(5):2424–30
Homsher E, Nili M, Chen IY, Tobacman LS (2003) Regulatory proteins alter nucleotide binding to acto-myosin of sliding filaments in motility assays. Biophys J 85(2):1046–1052. https://doi.org/10.1016/S0006-3495(03)74543-3
Kohmoto O, Spitzer KW, Movsesian MA, Barry WH (1990) Effects of intracellular acidosis on [Ca2+]i transients, transsarcolemmal Ca2+ fluxes, and contraction in ventricular myocytes. Circ Res 66(3):622–632. https://doi.org/10.1161/01.res.66.3.622
Kopylova GV, Shchepkin DV, Nabiev SR, Matyushenko AM, Koubassova NA, Levitsky DI, Bershitsky SY (2019) Cardiomyopathy-associated mutations in tropomyosin differently affect actin–myosin interaction at single-molecule and ensemble levels. J Muscle Res Cell Motil 40(3–4):299–308
Lehman W, Medlock G, Li XE et al (2015) Phosphorylation of Ser283 enhances the stiffness of the tropomyosin head-to-tail overlap domain. Arch Biochem Biophys 571:10–15. https://doi.org/10.1016/j.abb.2015.02.026
Lu X, Heeley DH, Smillie LB, Kawai M (2010) The role of tropomyosin isoforms and phosphorylation in force generation in thin-filament reconstituted bovine cardiac muscle fibres. J Muscle Res Cell Motil 31(2):93–109. https://doi.org/10.1007/s10974-010-9213-x
Mak A, Smillie LB, Barany M (1978) Specific phosphorylation at serine-283 of alpha tropomyosin from frog skeletal and rabbit skeletal and cardiac muscle. Proc Natl Acad Sci USA 75(8):3588–3592. https://doi.org/10.1073/pnas.75.8.3588
Margossian SS, Lowey S (1982) Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol 85 Pt B:55‐71. doi:https://doi.org/10.1016/0076-6879(82)85009-x
Mashanov GI, Molloy JE (2007) Automatic detection of single fluorophores in live cells. Biophys J 92(6):2199–2211. https://doi.org/10.1529/biophysj.106.081117
Matyushenko AM, Artemova NV, Sluchanko NN, Levitsky DI (2015) Effects of two stabilizing substitutions, D137L and G126R, in the middle part of α-tropomyosin on the domain structure of its molecule. Biophys Chem 196:77–85. https://doi.org/10.1016/j.bpc.2014.10.001
Matyushenko AM, Shchepkin DV, Kopylova GV et al (2017) structural and functional effects of cardiomyopathy-causing mutations in the troponin T-binding region of cardiac tropomyosin. Biochemistry 56(1):250–259. https://doi.org/10.1021/acs.biochem.6b00994
Matyushenko AM, Kleymenov SY, Susorov DS, Levitsky DI (2018) Thermal unfolding of homodimers and heterodimers of different skeletal-muscle isoforms of tropomyosin. Biophys Chem 243:1–7. https://doi.org/10.1016/j.bpc.2018.09.002
McKillop DFA, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65:693–701. https://doi.org/10.1016/S0006-3495(93)81110-X
Monteiro PB, Lataro RC, Ferro JA, Reinach Fde C (1994) Functional alpha-tropomyosin produced in Escherichia coli. A dipeptide extension can substitute the amino-terminal acetyl group. J Biol Chem 269(14):10461–10466
Pardee JD, Spudich JA (1982) Purification of muscle actin. Methods Enzymol 85 Pt B:164‐181. doi:https://doi.org/10.1016/0076-6879(82)85020-9
Perry SV (2001) Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil 22:5–49
Potter JD (1982) Preparation of troponin and its subunits. Methods Enzymol 85 Pt B:241‐263. doi:https://doi.org/10.1016/0076-6879(82)85024-6
Rajan S, Jagatheesan G, Petrashevskaya N, Biesiadecki BJ, Warren CM, Riddle T, Liggett S, Wolska BM, Solaro RJ, Wieczorek DF (2019) Tropomyosin pseudo-phosphorylation results in dilated cardiomyopathy. J Biol Chem 294(8):2913–2923. https://doi.org/10.1074/jbc.RA118.004879
Rao VS, La Bonte LR, Xu Y, Yang Z, French BA, Guilford WH (2007) Alterations to myofibrillar protein function in nonischemic regions of the heart early after myocardial infarction. Am J Physiol Heart Circ Physiol 293(1):654–659. https://doi.org/10.1152/ajpheart.01314.2006
Rao VS, Marongelli EN, Guilford WH (2009) Phosphorylation of tropomyosin extends cooperative binding of myosin beyond a single regulatory unit. Cell Motil Cytoskeleton 66(1):10–23. https://doi.org/10.1002/cm.20321
Reiser PJ, Kline WO (1998) Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am J Physiol 274(3):1048–1053. https://doi.org/10.1152/ajpheart.1998.274.3.H1048
Sano K, Maeda K, Oda T, Maéda Y (2000) The effect of single residue substitutions of serine-283 on the strength of head-to-tail interaction and actin binding properties of rabbit skeletal muscle alpha-tropomyosin. J Biochem 127(6):1095–1102. https://doi.org/10.1093/oxfordjournals.jbchem.a022703
Sata M, Sugiura S, Yamashita H, Fujita H, Momomura S, Serizawa T (1995) MCI-154 increases Ca2+ sensitivity of reconstituted thin filament. A study using a novel in vitro motility assay technique. Circ Res 76(4):626–633. https://doi.org/10.1161/01.res.76.4.626
Schulz EM, Wieczorek DF (2013) Tropomyosin de-phosphorylation in the heart: what are the consequences? J Muscle Res Cell Motil. 34(3–4):239–46. https://doi.org/10.1007/s10974-013-9348-7
Schulz EM, Correll RN, Sheikh HN, Lofrano-Alves MS, Engel PL, Newman G, Schultz Jel J, Molkentin JD, Wolska BM, Solaro RJ, Wieczorek DF (2012) Tropomyosin dephosphorylation results in compensated cardiac hypertrophy. J Biol Chem 287(53):44478–89. https://doi.org/10.1074/jbc.M112.402040
Schulz EM, Wilder T, Chowdhury SA, Sheikh HN, Wolska BM, Solaro RJ, Wieczorek DF (2013) Decreasing tropomyosin phosphorylation rescues tropomyosin-induced familial hypertrophic cardiomyopathy. J Biol Chem 288(40):28925–28935. https://doi.org/10.1074/jbc.M113.466466
Shchepkin DV, Nabiev SR, Kopylova GV, Matyushenko AM, Levitsky DI, Bershitsky SY, Tsaturyan AK (2017) Cooperativity of myosin interaction with thin filaments is enhanced by stabilizing substitutions in tropomyosin. J Muscle Res Cell Motil 38(2):183–191
Solaro RJ (2011) Modulation of cardiac myofilament activity by protein phosphorylation. In: Page E, Fozzard HA, Solaro RJ (Eds) Handbook of physiology: section 2. The cardiovascular system. Oxford University Press, New York, NY, pp 264–300
Swietach P, Spitzer KW, Vaughan-Jones RD (2015) Na+ ions as spatial intracellular messengers for co-ordinating Ca2+ signals during pH heterogeneity in cardiomyocytes. Cardiovasc Res 105(2):171–181. https://doi.org/10.1093/cvr/cvu251
VanBuren P, Harris DE, Alpert NR, Warshaw DM (1995) Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circ Res 77(2):439–44. https://doi.org/10.1161/01.res.77.2.439 (PMID: 7614728)
VanBuren P, Palmiter KA, Warshaw DM (1999) Tropomyosin directly modulates actomyosin mechanical performance at the level of a single actin filament. Proc Natl Acad Sci USA 96(22):12488–12493. https://doi.org/10.1073/pnas.96.22.12488
Warren CM, Arteaga GM, Rajan S, Ahmed RP, Wieczorek DF, Solaro RJ (2008) Use of 2-D DIGE analysis reveals altered phosphorylation in a tropomyosin mutant (Glu54Lys) linked to dilated cardiomyopathy. Proteomics 8(1):100–105. https://doi.org/10.1002/pmic.200700772
Woodward M, Previs MJ, Mader TJ, Debold EP (2015) Modifications of myofilament protein phosphorylation and function in response to cardiac arrest induced in a swine model. Front Physiol 6:199. https://doi.org/10.3389/fphys.2015.00199.eCollection2015
Acknowledgements
The authors thank Dr. Vera Borzova for assistance in the viscosity measurements and A. Kochurova for assistance in an in vitro motility assay experiments.
Funding
This work was funded by the Russian Foundation for Basic Research Grants 17-00-00065 (D.L.), 17-00-00070 (S.B.), 20-04-00130 (S.B.), and 18-34-20085 (D.S.); and State Program AAAA-A19-119010590010-3 (D.L.), and AAAA-A18-118020590135-3 (S.B.). This work was performed using the equipment of the Shared Research Center of Scientific Equipment of IIP UrB RAS. DSC measurements were carried out on the equipment of the Shared-Access Equipment Centre «Industrial Biotechnology» of Federal Research Center «Fundamentals of Biotechnology» of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kopylova, G.V., Matyushenko, A.M., Berg, V.Y. et al. Acidosis modifies effects of phosphorylated tropomyosin on the actin-myosin interaction in the myocardium. J Muscle Res Cell Motil 42, 343–353 (2021). https://doi.org/10.1007/s10974-020-09593-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-020-09593-4