Abstract
Previous studies on the characteristics of stromatolite and micrite have carried out a lot of work, but there was less research in thermochemical characteristics. Here, X-ray Diffraction (XRD) and Fourier-Transform Infrared Spectroscopy were used to determine the mineral composition and organic components of stromatolite and micrite. In addition, TG and DTG were used to analyze their thermochemical characteristics and the non-isothermal decomposition of stromatolite and micrite at multi-heating rates of 5, 10, 20 and 30 K min−1 in nitrogen atmospheres. Moreover, kinetic model function, kinetic parameters of apparent activation energy (E), and pre-exponential factor (A) were calculated by Popescu, Flynn–Wall–Ozawa and Kissinger–Akahira–Sunose. The results of XRD showed that the full width at half maximum (FWHM) of 104 crystal plane of calcite was different although the mineral composition of stromatolite and micrite was calcite. The FWHM value of stromatolites was less than that of micrite, which indicated that the crystallinity of stromatolite was higher. Furthermore, the results of TG analysis showed that the final mass loss of stromatolite was less than that of micrite. Moreover, DTG results showed that the maximum temperature point of mass loss of stromatolite was higher than that of micrite. The average value of E of stromatolite calculated by three different methods was about 252.2 kJ mol−1 but was about 208.2 kJ mol−1 for micrite. Hence, the thermal analysis results showed that the crystallinity of stromatolite were relatively higher. These results provided an important reference for analyzing the thermochemical characteristics of different Lingbi stones, especially stromatolite as a typical microbialite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbialites are biosedimentary rocks, that is, they produced by the growth and physiological activities of benthic microbial communities that cause binding and trapping of detrital sediments, surface mineral precipitation and biomineralization. Among them, the most common were thrombolite and stromatolite [1, 2]. For thrombolite, the dark colored clot are considered to have a large amount of microorganisms, which is considered to be the biogenic part, most of which are in the form of patches. This part is mainly composed of cyanobacteria such as epiphytes and some clastic substances (mineral debris and rock debris) captured or bound by epiphytes. The lighter colored cementation part is considered to have a small amount of microorganisms, which is considered to be the abiogenic part. This part is mainly composed of argillaceous or sparry calcite, as well as a small amount of dolomite, quartz and clay minerals. For stromatolite, another microbialite, the surface of stromatolite has obvious laminar structure, that is, a conjugated laminar unit composed of biological laminae and mineral laminae. The biological laminae is composed of cyanobacteria such as epiphytes and some clastic substances captured or bound by epiphytes, and the mineral laminae is composed of argillaceous or sparry calcite, as well as a small amount of dolomite, quartz and clay minerals [3]. Recently, numerous modern examples and rock records show that the ecosystems represented by microbial rocks are the earliest ecosystems on earth and have important influences on the evolution of the earth's atmosphere and hydrosphere [4, 5].
The thermal decomposition (calcination) process of limestone has been the subject of extensive research over the past few years due to its great practical significance, ranging from the manufacture of architectural paint to the production of industrial calcium oxide (quicklime or lime) [6, 7]. In addition, calcination is the most basic step and has been widely used in different industries. For example, the application of calcination ranges from the ceramic industry to the construction industry (about 80% of the raw material used in the production of cement was provided by calcium oxide in limestone). Recently, the mechanism functions and kinetic parameters of the calcination process were analyzed such as activation energy and pre-exponential factors [8, 9]. Yang used thermogravimetric analyzer to study the thermal decomposition characteristics of limestone at different heating rates and temperatures reached to 1274 K. Moreover, the kinetic parameters (e.g., activation energy and pre-exponential factor) of limestone were calculated by different kinetic models. And the activation energy was 230–350 kJ mol−1, which mainly depends on the heating rate and the composition of limestone [10].
Now, some characteristics of limestone in the process of thermal decomposition (such as mineral diversity, crystallinity and microstructure of minerals) were still difficult to analyze due to the complexity in the process of thermal decomposition. Therefore, important dynamic parameters in the analysis results, such as decomposition temperature, activation energy and mass loss, still have a large variation range. In addition, although a lot of work has been carried out on the different characteristics of micrite and stromatolite, such as thin section observation, petrological characteristic analysis, and major and trace element analysis, there was no exact answer to focus on thermochemical characteristics.
The previously reported by Han et al. (2017) mainly study the microbial calcites (biogenic mineral) induced by cyanobacteria Synechocystis sp. PCC6803 and chemical calcites (abiogenic minerals) prepared by the reaction of sodium carbonate (Na2CO3) with calcium chloride (CaCl2) in the laboratory. The results show that the crystallinity and thermal stability of microbial calcites (biogenic mineral) were higher than chemical calcites (abiogenic minerals) [11]. This result was found in the laboratory, but whether there are similar findings in the field. Therefore, the paper reported by Han et al. (2020) show that the crystallinity and thermal stability of the thrombolite (biogenic limestone) were higher than that of the micrite (abiogenic limestone) in Yishui area of the North China Platform, Shandong Province, China [12]. The most innovations of the current manuscript have two points. The first innovation was to explore whether different regions have similar results, that was, select Suzhou, Anhui Province, China, as current research area. The second innovation was to explore whether the crystallinity and thermal stability of another biogenic limestone (stromatolite), except for thrombolite, are higher than abiogenic limestone (micrite).
In this paper, the field investigation and sampling of Sinian stromatolite and micrite in Northern Anhui were carried out, and the location of sampling point was described in detail. The mineral compositions of the rocks were determined by X-ray Diffraction (XRD) and Fourier-Transform Infrared Spectroscopy (FTIR). Subsequently, the decomposition temperature and activation energy were investigated by using the technique of TG and DTG methods at different heating rates of 5, 10, 20 and 30 K min−1 from 323.15 to 1273.15 K, respectively. The kinetic parameters and mechanism functions were deduced by classical dynamics model (e.g., Flynn–Wall–Ozawa (FWO), Kissinger–Akahira–Sunose (KAS) and Popescu). It lays a foundation for further understanding stromatolite and how to distinguish limestone and stromatolite from the perspective of thermodynamics.
Materials and methods
The description of micrite and stromatolite
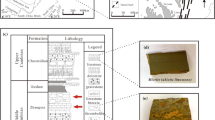
The sampling site of micrite and stromatolite was located in Suzhou, Anhui Province, China, which the Sinian strata in this area were well exposed, and the micrite and stromatolite were well developed and complete in variety as shown in Fig. 1a and b. The Sinian strata were dominated by carbonate rocks, interspersed with a few terrestrial clastic rocks, claystones and siliceous rocks. Limestone was the most abundant and dominant rock in this area, followed by dolomite and land-derived clastic rocks, but claystones and silica rock occurred in lower frequency. There were many types of limestones, of which micrite was the most common, followed by stromatolite, calcirudyte and a few oolitic limestones. Moreover, limestone can be divided into stromatolite (biogenic origin) and micrite (abiogenic origin) according to its origin, and calcite was contained in both stromatolite and micrite. The difference was that there were algae and clastic materials captured or bonded by algae in stromatolite. However, the micrite has no such structure. The micrite was mainly composed of microcrystalline calcite and exposed in the lower part of the Weiji Formation, Zhangqu Formation and Jiudingshan Formation. However, the stromatolite was only exposed in the lower part of the Zhangqu Formation. In this study, two different types of limestones including stromatolite and micrite were collected from the Zhangqu Formation as shown in Fig. 1c with red arrow.
From Fig. 2a and b, the micrite with fine mineral grains (0.01–0.02 mm) was gentle in appearance and stable in thickness, while the surface of stromatolite has obvious laminar structure. Besides, it was shown that this laminar structure contains dark organic laminae and light mineral laminae, that is, a conjugated laminar unit composed of biological laminae and mineral laminae.
X-ray diffraction (XRD) and scanning electron microscopy (SEM)
The mineralogical composition of stromatolite and micrite was analyzed by XRD (Rigaku SmartLab-SE). In this study, the fresh and unweathered stromatolites and micrite were collected from the Zhangqu Formation numbered from ZX-1 to ZX-12. Taking ZQ-1, ZQ-2, ZQ-3 and ZQ-4 as example, but the analysis steps of other samples and the results were similar. Three fresh and unweathered stromatolites were collected from the Zhangqu Formation numbered ZQ-1, ZQ-2 and ZQ-3, and the micrite collected from the same stratum was numbered ZQ-4. Some mineral powders were obtained from the fresh face using an electric engraving machine, and an appropriate amount of the powder was taken out for mineral composition analysis. The experimental conditions were set to a scanning angle (2θ) of 10°–80°, a step size of 0.02 and a scanning speed of 8° min−1. Besides, the sample were sprayed with gold in vacuum and observed by SEM (TESCAN MIRA4), and microstructures were observed simultaneously.
Mineralogical composition and organic functional group analyzed by Fourier-Transform Infrared (FT-IR)
The mineral powders were obtained as described above. Then, the sample was mixed with the dried potassium bromide powder, ground and prepared in an onyx mortar and tablet press (thin laminated transparent disks were most suitable). These two kinds of minerals were subsequently analyzed by using FTIR (Thermo Fisher Scientific IN10) in a scanning range from 4000 to 400 cm−1 with a resolution of 4 cm−1.
Thermochemical characteristics analyzed by thermogravimetric (TG) and derivative thermogravimetric (DTG)
The samples were ground to a powder through an agate mortar and then sieved (200 mesh) for the sake of the same sizes. Subsequently, the thermochemical characteristics of samples were analyzed by thermal gravimetric analyzer (Germany NETZSCH STA 449 F5), which the TG and DTG data were collected simultaneously during the same experiment. Besides, nitrogen was introduced at a flow rate of 50 cm3 min−1 to prevent oxidation at high temperatures, and the experimental temperatures were set at four heating rates of 5, 10, 20 and 30 K min−1 from 323.15 to 1273.15 K under non-isothermal conditions to ensure thermal equilibrium. The sample was weighed at approximately 8 mg in each experiment and all experiments were repeated three times to ensure reproducible results. The TG and DTG curves were plotted based on the relationship between mass and heat flow at different moments using Origin Lab 9.5 software.
Kinetic analysis
The kinetic parameters (activation energy and pre-exponential factor) of micrite and stromatolite were calculated by Flynn–Wall–Ozawa (FWO), Kissinger–Akahira–Sunose (KAS) and Popescu methods [13,14,15], and the mechanism functions were deduced by the Popescu method. Assuming that the initial mass of the sample is m0, the mass at moment t is mt, and the mass of the final remaining sample is m∞, the conversion rate α can be expressed as:
The reaction rate (dα/dt) is conventionally expressed as shown in Eq. (2)
Here, T is the decomposition temperature and \(f\left( \alpha \right)\) was determined according to the reaction mechanism [16, 17], k(T) value can be replaced by the Arrhenius equation [18].
where R is the universal gas constant.
Furthermore, the expression for the heating rate β can be defined as \(\beta = \frac{{{\text{d}}T}}{{{\text{d}}t}}\), therefore
By substituting dT into \(f\left( \alpha \right)\), the new expression is shown in Eq. (5)
The integral form of Eq. (5) can be determined as shown Eq. (6)
Then replacing the k(T) shown in Eq. (6) by Eq. (3), the new rate expression for \(G\left( \alpha \right)_{{{\text{mn}}}}\) is obtained and shown in Eq. (7).
Here, \(\alpha_{{\text{m}}}\) and \(\alpha_{{\text{n}}}\) are two different conversion rates, while Tm and Tn are their corresponding temperatures, besides, \(T_{{\upxi }}\) can be determined as presented in Eq. (8).
The \(G\left( \alpha \right)_{{{\text{mn}}}}\), which proved to be a desired mechanism, can be obtained from the slope of the plots between \(G\left( \alpha \right)_{{{\text{mn}}}}\) and \(1/\beta\) at different decomposition temperature.
From Eq. (7), the activation energy (E) and pre-exponential factor (lnA) can be further easily determined from the slope of the \(- E/RT_{{\upxi }}\) and the intercept of \(\ln \left[ {A/G\left( \alpha \right)} \right]\) as shown in Eq. (9)
In addition, there are two other methods of kinetic analysis (FWO and KAS) to determine the E and lnA, corresponding to the slope of \(- 1.0516E/RT\) and the intercept of \(\ln \left[ {0.0048\;AE/RG\left( \alpha \right)} \right]\), the slope of \(- E/RT\) and the intercept of \(\ln \left[ {AR/EG\left( \alpha \right)} \right]\).
FWO formula be expressed as shown in Eq. (10).
KAS formula be expressed as shown in Eq. (11)
Compared with the Popescu method, the E values calculated by the FWO method and the KAS methods can be obtained under the condition of an uncertain mechanism function (reaction model) [19, 20]. Moreover, three different methods can be compared and verified with each other to improve the accuracy of the results. The final E value was the average of the results obtained by different methods. All curves were obtained by plotting using Origin 9.0 software (OriginLab Corporation).
Results and discussion
SEM and XRD results and analysis
The analysis results of surface morphologies of stromatolite (ZQ-1, ZQ-2 and ZQ-3) and micrite (ZQ-4) are shown in Fig. 3. The results show that aggregate appears composed of calcite and where bioclasts could also observed in Fig. 3a–c marked with a red arrow. In addition, the samples consist of crystalline aggregates of calcite without displaying an apparent highly porous structure. Contrary, micrite (ZQ-4) show that irregular patches of calcite associated with rhombohedral single crystals are found in Fig. 3d and original higher porosity could also observed.
The results of XRD analysis show that the mineral composition of stromatolite and micrite were mainly calcite as shown in Fig. 4, and other mineral composition may have no diffraction peak in this experiment due to their low content and poor crystallinity. Taking ZQ-1 as an example, the main diffraction peak positions (2θ) of minerals found in the X-ray diffraction pattern were 22.9, 29.3, 35.9, 39.3, 43.0, 47.4 and 48.4, which corresponds to the crystal planes (hkl) of (012), (104), (110), (113), (202), (018) and (116), respectively. In addition, the results of full width at half maximum (FWHM) for crystal plane (104) are depicted in Fig. 5. Among them, the value of FWHM for crystal plane (104) of micrite (ZQ-4) was 0.167, while for stromatolite (ZQ-1, ZQ-2 and ZQ-3), the value of FWHM was 0.119, 0.135 and 0.128, which were lower than that of micrite (ZQ-4). It can be seen that the value of FWHM was different although mineral compositions was mainly calcite, which resulting in different crystallinity. The results of FWHM show that the crystallinity of stromatolite was higher than that of micrite.
FTIR results and analysis
As shown in Fig. 6a, the FT-IR results of stromatolite for the characteristic absorption bands of calcite at 712, 875 and 2514 cm−1 marked by the black arrow in the shaded part [21]. Furthermore, the clear vibrational bands at different absorption bands indicated that stromatolites (ZQ-1, ZQ-2 and ZQ-3) contained different types of organic functional groups. For example, the absorption band at 3436 cm−1 might be attributed to the polymeric O–H stretching vibration, and the absorbance peak at 2887 cm−1 may be due to the C–H asymmetric stretching vibration. Additionally, the absorption band at 1818 cm−1 may be due to the stretching vibration of C=O in the carboxylic groups or ester groups. The main CO32− band of calcite around 1430 cm−1 is assigned in the spectra shown in Fig. 6b–e [22, 23]. The appearance of organic functional groups maybe originated from various organic substances produced by microorganisms through their own metabolic activities, which preserved in rocks after death. It can be speculated that the formation of stromatolite may be closely related to organic functional groups [24]. However, there were not so many characteristic absorption bands can been observed from micrite (ZQ-4).
TG and DTG results and analysis
Figure 7a shows the results of TG analysis for stromatolite and micrite at heating rate of 10 K min−1. The final heat loss for stromatolite (ZQ-1, ZQ-2 and ZQ-3) was 43.7%, 44.6% and 44.9%, respectively, while the final heat loss for micrite (ZQ-4) was 58.9%. Moreover, the temperature at the curves to decarbonation of the ZQ-1, ZQ-2, ZQ-3 and ZQ-4 were 1034 K, 1021 K, 1005 K and 954 K, respectively. The higher heat loss indicates that the remaining mass of micrite was lower than stromatolite, which could be attributed to the higher crystallinity of stromatolites, so their stability was higher. Combined with the previous analysis results of the FWHM, the crystallinity of stromatolite was also higher than micrite. Moreover, this phenomenon mainly originated from the different formation conditions of stromatolite and micrite, where the surrounding environments of calcite crystals during the nucleation and growth process were distinctively different. The biogenic part in stromatolite played an important role in precipitation process, that is to say, microorganisms were thought to have greatly influenced in crystallinity and result in a higher decarbonation temperature of stromatolite.
The results of DTG analysis of stromatolite and micrite at a heating rate of 10 K min−1 are shown in Fig. 7b. The experimental results indicated that the pyrolysis process of stromatolite and micrite can be roughly divided into three stages (taking ZQ-4 as an example), where the temperature interval of the first stage ranges from the initial setting temperature of 324 K to T1. The mass loss of samples was due to the evaporation of free water and bound water, and the difference in mass loss was not significant. The results of thermal decomposition carried out at different heating rates showed that mass loss of limestones differed from thermal decomposition of calcium carbonate (the main component of calcite, CaCO3). Since the content of clay minerals in stromatolitic and in micritic were in negligible amount with respect to their CaCO3 content, and mass loss of samples considered were wholly result of decomposition of their CaCO3 content. Furthermore, the results of XRD analysis show that the mineral composition of stromatolite and micrite were mainly calcite as shown in Fig. 4. In current manuscript, the effect that clay minerals associated with limestone on the decarbonation of calcite during thermal analysis maybe the loss of adsorbed water on clay minerals at 474 K [25, 26].
As the temperature continued rising to T2, and this was the second stage where mass loss occurred. In addition, the maximum mass loss for micrite at this stage was 925 K, while the maximum mass loss for stromatolite (ZQ-1, ZQ-2 and ZQ-3) was 1030 K, 1002 K and 990 K, respectively, which were higher than micrite and corresponding to the maximum slope of TG curve. The main reason for mass loss occurring at this stage was the thermal decomposition of calcium carbonate (the main component of calcite). The specific chemical equation was as follows:
The third stage of the pyrolysis reaction was from T3 to the final temperature set by the instrument (1274 K). The graph showed that the curve at this stage remains essentially horizontal, which indicated that the mass of the sample was also essentially unchanged.
Kinetic analysis
During the second stage of thermal decomposition, different conversion rates with their corresponding temperatures were chosen for known heating rate conditions, and subsequently the conversion rates and corresponding temperatures at other heating rate conditions were obtained. The results were substituted into the 16 different mechanistic function models in Table 1 to obtain the most suitable mechanistic function [27, 28]. The conversion interval of stromatolite and micrite was chosen to be in the range of 0.3–0.5 and 0.6–0.8, and the optimum mechanism functions for stromatolite (ZQ-1, ZQ-2 and ZQ-3) and micrite (ZQ-4) are determined in Table 2. Moreover, the high correlation fitting coefficient (R = 0.94–0.99) indicates the reliability of the experimental results.
In addition, the kinetic parameters E and lnA were calculated using the non-isothermal integration methods (Popescu, FWO and KAS) according to Eqs. 9, 10 and 11, respectively, and all results are shown in Table 3. The results show that the E value of stromatolite (ZQ-1) obtained by different kinetic methods at different heating rates were 250.7, 246.5 and 259.2 kJ mol−1, respectively, with an average value of 252.2 kJ mol−1. Besides, the average values of E for stromatolite (ZQ-2, ZQ-3) and micrite (ZQ-4) were 244.5, 262.1, 208.2 kJ mol−1. Besides, the values of E between two samples calculated by different methods have extremely significant differences (P < 0.01). Moreover, the list of another analyzed samples numbered from ZX-1 to ZX-12 including XRD, TG and DTG analysis is shown in Table 4, which the E value of stromatolite range from 264.9 to 285.4 kJ mol−1. However, the E value of micrite were 214.8, 225.6 and 234.6 kJ mol−1. In summary, the activation energy of stromatolite was higher than micrite, and its thermal stability was also higher.
The results of this manuscript showed that similar findings have been found in the current research area (Suzhou, Anhui Province, China). Moreover, other biogenic limestone (stromatolite) also showed a higher crystallinity and thermal stability except for thrombolite, which further enriched the previous publications.
Kinetic compensation effect
The kinetic compensation effect is an important part of thermal analysis kinetics. Generally speaking, the kinetic compensation effect refers to the phenomenon that lnA and E exhibit a linear relationship. So, the mathematical equation can be described as follows:
Equation. 13 indicates that the effect of lnA on the changes of E has partially compensation, in other words, the experimental values of lnA could be predicted from known E values, or the E values can also be predicted from known lnA values. In this paper, Fig. 8a–c shows that kinetic compensation effects of stromatolite (taking ZQ-1 as an example) in FWO, KAS and Popescu methods were \(\ln A = 0.04557E - 6.49543,\;\ln A = 0.06736E - 2.02765\;{\text{and}}\;{\text{ln}}A = 0.20942E - 31.029\) in response to E and lnA values. Moreover, the high correlation fitting coefficient (R = 0.92–0.93) indicates the reliability of the experimental results.
Conclusions
In this study, the thermal decomposition and crystallinity of stromatolites and micrite in northern Anhui (Sinian) were analyzed by using mineralogical, petrological and thermochemical methods, and the following conclusions were obtained. XRD, SEM and FTIR with different crystallinity, surface morphology and crystal structure were analyzed as a foundation of thermal analysis.
Firstly, the XRD results of stromatolite and mudstone show that the crystallinity was completely different, that is, the FWHM for crystal plane (104) of stromatolite was smaller than micrite, which shows that their crystallinity was different, and the crystallinity of stromatolite was higher than micrite although their mineral components were both calcites. Secondly, the results of thermal analysis show that the activation energy and thermal stability of stromatolite were also relatively higher, and the XRD results show that the crystallinity of the stromatolite was higher than micrite. This was also an important reason for the higher activation energy and thermal stability shown by the stromatolites. Therefore, the above experimental results not only provide a full understanding of the mineralogical characteristics for stromatolites, including the crystallinity and thermal stability characteristics, but also provide a very effective way to further distinguish the characteristics of the microbialite.
References
Mingxiang MEI. Revised classification of microbial carbonates: complementing the classification of limestones. Earth Sci Front. 2007;14:222–32.
Corbett P, Hayashi FY, Alves MS. Microbial carbonates: a sampling and measurement challenge for petrophysics addressed by capturing the bioarchitectural components. J Geol Soc Lond. 2015;418:69–85.
Altermann W, Kazmierczak J, Oren A. Cyanobacterial calcification and its rock-building potential during 3.5 billion years of Earth history. Geobiology. 2006;4:147–66.
Chan Y, Lacap DC, Lau MCY. Hypolithic microbial communities: between a rock and a hard place. Environ Microbiol. 2012;14:2272–82.
Cirigliano A, Mura F, Cecchini A. Active microbial ecosystem in iron-age tombs of the Etruscan civilization. Environ Microbiol. 2021;23:3957–69.
Marinoni N, Allevi S, Marchi M. A kinetic study of thermal decomposition of limestone using in situ high temperature X-ray powder diffraction. J Am Ceram Soc. 2012;95:2491–8.
Ávila I, Crnkovic PM, Milioli FE. Thermal decomposition kinetics of Brazilian limestones: effect of CO2 partial pressure. Environ Technol. 2012;33:1175–82.
Vejmelková E, Koňáková D, Doleželová M. Effect of calcined Czech claystone on the properties of high performance concrete: microstructure, strength and durability. Constr Build Mater. 2018;168:966–74.
Belmokhtar N, Ammari M, Brigui J. Comparison of the microstructure and the compressive strength of two geopolymers derived from Metakaolin and an industrial sludge. Constr Build Mater. 2017;146:621–9.
Yang M, Chen X, Yuan B. Inhibition effect of ammonium dihydrogen phosphate on the thermal decomposition characteristics and thermal sensitivity of ammonium nitrate. J Anal Appl Pyrolysis. 2018;134:195–201.
Han Z, Zhuang D, Yan H. Thermogravimetric and kinetic analysis of thermal decomposition characteristics of microbial calcites induced by cyanobacteria Synechocystis sp. PCC6803. J Therm Anal Calorim. 2017;127:1371–9.
Han Z, Zhuang D, Zhao H. Comparative study on thermal behaviors between micrites and thrombolites using thermogravimetric analysis. J Therm Anal Calorim. 2020;139:1229–42.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B. 1966;4:323–8.
Popescu C. Integral method to analyze the kinetics of heterogeneous reactions under non-isothermal conditions-a variant on the Ozawa-Flynn-Wall method. Thermochim Acta. 1996;285:309–23.
Vyazovkin S, Burnham AK, Criado JM. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Pham QT, Zhan YX, Wang FM. Mechanisms and kinetics of non-isothermal polymerization of N, N′-bismaleimide-4, 4′-diphenylmethane with barbituric acid in dimethyl sulfoxide. Thermochim Acta. 2019;676:139–44.
Punyodom W, Meepowpan P, Limwanich W. Determination of the activation parameters for the ring-opening polymerization of ε-caprolactone initiated by Sn (II) and Zn (II) chlorides using the fast technique of DSC. Thermochim Acta. 2022;710:160–79.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Limwanich W, Khunmanee S, Kungwan N. Effect of tributyltin alkoxides chain length on the ring-opening polymerization of ϵ-caprolactone: kinetics studies by non-isothermal DSC. Thermochim Acta. 2015;599:1–7.
Zhou GT, Guan YB, Yao QZ. Biomimetic mineralization of prismatic calcite mesocrystals: relevance to biomineralization. Chem Geol. 2010;279:63–72.
Pan J, Ge X, Liu R. Characteristic features of Bacillus cereus cell surfaces with biosorption of Pb (II) ions by AFM and FT-IR. Colloid Surf B. 2006;52:89–95.
Wu YH, Feng SX, Li B. The characteristics of Escherichia coli adsorption of arsenic (III) from aqueous solution. World J Microbiol Biotechnol. 2010;26:249–56.
Nardi S, Ertani A, Francioso O. Soil–root cross-talking: the role of humic substances. J Plant Nutr Soil Sc. 2017;180:5–13.
Hartshorn SA, Sharp JH, Swamy RN. The thaumasite form of sulfate attack in Portland-limestone cement mortars stored in magnesium sulfate solution. Cem Concr Comp. 2002;24:351–9.
González-Gómez WS, Quintana P, May-Pat A. Thermal effects on the physical properties of limestones from the Yucatan Peninsula. Int J Rock Mech Min. 2015;75:182–9.
Tian L, Chen H, Chen Z. A study of non-isothermal kinetics of limestone decomposition in air (O2/N2) and oxy-fuel (O2/CO2) atmospheres. J Therm Anal Calorim. 2014;115:45–53.
Xu ZX, Cheng JH, Wang Q. The influence of dissociation reaction on ammonium nitrate thermal decomposition reaction. J Therm Anal Calorim. 2019;136:1415–24.
Acknowledgements
This work was supported by the Doctoral Research Start-up Fund of Suzhou University (2020BS008, 2019jb16, 2017jb01); Postdoctoral Research Start-up Fund of Suzhou University (2022BSH001, 2023BSH001); Postdoctoral Research Start-up Fund of Suzhou University (2023BSH002, 2023BSH003); Postdoctoral Research Start-up Fund of Suzhou University (2023BSH004); Fundamental Geology Research Center Project of Suzhou University (2021XJPT55); Youth project of Anhui Natural Science Foundation (2008085QD175); the University Natural Science Research Project of Anhui Province (No. 2022AH051382, KJ2021A1113); Innovation and entrepreneurship training program of Suzhou University (ZCXM22-306, ZCXM22-319, ZCXM22-320). The authors would like to thank Chao Wang from Shiyanjia Lab (www.shiyanjia.com) for the XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhuang, D., Cao, A., Pan, L. et al. Comparative study on thermal decomposition of stromatolite and micrite using the technique of TG and DSC. J Therm Anal Calorim 148, 5529–5541 (2023). https://doi.org/10.1007/s10973-023-12112-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12112-5