Abstract
Effects of Sn and Gd additions on the (β1 + β3) field in the Cu-11%Al-10%Mn alloy were studied by in situ X-ray diffraction, differential scanning calorimetry, non-isothermal kinetics, and magnetization measurements as a function of the temperature. The microstructures were investigated using optical microscopy and scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy. The results showed that the \({\upbeta }_{3} \left( {{\text{Cu}}_{{2}} {\text{AlMn}}} \right) + {\upbeta }_{1} \left( {{\text{Cu}}_{{3}} {\text{Al}}} \right) \to {\upbeta }_{3} \left( {{\text{Cu}}_{{2}} {\text{AlMn}}} \right)\) reaction is strongly dependent on the heating and cooling rates. The Sn addition to the Cu-11%Al-10%Mn alloy stabilizes the ferromagnetic β3 phase. In the Sn-containing alloy, the miscibility gap transition is followed by the martensitic transformation at about 560 K. Both Sn and Gd additions have extended the stability field of the ferromagnetic β3 phase. Nonetheless, the formation of Cu5Gd precipitates slows the miscibility gap transition on heating, which may be associated with the reduction of the ferromagnetic phase amount at room temperature in the Cu11%Al10%Mn3%Gd alloy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shape memory alloys play a key role in many innovative applications, such as sensors, aerospace actuators, energy harvesting, and biomedical devices [1,2,3,4]. An important property of the development of these devices is the magnetic behavior of the material that composes them. For instance, in a temperature or strain sensor based on the shape memory effect [3, 5], it is interesting that the alloy does not strongly respond to the magnetic field, so the sensor signal will not suffer the influence of changes in the magnetic field. In contrast, some actuators and energy harvesting systems that use these materials must present a fast response to the magnetic field [4, 6]. Designing shape memory alloys with a controllable magnetic response may increase the versatility of their application, and the control of magnetic response can be done by adding other elements.
Some Cu-Al-Mn alloys are known as shape-memory materials, and their shape recovery is dependent on the Al and Mn contents and also on the heating rate [7, 8]. Yang et al. showed that the shape recovery of Cu-17.0%at.Al-10.5at.%Mn decreased from 85% at 20 K min−1 to 10% at 1 K min−1, but the addition of 2 at.% of Ni was sufficient to keep the shape recovery above 91% at both heating rates [9]. In the presence of Cr, the quaternary alloy showed abnormal grain growth and potential for superelasticity applications [10]. Furthermore, the ferromagnetic behavior of these alloys has been extensively studied [11,12,13,14,15,16,17] and can be associated with the \({\upbeta }_{3} (\mathrm{L}{2}_{1})\) phase formation with Cu2AlMn stoichiometry [18]. Bouchard and Thomas [19] investigated the phase transition changes by Mn addition from Cu3-xAlMnx alloys and detected a spinodal decomposition below 598 K. Prado et al. [13] studied the magnetic properties of Cu-Al-Mn alloys with Mn content up to 20 at.% and observed micromagnetism below 90 K and superparamagnetism between 90 K and room temperature. Kainuma et al. [20] showed that the change in concentration of Mn and Al in the \(\mathrm{L}{2}_{1}\) alloy can be used to set the Curie temperature (TC) and this parameter increases the higher the content of Mn. Sasmaz et al. [11] observed that the saturation magnetization also increases for higher Mn concentrations in the Cu-Al-(3.21–15.67%)Mn alloys. Marcos et al. [21] investigated the magnetic behavior of Cu-Al-Mn alloys at low temperatures and found a high magnetic hysteresis loop due to the existence of ferromagnetic clusters when the concentration of Mn is around 13 at.%.
The addition of a fourth alloying element in ferromagnetic Cu-Al-Mn alloys has also been reported. Santos et al. [22] suggested that the addition of 3 mass% Ag increases the \({\upbeta }_{1}\) and \({\upbeta }_{3}\) stability field. Canbay et al. [23] showed that the Ni addition increases the slope of the magnetization curve, while the Ti addition showed almost the same behavior as the ternary alloy. The role of Sn and Gd additions in Cu-Al-Mn alloys was reported in a few studies related to phase transformations. Sutou, Kainuma, and Ishida studied the effects of several fourth alloying elements in the Cu-17at.%Al-10at.%Mn alloy, and the addition of Sn decreased the martensitic start temperature and reduced the cold workability of the alloy [24]. Souza, Modesto, and Silva showed that Sn addition to the Cu-11mass%Al-10mass%Mn alloy stabilized the β phase, suppressed ordering reactions, and increased the saturation magnetization of the alloy. They also showed that the Gd addition to the same alloy reduces the saturation magnetization [27]. Oliveira and Silva studied the Cu-9mass%Al-10mass%Mn-3mass%Gd alloy, the Gd addition changed the martensitic transformation, and in its presence, the alloy showed a magnetic behavior strongly dependent on the cooling rate [26]. Brazolin et al. showed that Gd decreased the stability of the martensitic phase and induced the formation of Gd-rich precipitates [25].
In this study, the effects of Sn and Gd additions on the ferromagnetic phase in the Cu-11%Al-10%Mn alloy were investigated based on its dependence on the miscibility gap transition. The phase transition and stable phases in the region outside and inside the miscibility gap were identified using differential scanning calorimetry (DSC), non-isothermal kinetics, in situ X-ray diffraction (XRD), optical microscopy (OM), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), and magnetization measurements as a function of the temperature.
Experimental
The Cu-11%Al-10%Mn, Cu-11%Al-10%Mn-3%Gd, and Cu-11%Al-10%Mn-3%Sn alloys (compositions in mass%) were obtained from pure raw materials (≥ 99.5%) using an arc melting furnace under argon atmosphere. In order to achieve homogenization and more stable phase formation (annealed samples), the alloy ingots of 10 g were kept for 120 h at 1123 K in an argon atmosphere, followed by slow cooling at 1 K min−1. In sequence, the annealed ingots were cut and the samples were prepared for different characterizations. Optical micrographs (OM) were registered using an Olympus SC 30 camera in an Olympus BX41M-LED microscope. A JEOL JSM-6610LV scanning electron microscope (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) was used to investigate the microstructures. Both SEM and OM micrographs were acquired from etched samples, and the etching solution was composed of FeCl3 0.1 mol L−1 and HCl 0.5 mol L−1. Non-powdered samples were used to acquire in situ X-ray diffraction patterns at temperatures inside and outside of the miscibility gap. For in situ X-ray diffraction the furnace with helium atmosphere heated the samples up to 573 K (out of the miscibility gap), maintaining this temperature for 15 min to ensure stabilization, and then the first diffractogram was acquired. In sequence, the samples were cooled at a rate of 1 K min−1 down to 473 K and after 15 min the equipment performed the X-ray diffractogram. A Q20 TA instrument carried out differential scanning calorimetry (DSC) curves using platinum pans, argon flow of 50 mL min−1, and heating/cooling rate of 10 K min−1, as seen in Fig. 1. DSC curves at 2, 4, 6, 8, and 10 K min−1 were also obtained to perform the kinetic analysis on heating. All DSC curves were normalized in relation to the sample mass. In order to provide information about the formation and decomposition of the ferromagnetic phase, a VSM 4500 EG&G Princeton Applied Research with electromagnetic Walker Scientific Inc measured the magnetic moment as a function of the temperature with a heating rate of 10 K min−1 and uncontrolled cooling rate for an applied magnetic field of 600 Oe.
The kinetic analyses were performed from DSC curves of annealed samples from 313 to 673 K at rates of 2, 4, 6, 8, and 10 K min−1 on heating. The transformed phase fraction (y) was calculated from the enthalpy of transition, as described in Eq. 1, where ΔHT is the enthalpy change of the thermal event and ΔHi is the enthalpy change from the start temperature to a selected one. The activation energy (Ey) was calculated at y values between 0.1 and 0.9, with a step of 0.005, using the linear adjustment of the experimental data to Friedman’s Equation in Eq. 2, where R is the gas constant, \({\alpha }_{\mathrm{i}}\) is the heating rate, and Ty, i is the absolute temperature at a selected y and α.
Results and discussion
The microstructures that were obtained for the Cu-11%Al-10%Mn, Cu-11%Al-10%Mn-3%Gd, and Cu-11%Al-10%Mn-3%Sn alloys after cooling at 1 K min−1 are shown in Fig. 2. These OM images exhibit the microstructure of the alloys inside the miscibility gap. In this case, it is possible to observe different microstructures with the additions of Gd and Sn. The \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) and \({\upbeta }_{1}({\mathrm{Cu}}_{3}\mathrm{Al})\) phases were expected as a product of a spinodal reaction that is a well-known phase transition for Cu-Al-Mn alloys [20]. Figure 2a shows the microstructure for the Cu-11%Al-10%Mn alloy after slow cooling, showing grains of β phases, while in Fig. 2b some different phases can be noticed in the Cu-11%Al-10%Mn-3%Gd alloy, but the β phases grains are not well-defined. This phase can be related to the Gd-rich precipitates. Figure 2c shows the optical micrograph obtained for the Cu-11%Al-10%Mn-3%Sn alloy. In this image, it is possible to see a homogeneous surface without a clear definition of the grains of β phases, as seen in Fig. 2a. This does not mean that the \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) and \({\upbeta }_{1}({\mathrm{Cu}}_{3}\mathrm{Al})\) phases were not produced, but it indicates that the alloy microstructure may be associated with the formation of another characteristic phase, in this case, the martensitic one, since tin is a stabilizing element of the β phase in Cu-Al alloys.
Figure 3 shows SEM image and EDX spectra obtained for the Gd-containing alloy from the precipitate (region 1) and matrix (region 2), as indicated in Fig. 3a. The matrix and precipitates of alloy are constituted by Gd and Cu atoms, and their stoichiometric suggests the formation of precipitates with Cu5Gd and Cu5.44Gd0.78 compositions, as already reported in [27]. These precipitates have the same composition as those detected in the Cu-11%Al-3%Gd alloy [28].
Figure 4 shows DSC curves acquired during the heating, cooling, and reheating cycle from the samples initially as-annealed, as well the enthalpy changes for each thermal event are shown in Table 1. The thermal event in Fig. 4a refers to the \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})+{\upbeta }_{1}({\mathrm{Cu}}_{3}\mathrm{Al})\to {\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) reaction [20]. Therefore, inside the miscibility gap the ferromagnetic \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) and paramagnetic \({\upbeta }_{1}({\mathrm{Cu}}_{3}\mathrm{Al})\) phases are stable and out of this field the paramagnetic \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) phase is found. This suggests the occurrence of a magnetic disordering from the ferromagnetic \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) phase at temperatures around the \({\upbeta }_{1}({\mathrm{Cu}}_{3}\mathrm{Al})\) phase dissolution. This thermal event was observed in all studied alloys on heating (Fig. 4a), but the peak area in each thermal event was dependent on the alloying element added. The peak area corresponds to the enthalpy change and can be associated with the transformed phase fraction. For the Cu-11%Al-10%Mn alloy, it is possible to see that on heating (Fig. 4a) and cooling (Fig. 4b) there is a decrease of 7.6 times in enthalpy values. This was already expected because of the difference in the applied cooling rates. Before the initial heating, the samples were cooled with a rate of 1.0 K min−1 and, in DSC experiment, the cooling rate was 10 K min−1. This can reduce the fraction of the produced β1 and β3 phases, but on reheating (Fig. 4c) the enthalpy value was higher than that obtained on cooling, suggesting that other phases are decomposed in this temperature range. In this alloy, the α, T3, and γ1 phases can also be formed on slow cooling; any amount of these could be decomposed on reheating. When Gd is added, the peak intensity is modified, indicating that this element interferes with β1 and β3 phase formation. Enthalpy changes were not verified on cooling and reheating of the Gd-containing alloy, showing that the modification in cooling rate is the dominant factor for the β1 and β3 formation in the Cu-11%Al-10%Mn-3%Gd alloy. For Sn-containing alloy the enthalpy values are few modified on heating, cooling, and reheating. This indicates that this alloy is not sensible to the cooling/heating rates used in this study. Concerning the three studied alloys, the Sn-containing one exhibited a higher value of enthalpy change on the initial heating and it is less dependent on the heating/cooling rate, thereby suggesting the presence of a possible parallel diffusionless reaction.
It can be noticed in Fig. 5a that the peak position of the thermal event in the Cu-11%Al-10%Mn alloy shifts when the heating rate increases, showing a relation between the heating rate and the temperature of the thermal event. This is also noticed in the transformed phase fraction curve in Fig. 5b, indicating that the reaction starts temperature and ending temperature changes with the heating rate. The Gd-containing alloy also shows the same dependence on the heating rate, as seen in Fig. 5c, d. However, in Fig. 5e, the DSC curves obtained for the Cu-11%Al-10%Mn-3%Sn alloy almost were not shifted up when the heating rate increased, which shows that this thermal event may have a diffusionless contribution due to the presence of a parallel reaction. Even though the thermal events from the Cu-11%Al-10%Mn and Cu-11%Al-10%Mn-3%Gd alloys have the main contribution from the miscibility gap transition, in the Cu-11%Al-10%Mn-3%Sn, a multiple peak analysis must be made to fully understand the phase transformations associated with the thermal event.
The multiple peak analysis from the thermal event in the Cu-11%Al-10%Mn-3%Sn alloy is shown in Fig. 6a, and two parallel reactions can be attributed to it. On the one hand, the first event P1, at about 525 K, is a thermally activated process because it is shifted up when the heating rate is increased as seen in Fig. 6b. In addition, P1 presents the average enthalpy change of (8.15 ± 0.66) J g−1, whose value is in the same range of that observed for the \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})+{\upbeta }_{1}({\mathrm{Cu}}_{3}\mathrm{Al})\to {\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) reaction in the Cu-11%Al-10%Mn and Cu-11%Al-10%Mn-3%Gd alloys on first heating (see Table 1). Therefore, it can be the same reaction detected in P1. On another hand, the second peak P2, at about 560 K, is a diffusionless transition because it remains at the same temperature even when the heating rate is changed. In this same temperature range, Canbay et al. [29] showed that the as-quenched Cu-10.6Al-1.26Sn (at.%) alloy undergoes reverse martensitic transformation. Therefore, the event P2 could be associated with the reverse martensitic transformation, which indicates that even on slow cooling the austenite β phase does not fully decompose in the Cu-11%Al-10%Mn-3%Sn alloy.
a Multiple peak analysis of the thermal event in Fig. 4a, b the results of multiple peak analysis at a heating rate of 2, 4, 6, 8, and 10 K min−1, and c transformed phase fraction as a function of the temperature for the first peak in (b)
The kinetic effects of the Sn and Gd additions on the \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})+{\upbeta }_{1}({\mathrm{Cu}}_{3}\mathrm{Al})\to {\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) reaction that occurs in the Cu-11%Al-10%Mn alloy can be evaluated using the isoconversional model-free kinetics as described in the ‘Experimental’ section. Considering the curves from the transformed phase fraction in Figs. 5b, d and 6c, the activation energy as a function of the transformed phase fraction was calculated by linear adjustment of Friedman’s equation to the experimental data, as seen in Fig. 7. The regression coefficient (r2) shows a good adjustment of the model to the experimental data with r2 values above 0.9 in the analyses for the Cu-11%Al-10%Mn and Cu-11%Al-10%Mn-3%Gd alloys, while the values for the Sn-containing alloy are above 0.8 until the 64% of transformed phase fraction. Even though the miscibility gap transition and the reverse martensitic transformation events were separated in the multiple-step analysis (see Fig. 6), the Ey calculation was strongly affected by the thermal peaks overlapping. Therefore, the regression coefficient decreasing at higher y values was observed (see insert in Fig. 7) where those transformations achieved a huge overlapping. It can also be observed by the progressive increasing of the error bars. The studied reaction presents an average Ey of 249 kJ mol−1 in the Cu-11%Al-10%Mn alloy, and in all studied alloys, the process slows as y increases. This transition seems to be favored by the Sn and Gd addition at the beginning of the reaction, which may be related to nucleation steps. Nonetheless, the Cu5Gd precipitates can act as a physical barrier to the \({\upbeta }_{3}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) phase growing, and the Gd-containing alloy had its activation energy strongly increased (113%) as the transformation was processed, once Ey is 151 kJ mol−1 at 10% and 322 kJ mol−1 at 90% of the transformed fraction.
In order to confirm the structure of the expected phases, in situ X-ray diffraction patterns were acquired out of the miscibility gap (573 K) and inside it (473 K) after cooling at a rate of 1 K min−1. In the XRD patterns at 573 K, the Cu-11%Al-10%Mn alloy (Fig. 8a) presented more intense \({\upbeta }_{3}\) phase peaks than \(\mathrm{\alpha }(\mathrm{Cu})\), \({\mathrm{T}}_{3}({\mathrm{Cu}}_{3}{\mathrm{Mn}}_{2}\mathrm{Al})\), and \({\upgamma }_{1}({\mathrm{Cu}}_{9}{\mathrm{Al}}_{4})\) phases, which was expected considering the literature data [25, 30, 31]. At 473 K, the peaks for the \({\beta }_{3}\) phase became less intense at about 2θ = 95° and the \({\upbeta }_{1}\) phase was detected at about 2θ = 42° and 2θ = 74°. When the \({\upbeta }_{3}\) and \({\upbeta }_{1}\) phases coexist, the \({\upbeta }_{3}\) phase may have ferromagnetic behavior as previously mentioned.
Considering the X-ray diffraction pattern performed at 473 K for the Cu-11%Al-10%Mn-3%Gd alloy and shown in Fig. 8b, the \({\upbeta }_{1}\) phase was observed at 2θ = 42° and 2θ = 54°. Nevertheless, no intensity reduction for the \({\upbeta }_{3}\) phase was identified. In this case, the \(\mathrm{\alpha }\), \({\mathrm{T}}_{3}\), Cu5Gd, and Cu5.44Gd0.78 phases grow as the temperature reduces. The high content of Cu in the Cu5Gd and Cu5.44Gd0.78 precipitates increases the available Al amount and contributes to widening the stability field in which the ferromagnetic phase exists, but it is not sufficient to overcome the stabilization of paramagnetic \({\upbeta }_{3}\) phase at room temperature by the Gd addition, as previously mentioned [25].
In Fig. 8c, the XRD pattern acquired for the Cu-11%Al-10%Mn-3%Sn alloy at 573 K presented only two significant peaks. However, the diffractograms at both temperatures are useful to identify the \(\upbeta\)Mn phase formation on cooling in the same temperature range in which the paramagnetic β3 phase decomposition is observed. The β1 and the martensitic β1’ phases were detected at 473 K.
DSC curves are not enough to predict the effect of the ferromagnetic β3 phase considering the temperature of the thermal event, since the β1 phase dissolution and magnetic disordering of the β3 phase can assume slightly different temperatures [32]. This way, the investigation of the alloy magnetic behavior can contribute to this discussion, considering that only the \({\upbeta }_{3}\) phase can show ferromagnetism.
Figure 9 shows the magnetization change as a function of the temperature acquired during heating and cooling. On heating, the initial point of the magnetization measurements is associated with as-annealed samples and inside the miscibility gap. At this initial point, the Gd-containing alloy showed the lowest magnetization value at room temperature, thus suggesting that the Gd addition reduced the relative fraction of the ferromagnetic \({\upbeta }_{3}\) phase. Considering that the enthalpy values obtained on heating of the Cu-11%Al-10%Mn and Cu-11%Al-10%Mn-3Gd alloys (shown in Table 1) are similar, the decrease in relative fraction of the ferromagnetic \({\upbeta }_{3}\) phase can be related to the increase in β1 phase fraction or stabilization of the paramagnetic \({\upbeta }_{3}\) phase at room temperature. This behavior may be attributed to the increase in activation energy values of the \(\upbeta\) phase decomposition on cooling because of the presence of Cu5Gd, and Cu5.44Gd0.78 precipitates. In addition, it was reported that the presence of Gd [25] may stabilize the paramagnetic \({\upbeta }_{3}\) phase at room temperature [33].
On samples heating (red line in Fig. 9), the magnetization values reach zero at 535, 543, and 578 K for the Cu-11%Al-10%Mn, Cu-11%Al-10%Mn-3%Gd, and Cu-11%Al-10%Mn-3%Sn alloys, respectively. These values provide the temperature of magnetic disordering of the entire β3 phase on heating and also show that a modification in this process occurs with the addition of Sn and Gd alloying elements. It is important to notice that on cooling (blue curve, in Fig. 9) all alloys presented a magnetization change, but the Cu-11%Al-10%Mn and Cu-11%Al-10%Mn-3%Gd alloys always exhibited a lower magnetization. This suggests that the fraction of ferromagnetic \({\upbeta }_{3}\) phase produced during uncontrolled cooling is lower than that formed on cooling at a rate of 1 K min−1. For the Sn-containing alloy, the magnetization is slightly shifted in relation to the heating, but at room temperature the values found are similar. This is in agreement with the results seen in Table 1, which indicated enthalpy values nearby during the thermal cycle.
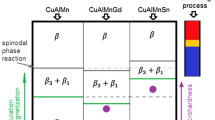
Figure 10a shows a schematic diagram considering the critical temperature of the \({\upbeta }_{3(\mathrm{f})}({\mathrm{Cu}}_{2}\mathrm{AlMn})+{\upbeta }_{1}({\mathrm{Cu}}_{3}\mathrm{Al})\to {\upbeta }_{3(\mathrm{p})}({\mathrm{Cu}}_{2}\mathrm{AlMn})\) transition as a function of the Al content. The results of this study were compared to those found in the literature [22]. In Fig. 10a, the Gd and Sn additions seem to widen the stability field of the \({\upbeta }_{1}\) and ferromagnetic \({\upbeta }_{3}\) phases in the Cu-11%Al-10%Mn-3%Sn and Cu-11%Al-10%Mn-3%Gd alloys. As seen in Fig. 10b, the Cu-11%Al-10%Mn-3%Sn alloy showed the highest transition temperature. Results about the Sn-containing alloy were not reported in the consulted literature. Nonetheless, the possible increase in \(\left({\upbeta }_{3\left(\mathrm{f}\right)}+{\upbeta }_{1}\right)\) stability field and also the martensitic transformation occurrence in the same range of the miscibility gap, as seen for the Cu-11%Al-10%Mn-3%Sn alloy, may be useful for magnetic and shape memory applications.
Conclusions
In this study, the relation between the ferromagnetic behavior and the miscibility gap transition of Cu-11%Al-10%Mn, Cu-11%Al-10%Mn-3%Gd, and Cu-11%Al-10%Mn-3%Sn alloys was evaluated on heating and at different cooling rates. The main conclusions can be listed as follows:
-
(i)
The miscibility gap transition occurred in all studied alloys on heating after the initial thermal treatment at 1 K min−1. The thermal events on heating were registered between 420 and 600 K and presented enthalpies of transition around of 7.0 J g−1.
-
(ii)
The miscibility gap transition was showed to be dependent on the cooling/heating rates in the Cu-11%Al-10%Mn and Cu-11%Al-10%Mn-3%Gd alloys. The enthalpy of transition on heating was reduced from 7.3 J g−1 after cooling at 1 K min−1 to 3.9 J g−1 after cooling at 10 K min−1 in the Cu-11%Al-10%Mn alloy. In another hand, when Gd was added, the transition was suppressed after the cooling at 10 K min−1.
-
(iii)
The Sn addition to the Cu-11%Al-10%Mn alloy added a parallel reaction, possibly the martensitic transformation, at the temperature range of the miscibility gap transition.
-
(iv)
In all studied alloys, the miscibility gap transition is slowed down when the transformed phase fraction increases. The initial activation energy of that process is reduced by the Sn and Gd addition, but the presence of precipitates in the Cu-11%Al-10%Mn-3%Gd alloys slows the growing steps in this transformation.
-
(v)
The Gd addition to the Cu-11%Al-10%Mn alloy increased the Al atoms availability due to the precipitation of the Cu5Gd and Cu5.44Gd0.78 phases, which widened the stability field of the ferromagnetic phase.
References
Roubíček T, Stefanelli U. Magnetic shape-memory alloys: thermomechanical modelling and analysis. Contin Mech Thermodyn. 2014;26:783–810. https://doi.org/10.1007/s00161-014-0339-8.
Zakharov D, Lebedev G, Cugat O, Delamare J, Viala B, Lafont T, et al. Thermal energy conversion by coupled shape memory and piezoelectric effects. J Micromech Microeng. 2012;22:094005. https://doi.org/10.1088/0960-1317/22/9/094005.
Nespoli A, Rigamonti D, Villa E, Passaretti F. Design, characterization and perspectives of shape memory alloy elements in miniature sensor proof of concept. Sens Actuators A Phys. 2014;218:142–53.
Hartl DJ, Lagoudas DC. Aerospace applications of shape memory alloys. Proc Inst Mech Eng G J Aerosp Eng. 2007;221:535–52. https://doi.org/10.1243/09544100JAERO211.
Amarante dos Santos F. Shape-memory alloys as macrostrain sensors. Struct Control Health Monit. 2017;24:e1860. https://doi.org/10.1002/stc.1860.
Lindquist P, Hobza T, Patrick C, Müllner P. Efficiency of energy harvesting in Ni–Mn–Ga shape memory alloys. Shape Mem Superelast. 2018;4:93–101. https://doi.org/10.1007/s40830-018-0158-z.
Milhorato FR, Mazzer EM. Effects of aging on a spray-formed Cu-Al-Ni-Mn-Nb high temperature shape memory alloy. Mater Sci Eng A. 2019;753:232–7.
Zhou L, Lan J, Liu J, Li X, Shi B, Zheng S. Effect of gradient heat treatment on microstructure and properties of Cu-Al-Mn shape memory alloy. Materials. 2019;12:1–11.
Yang Q, Yin DQ, Ge JQ, Chen J, Wang SL, Peng HB, et al. Suppressing heating rate-dependent martensitic stabilization in ductile Cu-Al-Mn shape memory alloys by Ni addition: an experimental and first-principles study. Mater Charact. 2018;145:381–8.
Yang S, Zhang J, Chi M, Yang M, Wang C, Liu X. Excellent superelasticity of Cu-Al-Mn-Cr shape memory single crystal obtained only through annealing cast polycrystalline alloy. Scr Mater. 2019;165:20–4.
Şaşmaz M, Bayri A, Aydoğdu Y. The magnetic behavior and physical characterization of Cu–Mn–Al ferromagnetic shape memory alloy. J Supercond Nov Magn. 2011;24:757–62. https://doi.org/10.1007/s10948-010-0934-2.
Emre Gerdan Y, Aldirmaz E, Guler M, Tanak H, Guler E. Martensitic transformation and magnetic properties of the CuAl, CuAlMn, and CuAlMnZn alloys. J Supercond Nov Magn. 2018;31:3919–23. https://doi.org/10.1007/s10948-018-4658-z.
Prado MO, Lovey FC, Civale L. Magnetic properties of Cu–Mn–Al alloys with shape memory effect. Acta Mater. 1998;46:137–47.
Lanzini F, Alés A. The role of magnetism in the formation of the two-phase miscibility gap in β Cu-Al-Mn. J Magn Magn Mater. 2015;395:234–9. https://doi.org/10.1016/j.jmmm.2015.07.068.
Wachtel E, Deissenrieder F. Magnetothermal investigations of Cu-Al-Mn alloys (field of the Heusler-phase). J Magn Magn Mater. 1976;4:292–301.
Obradó E, Vives E, Mañosa L, Planes A, Martínez B. Spin-glass phase in the intermetallic Cu-Al-Mn compound. J Magn Magn Mater. 1999;196–197:634–6.
Zárubová N, Novák V. Phase stability of CuAlMn shape memory alloys. Mater Sci Eng A. 2004;378:216–21.
Marcos J, Mañosa L, Planes A, Romero R, Castro ML. Kinetics of the phase separation in Cu–Al–Mn alloys and the influence on martensitic transformations. Philos Mag. 2004;84:45–90. https://doi.org/10.1080/14786430310001604994.
Bouchard M, Thomas G. Phase transitions and modulated structures in ordered (Cu-Mn)3Al alloys. Acta Metall. 1976;23:1485–500.
Kainuma R, Satoh N, Liu XJ, Ohnuma I, Ishida K. Phase equilibria and Heusler phase stability in the Cu-rich portion of the Cu-Al-Mn system. J Alloys Compd. 1998;266:191–200.
Marcos J, Vives E, Mañosa L, Planes A, Duman E, Acet M. Criticality in Cu–Al–Mn hysteresis loops. J Magn Magn Mater. 2004;272–276:E515–6.
Santos CMA, Adorno AT, Stipcich M, Cuniberti A, Souza JS, Bessa CVX, et al. Effects of Ag presence on phases separation and order-disorder transitions in Cu-xAl-Mn alloys. Mater Chem Phys. 2019;227:184–90. https://doi.org/10.1016/j.matchemphys.2019.02.016.
Canbay CA, Genc ZK, Sekerci M. Thermal and structural characterization of Cu-Al-Mn-X (Ti, Ni) shape memory alloys. Appl Phys A Mater Sci Process. 2014;115:371–7.
Sutou Y, Kainuma R, Ishida K. Effect of alloying elements on the shape memory properties of ductile Cu-Al-Mn alloys. Mater Sci Eng. 1999;A275–275:375–9.
Souza JS, Modesto DA, Silva RAG. Thermal behavior of the as-cast Cu–11Al–10Mn alloy with Sn and Gd additions. J Therm Anal Calorim. 2019;138:3517–24. https://doi.org/10.1007/s10973-019-08277-7.
Oliveira AB, Silva RAG. Thermomagnetic behavior of an as-quenched Cu-Al-Mn-Gd alloy. Mater Chem Phys. 2018;209:112–20.
Brazolin GF, Canbay CA, Ozgen S, Oliveira AB, Silva RAG. Effects of Gd addition on the thermal and microstructural behaviors of the as-cast Cu–9 %Al and Cu–9 %Al–10 %Mn alloys. Appl Phys A. 2016;22:928. https://doi.org/10.1007/s00339-016-0474-0.
Souza JS, Silva RAG. Microstructure evolution and phase transitions of the annealed Cu–11%Al alloy with Sn and Gd additions. Metallogr Microstruct Anal. 2019;8:782–94. https://doi.org/10.1007/s13632-019-00586-5.
Canbay CA, Karaduman O, Ünlü N, Özkul İ. An exploratory research of calorimetric and structural shape memory effect characteristics of Cu–Al–Sn alloy. Phys B Condens Matter. 2020;580:411932.
Counioux JJ, Macqueron JL, Robin M, Scarabello JM. Phase transformations and shape memory effect in copper-aluminium-manganese alloys. Scr Metall. 1988;22:821–5.
Obradó E, Frontera C, Mañosa L, Planes A. Order-disorder transitions of Cu-Al-Mn shape-memory alloys. Phys Rev B Condens Matter Mater Phys. 1998;58:14245–55.
Pilz CB, Matsumura EL, Paganotti A, Cornejo DR, Silva RAG. Microstructure and phase stability of CuAlMnAgZr multicomponent alloys. Mater Chem Phys. 2020;241:122343. https://doi.org/10.1016/j.matchemphys.2019.122343.
Brazolin GF, Silva CCS, Silva LS, Silva RAG. Phase transformations in an annealed Cu–9Al–10Mn–3Gd alloy. J Therm Anal Calorim. 2018;134:1405–12. https://doi.org/10.1007/s10973-018-7586-z.
Acknowledgements
The authors are thankful to LNLS by Synchrotron radiation (Proposal 20170704) and Sérgio Antônio Romero from the Laboratory of Magnetic Materials of the Institute of Physics of USP by magnetic measurements. The authors thank CNPq (grant no. 153962/2018-6). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)- (Grant no. 2019/06717-0).
Author information
Authors and Affiliations
Contributions
JSS helped in investigation, formal analysis, visualization, writing—original draft. LSS performed investigation, writing—original draft, formal analysis, software. CVXB contributed to investigation, formal analysis, visualization, writing—original draft. RAGS was involved in writing—review & editing, supervision, resources, funding acquisition.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Souza, J.S., Silva, L.S., Bessa, C.V.X. et al. Effects of Sn and Gd on the (β1 + β3) stability field in the Cu-11%Al-10%Mn alloy. J Therm Anal Calorim 148, 2357–2366 (2023). https://doi.org/10.1007/s10973-022-11918-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11918-z