Abstract
In this article, data on the distribution of sulfur in Estonian kukersite shale oil obtained using pyrolysis at temperatures below 520 °C were presented. It was found that the highest concentration of sulfur is present in the fractions boiling between about 150 and 190 °C. Also, we studied the effect of N2, N2/steam, CO2, and CO2/steam environments on the distribution of sulfur in pyrolysis products and concentrations of some sulfur compounds in shale oil. The results show that thiophenic compounds comprise most of the sulfur compounds in crude kukersite shale oil. Furthermore, CO2 increased the concentration of some of the identified sulfur compounds in the shale oil as compared to N2. The presence of steam increased the concentration of sulfur compounds in the shale oil as compared to dry sweep gases and significantly increased the sulfur concentration in pyrolysis gas. This was also supported by the investigation of the release of H2S and SO2 gases during the pyrolysis of oil shale in which steam contributes to an increase in the formation of sulfurous gases. The presence of steam not only enhances the release of sulfur compounds from oil shale, but also causes it to occur at lower temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increase in demand for energy resources, shale oil has drawn attention as an alternative fuel [1]. A promising approach is to utilize shale oil for producing valuable chemicals. Estonian kukersite shale oil is extensively studied and the corresponding oil shale is considered to be the most industrially utilized oil shale resource in the world [2, 3]. Vast exploitation in Estonia, which has mainly been used for electricity generation, made the country one of the least energy-dependent countries in the EU [4]. However, the increase in the price of CO2 emissions in the European Emissions Trading System has led to a decrease in electricity production from oil shale in Estonia. In 2019 and 2020, Estonia produced 1.156 and 1.173 million tons of shale oil. However, the electricity generated from oil shale in 2020 was 1.834 TWh which is 40% less compared to 2019. It is worth noting that the total electricity production from the oil shale industry in 2020 was 2.949 TWh. This accounts for the electricity produced from oil shale and pyrolysis gas. The corresponding value in 2019 was 4.873 TWh [5]. 99% of shale oil produced in Estonia is sold to other countries and the export of chemical and phenolic products rose by 33% [5, 6].
To produce shale oil, oil shale is pyrolyzed, i.e., thermally processed at temperatures around 500 °C in the absence of oxygen [7, 8]. The solid heat carrier (SHC) method is preferred over the gaseous heat carrier method for shale oil production mainly because of the higher production rate, ability to use lower quality oil shale, and lower environmental impact [9]. In the SHC method, the shale ash produced from the combustion of solid pyrolysis residue (semi-coke) is used as the heat carrier. For the pyrolysis process, dried oil shale is mixed with hot shale ash to produce liquid and gaseous products [10]. These products are made up mainly of the elements found in kerogen (the organic matter in oil shale): carbon and hydrogen as well as organic oxygen, nitrogen, and sulfur [11]. However, inorganic compounds may also contribute. This is especially relevant in the case of sulfur compounds. Estonian oil shale contains about 1–2% of sulfur [12], which is further classified as either organic or inorganic sulfur [13]. In the molecular model proposed for the structure of Estonian kukersite kerogen, mercaptans, thiophene, and thioether compounds are mainly present [13, 14]. Pyrite, marcasite, or small amounts of sulfates are categorized as inorganic sulfur [16]. The formation and distribution of sulfur compounds depend on covalent bond dissociation and the thermochemical characteristics of kerogen [15].
Thiols, sulfides, and thiophenes are the main sulfur compounds in kukersite shale oil [17], among which alkyl thiophenes are the most common [18]. Because of the properties that kukersite shale oil has, it finds use as a component in marine fuels. However, in order to avoid the environmental issues caused by sulfur, the International Maritime Organization (IMO) has applied strict regulations for marine fuels. The sulfur content of the marine fuels in SOx Emission Control Areas has been limited to 0.1% by mass since 2015. The global sulfur limit was also lowered to 0.5% from 2020 onward [19].
Due to the detrimental effect of sulfur on the oil shale upgrading process, the connection to environmental problems, and the strict regulations set on the sulfur content of marine fuels, information on the distribution of sulfur in different fractions of kukersite shale oil is needed. Therefore, the primary aim of this work is to analyze the distribution of sulfur in different fractions of Estonian shale oil. Also, the effect of the gaseous atmosphere used in pyrolysis (N2, CO2, and their mixtures with steam) on shale oil sulfur compounds was investigated. The results of this study would be beneficial to the development and improvement of sulfur removal.
Experimental
Sample preparation
Shale oil samples for studying the sulfur distribution were obtained from the Enefit Oil Plants in Narva, Estonia, which use the solid heat carrier (also known as Galoter) method [10]. The crude oil is separated at the plant into three fractions: gasoline, fuel oil (also referred to as the “middle oil fraction” in some older Galoter process based publications), and heavy oil. Two fuel oil samples and one shale gasoline sample were used. One fuel oil sample was from the newer Enefit-280 technology, and the other two samples were from the Enefit-140 technology (also called the UTT 3000 process in older publications).
The samples from the SHC process were further separated into narrow boiling range fractions by laboratory distillation. The fuel oil distillations were performed in a vacuum (around 7–70 mbar) to avoid the decomposition that can otherwise occur at higher temperatures. The distillation fractions were volume based, so each narrow fraction was taken to have roughly the same volume as the other fractions. Most of the fractions spanned distillation temperature intervals of about 5–10 °C and had average atmospheric boiling points ranging from about 80 to 420 °C. More information on how the samples were prepared, along with basic characteristic data for the samples, can be found in our earlier article [20].
In order to analyze the sulfur compounds of crude kukersite shale oil, a modified Fischer assay method was used to produce shale oil from an oil shale sample. The oil shale sample was crushed to the particle size of 500–710 μm. Then, 50 g of oil shale sample was subjected to N2, CO2, N2/steam, and CO2/steam environments for pyrolysis. The temperature program used for the Fischer assay method is as follows: heating up to 500 °C at 10 °C min–1heating from 500 to 520 °C at 1 °C min−1, and holding at 520 °C for 20 min. For the tests without steam, a flow rate of approximately 20 mL min−1 was used to inject N2 and CO2. For experiments using steam, water and CO2/N2 were injected at 0.1 mL min−1 and 10 mL min−1, respectively. The oil yield of above-mentioned technologies, Enefit-280, Enefit-140, and Fischer assay, are about 13.2%, 11.5%, and 16.1%, respectively, on a dry oil shale basis [6, 20].
Analytical methods
Sulfur analysis
The sulfur content of the shale oil fractions was measured using a Lab-X 3500 Benchtop XRF Analyser (Oxford Instruments, Abingdon, United Kingdom), which uses the ASTM D 4294 method. The estimated standard uncertainty of the sulfur measurements was 0.0024 mass% (expanded uncertainty of 0.0043 mass% at the 95% level). The sulfur content of the oils and semi-cokes obtained using Fischer assay was measured using elemental analysis (Vario MACRO CHNS Cube, Elementar Analysensysteme GmbH, Langenselbold, Germany) as reported in our previous work [22].
TGA-MS analysis
The evolution of H2S and SO2 gases during pyrolysis of the oil shale sample (approximately 8 mg) was analyzed with a NETZSCH STA 449 F3 Jupiter® TG-DSC coupled with a NETZSCH QMS Aëolos® mass spectrometer. To pyrolyze the shale samples, conditions similar to those of a Fischer assay were used, such as the sweep gas flowrate and temperature profile. The relative abundance of the gases can be fragmented at different mass-to-charge ratios. On the other hand, even though m/z of 32, 33, and 34 can be used to detect the H2S fragment, the relative abundance of H2S at m/z 34 is considered 100%, and other mass-to-charge ratios are calculated with respect to that. Similarly, m/z 64 was used to analyze the evolution of sulfur dioxide gases as this mass-to-charge ratio refers to the relative intensity of 100% for this gas. Because the highest intensity for the evolution H2S and SO2 compounds are recorded at mass-to-charge ratios (m/z) of 34 and 64, respectively, these m/z ratios were mainly used to interpret the evolution of these sulfurous gases. The tests in each carrier gas were conducted at least three time to make sure the results were reproducible. Initially, the baseline was subtracted so that the background noise was removed. Then, a cubic spline interpolation method was used to smooth the intensity data using a Python script. The smoothed peaks were normalized by dividing the intensity by the sample mass to eliminate the effect of sample mass on the peak intensity values and multiplied by a factor of 1012. The standard deviation of the intensities of these runs at similar temperatures were grouped and then averaged so that the final expanded uncertainty (95% level) could be calculated. The value for the normalized intensity of the TGA measurements was computed to be 0.02.
GC–MS analysis
To analyze the sulfur compounds in shale oil samples produced in a Fischer assay, an Agilent 7890B gas chromatograph coupled to an Agilent 5975C Inert MSD mass spectrometer and Agilent Flame Photometric Detector (FPD) Plus was employed. The samples were injected using a CTC Combi/GC-PAL 80 autosampler. Injections (1 µl) were made with a split ratio of 10:1 at an injector temperature of 260 °C. Helium (0.9 mL min−1, 99.9999% purity) was used as the carrier gas. The temperature program was as follows: initial temperature of 40 °C held for 5 min, then ramped up to 160 °C at 3 °C min−1, and finally from 160 °C to 280 °C at a rate of 20 °C min−1. The total run time was 51 min. The temperature of the FPD was set to 300 °C and the detector flow rates were 60 mL min−1 for both hydrogen and air (1:1). The column was a DB Petro (100 m × 250 µm × 0.5 µm). Also, the capillary to the MS had dimensions of 1 m × 250 µm × 0 µm and to the FPD + the dimensions were 5 m × 200 µm × 0 µm. Components were identified based on mass spectra compared to the National Institute of Standards and Technology (NIST) database and high purity standards. Three shale oil samples produced under each test atmosphere were used to calculate the concentration of sulfur compounds, and then an average was reported. Based on these repeat measurements, the calculated absolute standard uncertainty for the parallel runs and the estimated expanded uncertainty (95% level) for each compound was calculated separately and is presented in Table 1.
Results and discussion
Sulfur distribution
Information on the presence of sulfur compounds in different fractions of Estonian shale oil is important to understand so that essential processing for sulfur removal can be developed. According to Fig. 1, crude kukersite shale oil usually has a sulfur content between 0.3 and 1.5 mass%, which is higher than the sulfur limit of the marine fuels: 0.1%. As seen from the distribution of sulfur in shale oil, the concentration of sulfur in the gasoline fraction of shale oil increases with temperature and is greatest in the fractions boiling between about 150 and 190 °C. However, at higher temperatures, the shale oil sulfur content becomes lower and remains level at around 0.7 mass%. The experimental data for the kukersite samples measured in this study are presented in Table S1 in the supplementary material. Also, earlier elemental composition data for the shale oil produced from other processing technologies can be found in the literature [18,19,20,21,22,23,24], and much of this data is presented in Table S2.
Past studies have determined that the sulfur compounds in kukersite shale oil are mostly alkylthiophenes and thioesters, although some mercaptans and disulfides are also present [29, 30].
The shape of the sulfur distribution is different from that normally seen with conventional petroleum. For conventional fuels, the sulfur content generally continues to increase with the average boiling point of the fraction [31, 32]. Coal liquid fractions also show a continual increase in the sulfur content [33]. Other shale oils, however, seem to have a distribution that resembles that of kukersite shale oil, as Fig. 2 shows. Even Jordanian El-Lajjun shale oil, which is known for its high sulfur content, shows a decrease in sulfur content in the heavier fractions. That is, the heavier fractions contained less sulfur.
Identification of sulfur compounds in kukersite shale oil
To study the effect of various sweep gases on sulfur compounds, a quantitative analysis was performed to compare the presence of such compounds in the shale oil produced in different atmospheres. The chromatography analysis technique was used to identify several sulfur compounds however, due to the complexity of shale oil, it is quite difficult to detect all of them. Table 1 presents several common sulfur compounds identified using in the GC–MS-FPD, although it is worth noting that there are other sulfur compounds present that were not quantitatively identified. The detailed analysis of kukersite oil shale and corresponding shale oil used in this study can be found in the literature [4, 22].
It can be seen from the table that a large number of sulfur compounds can be found in the oil samples. As shown in the Table 1, most of the sulfur compounds in the shale oil samples are different forms of thiophenes originating from organic sulfur in kukersite oil shale (Table 2). Thiophenes are the main sulfur compounds and comprise about 80% of the sulfur. The rest of the sulfur compounds are thiols, sulfides, and disulfides [43]. The dominance of thiophene compounds can be explained by their thermal stability, which means they are not easily decomposed [44]. According to the analysis conducted on Huadian shale oil by Cui et al. [44], they reported that 87% of sulfur compounds consist of H2S, thiophene, benzophenthiophene, and dibenzophenthiophene compounds, and also implied that the sulfur atoms are mostly present in the thiophene structures of kerogen macromolecules. The generation of H2S which is mainly in the gaseous phase, but also dissolves in the shale oil, is partly formed through the decomposition of aliphatic and aromatic sulfur in the kerogen or conversion of mercaptans to thioethers or sulfur compounds and C = C double bonds [44]. Therefore, it can be inferred that the organic sulfur in oil shale is converted mainly into H2S and thiophene derivatives as well as other thermostable heterocyclic compounds [43], especially at lower temperatures. The type of oil shale and the production process used will have an impact on properties of the shale oil produced. Therefore, differences in the production and distribution of sulfur compounds are not only dependent on experimental conditions, but is also associated with the covalent bond dissociation and thermochemical characteristics of kerogen during decomposition [15, 45].
As is seen from Table 1, there is a minor increase in the content of several sulfur compounds for CO2 compared to N2, although for other compounds the difference is too small to be considered significant. This suggests that CO2 could slightly increase the concentration of sulfur compounds in the shale oil. On the other hand, the addition of steam has a significant effect on the content of sulfur compounds and increases the concentration of sulfur in the oil. This indicates that the use of reactive gases (CO2 and H2O) promotes the release of sulfur from mineral and organic part of oil shale and transforms it into volatile sulfurous compounds, which later get incorporated into the liquid product or released as sulfurous gases. Similarly, as reported by Huang et al. [46], the release of sulfur in CO2 environment by means of the pyrite decomposition is promoted by H2O and this increases along with increasing H2O concentration in the sweep gas. In a study done on coal, it was found that when CO2 was added to dry N2, more sulfur ended up in the liquid product rather than in the gaseous product at temperatures below 500 °C [47]. Based on the literature data the increase in sulfur compounds (Table 1) in the presence of reactive gases is mainly due to the decomposition of pyrite to pyrrhotite (FeSx) as an intermediate step according to the following reaction [46]:
Also, Al-Ayed and Matouq [48, 49] investigated the sulfur content of El-Lajjun shale oil obtained in different atmospheres and concluded that steam plays an important role in increasing the shale oil sulfur content. According to the reactions suggested by them the increase in sulfurous compounds in shale oil is caused by the decomposition of pyrite and pyrrhotites in the mineral part of oil shale in the presence of steam:
In the current study, a remarkable increase in concentration of H2S in shale oil (Table 1) was noticed, while the increase in SO2 concentration was small (within the measurement uncertainty). Therefore, for the shale oil produced under CO2 atmosphere, a slight increase in concentration of SO2 is expected considering the following reactions [46, 50]:
It should also be stated that since water vapor is a by-product in a pyrolysis process, the reactions 2–7 may take place in CO2 environment as well, however, the effect of these reaction is not as significant simply because of lower amount of H2O in pyrolysis atmosphere.
Analysis of sulfur in semi-coke
Prior to analyzing the concentration of sulfur in semi-coke and also, release of sulfurous gases during pyrolysis, it is beneficial to know about the forms of sulfur that are present in the shale sample. Therefore, Table 2 is provided to present the corresponding data for the sample used in the study.
According to Table 2, majority of sulfur in the oil shale sample is in the form of pyrite. In addition, the elemental analysis conducted on semi-coke derived after pyrolysis is shown in Table 3. The results indicate that the semi-cokes from the tests in CO2 and steam atmospheres contain smaller amount of sulfur. This demonstrates that presence of steam as well as CO2 has an impact on the release of sulfur from the oil shale sample as explained previously.
Table 4 demonstrates the distribution of sulfur in pyrolysis products under different atmospheres. The sulfur content of the oil shale sample and the produced shale oil was taken from the authors’ previous work [22]. This data were then used to calculate the sulfur mass balance. Subsequently, the amount of sulfur in the pyrolysis gas was computed by difference. The data in Table 4 show that majority of sulfur will remain in semi-coke while large part of the liberated sulfur is released as non-condensable gases. The steam, however, is remarkably increasing the release of sulfur from the char in pyrolysis process. This result is similar to that reported in the literature [51].
Evolution of H2S and SO2 gases during pyrolysis
To investigate further the evolution of SO2 and H2S, oil shale samples were also run in a TGA-MS to observe the effect of sweep gases on the evolution of these sulfur compounds. Using this technique, it is possible to determine the relative abundance of H2S and SO2 during oil shale pyrolysis.
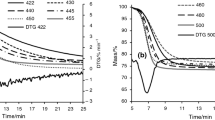
Figures 3 and 4 depict the intensity trends for the evolution of H2S and SO2 gases during the pyrolysis of the oil shale sample. The intensity values were normalized based on the sample mass used. These figures compare the effect of different sweep gases on the release of sulfur compounds. Also, the expanded uncertainty (95% level) of 0.02 is presented as a dotted line in the graphs. Comparing the figures demonstrates that the generation of H2S is generally greater than SO2 during the pyrolysis process at low temperatures implying that H2S accounts for most of the sulfurous gas released during pyrolysis [52]. According to literature, pyrolysis of the sample in a standard Fischer assay (under N2) generated about 16% v/v H2S of pyrolysis gas [4]. In one study, it was reported that the addition of steam to argon increased the yield of H2S gas while reducing the formation of SO2 [51]. That could be the major reason that our analysis showed that the generation of H2S in oil was significantly higher than that of SO2.
It is worth noting that the generation of various gases during pyrolysis would have a great effect on the release of sulfur in the process. Studying profiles of the generated gases during pyrolysis would be very beneficial to understand how the presence of pyrolysis gases plays a role in transforming sulfur from the sample and thus, the generation of sulfurous gases. Such information can be used to optimize sulfur removal processes. According to the study done by Zhou et al. [47] on pyrolysis of several coals in different atmospheres, it was found that the presence of H2 in the pyrolysis environment noticeably intensified the evolution of H2S gas as compared to pure N2. Whereas the presence of CO2 reduced the intensity of H2S at temperatures below 500 °C. Although they found that CO2 reduced the intensity there was only 10% of CO2 in the gaseous environment. A decrease in H2S intensity could be due to the presence of an insufficient amount of CO2 gas in the environment to react with the sample. Moreover, greater formation of H2S in an H2 atmosphere was attributed to hydrogenation and reduction in sulfur compounds as compared to CO2 and N2 sweep gases [50]. In another study, it was described that the addition of CO2 to an inert gas enhances the release of sulfur compounds such as H2S and SO2 during coal thermal decomposition [53]. Gu et al. also found that the release of volatile sulfur compounds such as SO2 and thiophenes is mostly higher in CO2 atmosphere than in N2 because of oxidizing effect that CO2 has during pyrolysis [50]. Sinha and Walker also compared several different gaseous environments and found that the amount of sulfur released from a coal sample increased in the following order: air, CO-steam, CO, N2 [54].
It is seen from Fig. 3 that the formation of H2S in a steam atmosphere exhibited a different behavior than that of dry sweep gases. Although the H2S is formed during decomposition of both organic sulfur and pyrite [51], the suggested reactions 2–7 explain the enhancement of the formation of H2S in the steam environment [49]. Also, it can be noticed that steam lowers the liberation temperature of sulfurous volatile compounds as well. More specifically, the intensity of peaks was at around 440 °C and 410 °C for N2 and CO2, respectively, and below 400 °C for the atmospheres with steam. Moreover, it can be noticed that in steam environment the H2S starts forming at temperatures between 200 °C and 300 °C, while this for the dry sweep gases is a little above 300 °C. This indicates that the presence of steam in a pyrolysis environment causes the increase in H2S concentration in pyrolysis gas at lower temperatures. Furthermore, it can also be derived from Fig. 3 that the formation of H2S is lowest in both dry carrier gases, in particular N2. This could be due to the low extent of hydrogenation/reduction reactions taking place in dry pyrolysis atmospheres [50].
In our previous work, it was shown how different gases such as H2 evolve during oil shale pyrolysis [55]. Our earlier results indicated that during oil shale pyrolysis the concentration of H2 in the pyrolysis environment intensifies with an increase in temperature and the generation rate was much sharper at around 400–500 °C. This could be one of the reasons that a higher H2S intensity within this range is observed. Yan et al. [56] reported that generation of H2S increased at a slow rate up to a temperature of 280 °C. However, after this temperature, the H2S was formed at a higher rate and reached a maximum at 360 °C. The decomposition reaction of most of the pyrite in oil shale begins at around 280 °C and H2S formation is dependent on the interaction of pyrite with the hydrogen donor [57]. Burnham et al. [58] described that oxidation of pyrite to form iron oxide is the main cause of H2S increase in the steam environment. As stated previously, the intensity of the peaks for pyrolysis in CO2/steam and N2/steam was significantly higher than in dry sweep gases. Subsequently, a greater amount of H2S compounds were dissolved in the corresponding shale oils.
From Fig. 4, the intensity of the SO2 peak for all test atmospheres can be seen. It is obvious that by increasing the temperature, the generation of SO2 increased as well. This can be explained by a higher reactivity of oxygen-bearing groups when temperature increased, which resulted in larger generation of SO2 [59]. Also, it can be seen that the intensity of SO2 in all environments does not generally get affected by the type of sweep gas applied at temperatures below 500 °C, which means that the steam effect is insignificant in this range. Hence, the yield of SO2 is observed to be lower, resulting in a lower SO2 content, accordingly. Although little SO2 is observed to form, it can be perceived from the figure that the generation of SO2 was initiated at around 300–350 °C. Moreover, from Fig. 4 it can be derived that until below 400 °C, the SO2 generation is small. However, at about 400 °C, due to the higher oxidizing effect of CO2 as compared to N2, higher amount of SO2 gas was formed under CO2 and CO2/steam atmospheres. This formation of SO2 could originate from decomposition of aliphatic and elemental sulfur species at around 330 °C, with subsequent decomposition of aromatic and pyritic sulfur at higher temperatures leading to the larger peaks [60]. Therefore, appearance of a more intense peak could be as a result of overlapping of both decomposition peaks, and also the presence of larger amount of pyrite than organic sulfur in the shale sample (Table 2). However, it was reported in the literature that for this sample, about 60% of organic sulfur were released as pyrolysis gas [4].
The formation of SO2 is from the reaction of oxygen-containing compounds with pyrite and unstable elemental sulfur when an inadequate amount of hydrogen is present to form H2S. In the presence of steam, however, since more hydrogen radicals are present in the pyrolysis atmosphere, a bigger amount of H2S is generated [51]. Moreover, when H2O is present in pyrolysis atmosphere, because of lower molar mass of H2O, the diffusion rate of sulfur gas is higher. Consequently, the sulfur gas escape the sample at higher rate [46]. According to the results of Wang et al. on coal pyrolysis, employing CO2 in place of an inert gas (argon) not only increased the intensity of H2S and SO2 but also lowered the maximum peak temperature in the evolution of these gases as well [61]. Maaten et al. [9] investigated the release of H2S and SO2 gases in different oil shales with different origins under N2 and concluded that keeping the pyrolysis temperature below 480 °C would be effective in reducing the sulfur content in the oil. However, they specified that this would not be the case for some oil shales such as Estonian oil shale because the evaporation maximum for this type of oil shale is approximately 400 °C and above this temperature the intensification lowers.
Combining analysis of the mass spectrometry results, it can be perceived from Figs. 3 and 4 that the release of sulfurous gases intensified starting at around 300–450 °C, even though for some curves it is small. The first peak could mainly take place as a result of decomposition of organic sulfur compounds—occurring at around 350 °C, and it further intensified above 400 °C as the pyrolysis of pyrite begins. As reported, pyrite decomposes at 400–500 °C [12]. This suggests that the decomposition of organic matter could increase the extent of the release of sulfurous gases. Also, comparing the effect of CO2 and N2 in shale oils, the difference in concentration of sulfur compounds was significant only for some of the identified compounds at low temperatures. In addition, the difference in concentrations of H2S and SO2 under CO2 could be mainly as a result of the noticeable difference that was observed in the evolution of SO2 and H2S gases during pyrolysis. This could confirm that CO2 is more effective in the liberation of volatile sulfur compounds as well as sulfurous gases from oil shale due to its higher reactivity, however, at low temperature the difference seems to be small. Unlike N2 in which the volatiles are released as a result of pyrolysis, CO2 can increase the release of volatiles even more by reacting with the semi-coke throughout the pyrolysis, although the effect of CO2 seems to be larger at higher heating rates and temperatures [62]. Another conclusion that can be drawn from applying steam in a pyrolysis environment is that steam increased the evolution of H2S in contrast with SO2. It can be deduced from the analysis that steam considerably enhances the concentration of sulfur compounds in shale oil.
Conclusions
In this study, the sulfur content in different fractions of Estonian kukersite shale oil was analyzed. Also, the effect of various sweep gases on behavior of sulfur in the oil shale pyrolysis process at temperatures below 520 °C was investigated as well. The main results are as follows:
-
The analysis on the sulfur content of kukersite shale oil fractions from different retorts show that the concentration of sulfur in the fractions boiling between about 150 °C and 190 °C was the highest. In fractions with higher average boiling points, the sulfur content decreases until it becomes almost constant.
-
GC–MS analysis on shale oils produced in N2, CO2, and their mixtures with steam demonstrated that the majority of the identified sulfur compounds in the shale oils were thiophenic compounds. Also, CO2 increased the concentration of some of the sulfur compounds in the shale oil as compared to N2. In addition, steam promotes the volatilization of sulfur compounds, which consequently increases the concentration of sulfur compounds in the shale oil.
-
The analysis on the evolution trend of H2S and SO2 gases showed that steam significantly increased the release of H2S gas, and its dissolved amount in the oil was the highest in comparison with other sulfur compounds. Also, the effect of steam on the evolution of these gases suggests that steam contributes to an increase in the formation of H2S and SO2.
-
The elemental analysis conducted on semi-coke derived after pyrolysis indicates that the semi-cokes from the tests in CO2 and steam atmospheres contain smaller amount of sulfur. This explains that the use of reactive gases (CO2 and H2O) has an impact on the release of sulfur from the oil shale sample.
Overall, sulfur analysis gives insight into how to decrease the sulfur content in oil and assist in the reduction in sulfurous gases during oil shale and coal industrial processes such as pyrolysis and gasification.
References
Jiang Z, Zhang W, Liang C, Wang Y, Liu H, Chen X. Basic characteristics and evaluation of shale oil reservoirs. Pet Res. 2016;1(2):149–63. https://doi.org/10.1016/S2096-2495(17)30039-X.
Motlep R, Kirsimae K, Puura E. Mineral composition of Estonian oil shale semi-coke sediments. 8th Int Sci Conf Mod Manag Mine Prod Geol Environ Prot SGEM. 2008;2(3):309.
Mozaffari P, Baird ZS, Listak M, Oja V. Vapor pressures of narrow gasoline fractions of oil from industrial retorting of Kukersite oil shale. Oil Shale. 2020;37(4):288–303. https://doi.org/10.3176/oil.2020.4.03.
Maaten B, Järvik O, Pihl O, Konist A, Siirde A. Oil shale pyrolysis products and the fate of sulfur. Oil Shale. 2020;37(1):51–69. https://doi.org/10.3176/oil.2020.1.03.
“Eesti põlevkivitööstuse aastaraamat 2020 (in Estonian). 2020, [Online]. Available: http://data.vk.edu.ee/PKYB20/.
“Oil Shale Industry Yearbook. 2019. 2019, [Online]. Available: http://data.vk.edu.ee/pkk_aastaraamat_2019_eng/.
Neshumayev D, Pihu T, Siirde A, Järvik O, Konist A. Solid heat carrier oil shale retorting technology with integrated CFB technology. Oil Shale. 2019;36(2S):99–113. https://doi.org/10.3176/oil.2019.2S.02.
Astra H, et al. Yields and the selected physicochemical properties of thermobitumen as an intermediate product of the pyrolysis of Kukersite oil shale. Oil shale. 2021;38(4):295–316. https://doi.org/10.3176/oil.2021.4.02.
Maaten B, Loo L, Konist A, Pihu T, Siirde A. Investigation of the evolution of sulphur during the thermal degradation of different oil shales. J Anal Appl Pyrol. 2017;128:405–11. https://doi.org/10.1016/j.jaap.2017.09.007.
Golubev N. Solid heat carrier technology for oil shale retorting. Oil Shale. 2003;20(3):324–32.
Dijkmans T, Djokic MR, Van Geem KM, Marin GB. Comprehensive compositional analysis of sulfur and nitrogen containing compounds in shale oil using GC x GC - FID/SCD/NCD/TOF-MS. Fuel. 2015;140:398–406. https://doi.org/10.1016/j.fuel.2014.09.055.
Maaten B, Pikkor H, Konist A, Siirde A. Determination of the total sulphur content of oil shale by using different analytical methods. Oil Shale. 2018;35(2):144–53. https://doi.org/10.3176/oil.2018.2.04.
Qin H, Hao Z, Wang Q, Bai J. Sulfur release from oil shale in retort. Energy Procedia. 2012;17:1747–53. https://doi.org/10.1016/j.egypro.2012.02.307.
Lille Ü, Heinmaa I, Pehk T. Molecular model of Estonian kukersite kerogen evaluated by 13C MAS NMR spectra. Fuel. 2003;82(7):799–804. https://doi.org/10.1016/S0016-2361(02)00358-7.
Pan S, et al. Investigation of behavior of sulfur in oil fractions during oil shale pyrolysis. Energy Fuels. 2019. https://doi.org/10.1021/acs.energyfuels.9b02406.
Guffey FD, Barbour FA, Cummings RE. Analysis of oil shale retort production stream gases for sulfur species. Liq Fuels Technol. 1983;1(4):235–57. https://doi.org/10.1080/07377268308915324.
Speight J. Shale oil and gas production processes. Houston: Gulf Professional Publishing; 2019.
Al-Harahsheh A, Al-Otoom AY, Shawabkeh RA. Sulfur distribution in the oil fractions obtained by thermal cracking of Jordanian El-Lajjun oil Shale. Energy. 2005;30(15):2784–95. https://doi.org/10.1016/j.energy.2005.01.013.
Van Chu T, Ramirez J, Rainey T, Ristovski Z, Brown RJ. Global impacts of recent IMO regulations on marine fuel oil refining processes and ship emissions. Transp Res Part D Transp Environ. 2019;70:123–34. https://doi.org/10.1016/j.trd.2019.04.001.
Baird ZS, Järvik O, Oja V, Rannaveski R. Properties of kukersite shale oil. Oil Shale. 2021;38(4):265. https://doi.org/10.3176/oil.2021.4.01.
Oja V, Elenurm A, Rohtla I, Tali E, Tearo E, Yanchilin A. Comparison of oil shales from different deposits: oil shale pyrolysis and co-pyrolysis with ash. Oil Shale. 2007;24(2):101–8.
Mozaffari S, Järvik O, Baird ZS. Effect of N2 and CO2 on shale oil from pyrolysis of Estonian oil shale. Int J Coal Prep Util. 2021;00(00):1–15. https://doi.org/10.1080/19392699.2021.1914025.
Luts K. The Estonian Oil Shale Kukersite, its Chemistry, Technology and Analysis. Tartu, Estonia, 1934.
Hissin JO. Sulfur in shale and shale gasoline [in Russian]. Oil Shale, no. 10, 1932.
Klesment I, Hallik E. Comparative properties of pyrolysis oils from oil shale (Cpaвнитeльнaя xapaктepиcтикa cмoлпoлyкoкcoвaния гopючнx cлaнцeв) [in Russian]. Chem Technol oil shale Prod (Xимия и тexнoлoгия гopючиx cлaнцeв и пpoдyктoв иx пepepaбoтки). 1963;12:169–80.
Yefimov V, Lille Ü, Piik E, Tuln M, Murb A. The results of thermal processing of Estonian oil shale in a small pilot gas retort (Rezul’taty termicheskoy pererabotki estonskogo slanca na malom opytnom gazogeneratore) [in Russian]. Goryuchie slancy, khimija i Technol. 1963;12:90–105.
Lille Ü. On the chemical composition of the 60–150 °C light fraction from a solid heat carrier retort (o xимичecкoм cocтaвe фpaкции 60–150 c лeгкoй cмoльi ycтaнoвки c твepдьiм тeплoнocитeлeм) [in Russian]. Chem Technol oil shale Prod (Xимия и тexнoлoгия гopючиx cлaнцeв и пpoдyктoв иx пepepaбoтки). 1963;12:127–33.
M. Wittlich. About the sulfur in Kukersite and its pyrolysis products (Einiges über den Schwefel im Kukersit und dessen Verschwelungsprodukten) [in German]. Acta Comment. Univ. Dorpat., 1925.
Zelenin NI, Feinberg VS, Chernyshev KB. The chemistry and technology of shale oil. Leningrad: Chemistry Publishing House; 1968. (in Russian).
Derenne S, Largeau C, Casadevall E, Sinninghe Damsté JS, Tegelaar EW, de Leeuw JW. Characterization of Estonian Kukersite by spectroscopy and pyrolysis: Evidence for abundant alkyl phenolic moieties in an Ordovician, marine, type II/I kerogen. Org Geochem. 1990;16(4–6):873–88. https://doi.org/10.1016/0146-6380(90)90124-I.
Riazi MR. Characterization and properties of petroleum fractions. 1st ed. Pennsylvania: ASTM International; 2005.
Baird ZS, Rang H, Oja V. Desulfurization, denitrogenation and deoxygenation of shale oil. Oil Shale. 2021;38(2):137–54. https://doi.org/10.3176/oil.2021.2.03.
Gray JA, Brady CJ, Cunnlngham R, James R, Wllson GM. Thermophysical properties of coal liquids 1. Selected physical, chemical, and thermodynamic properties of narrow boillng range coal liquids. Ind Eng Chem Process Des Dev. 1983;22(3):410–24.
Mapstone GE. Some properties of crude shale oil and naphtha. Oil Shale and Cannel Coal. 1951;2:662–72.
Cady WE, Seelig HS. Composition of shale oil. Ind Eng Chem. 1952;44(11):2636–41.
Lovell PF. Production of Utah shale oils by the Paraho DH and Union ‘B’ retorting processes. In: Eleventh Oil Shale Symposium Proceedings, 1978, pp. 184–192.
Hill GR, Dougan P. The characteristics of a in situ shale low temperature oil. 1967.
Miknis, FP. Characterization of DOE reference oil shale: Tipton member, Green River formation oil shale from Wyoming. 1988.
Qian J, Liang Y. Oil shale: petroleum alternative. Beijing: China Petrochemical Press; 2010.
Marecaux PP. Essais de valorisatsion integrale des huiles de schiste de Severac. Oil Shale and Cannel Coal. 1951;2:673–89.
Miknis FP, Robertson RE. Characterization of DOE reference oil shales: Mahogany zone, Parachute Creek member, Green River formation oil shale, and Clegg Creek member, New Albany shale. Western Research Inst., Laramie, WY (USA); 1987.
Bunger JW. Shale oil value enhancement research. Salt Lake City, 2007.
Fainberg V, Garbar A, Hetsroni G. Secondary pyrolysis of the products of the thermal destruction of high-sulfur oil shale. Energy Fuels. 1997;11(4):915–9. https://doi.org/10.1021/ef9601733.
Cui D, et al. Compositional analysis of heteroatom compounds in Huadian shale oil using various analytical techniques. Energy Fuels. 2019;33(2):946–56. https://doi.org/10.1021/acs.energyfuels.8b03889.
Mozaffari P, Järvik O, Baird ZS. Vapor pressures of phenolic compounds found in pyrolysis oil. J Chem Eng Data. 2020. https://doi.org/10.1021/acs.jced.0c00675.
Huang F, Zhang L, Yi B, Xia Z, Zheng C. Effect of H2O on pyrite transformation behavior during oxy-fuel combustion. Fuel Process Technol. 2015;131:458–65. https://doi.org/10.1016/j.fuproc.2014.12.027.
Zhou Q, Hu H, Liu Q, Zhu S, Zhao R. Effect of atmosphere on evolution of sulfur-containing gases during coal pyrolysis. Energy Fuels. 2005;19(3):892–7. https://doi.org/10.1021/ef049773p.
Al-Ayed OS, Matouq M. Influence of pyrolysis environment on liquid product and sulfur of oil shale. Energy Sour Part A Recover Util Environ Eff. 2009;31(8):679–86. https://doi.org/10.1080/15567030701752529.
Al-Ayed OS, Matouq M. Factors affecting sulfur reactions in high sulfur oil shale pyrolysis. J Energy Resour Technol Trans ASME. 2009;131(1):0125011–4. https://doi.org/10.1115/1.3068338.
Gu Y, Yperman J, Vandewijngaarden J, Reggers G, Carleer R. Organic and inorganic sulphur compounds releases from high-pyrite coal pyrolysis in H2, N2 and CO2: test case Chinese LZ coal. Fuel. 2017;202:494–502. https://doi.org/10.1016/j.fuel.2017.04.068.
Wang M, Du Q, Li Y, Xu J, Gao J, Wang H. Effect of steam on the transformation of sulfur during demineralized coal pyrolysis. J Anal Appl Pyrol. 2019;140:161–9. https://doi.org/10.1016/j.jaap.2019.03.011.
Qin H, Zheng Z, Wang Q, Sun B, Bai J. H2S evolution during oil shale pyrolysis. In: Asia-Pacific Power Energy Eng Conf APPEEC, pp. 2–5, 2010, https://doi.org/10.1109/APPEEC.2010.5448345.
Yang N, Guo H, Liu F, Zhang H, Hu Y, Hu R. Effects of atmospheres on sulfur release and its transformation behavior during coal thermolysis. Fuel. 2018;215:446–53. https://doi.org/10.1016/j.fuel.2017.11.099.
Sinha RK, Walker PL. Desulphurization of coals and chars by treatment in various atmospheres between 400 and 600 °C. Fuel. 1972;51(4):329–31. https://doi.org/10.1016/0016-2361(72)90012-9.
Mozaffari S, Järvik O, Baird ZS. Composition of gas from pyrolysis of Estonian oil shale with various sweep gases. Oil Shale. 2021;38(3):215–27. https://doi.org/10.3176/oil.2021.3.03.
Yan Q, Peng P, Yu C, Liu J. The role of sulfur in the pyrolysis of kerogen. Chin Sci Bull. 2004;49:10–8. https://doi.org/10.1007/BF02890448.
Fadeff SK, Burnham AK, Richardson JH. Organic and pyritic sulfur determination in oil shale. No. 1983.
Burnham GJ, Bey AK, Koskinas NK. Hydrogen sulfide evolution from colorado oil shale. ACS Symp Ser. 1981. https://doi.org/10.1021/bk-1981-0163.ch005.
Li L, et al. Release of sulfur and nitrogen during co-pyrolysis of coal and biomass under inert atmosphere. ACS Omega. 2020;5(46):30001–10. https://doi.org/10.1021/acsomega.0c04372.
Frigge L, Elserafi G, Ströhle J, Epple B. Sulfur and chlorine gas species formation during coal pyrolysis in nitrogen and carbon dioxide atmosphere. Energy Fuels. 2016;30(9):7713–20. https://doi.org/10.1021/acs.energyfuels.6b01080.
Wang X, Guo H, Liu F, Hu R, Wang M. Effects of CO2 on sulfur removal and its release behavior during coal pyrolysis. Fuel. 2016;165:484–9. https://doi.org/10.1016/j.fuel.2015.10.047.
Zhao S, Sun Y, Lü X, Li Q. Kinetics and thermodynamics evaluation of carbon dioxide enhanced oil shale pyrolysis. Sci Rep. 2021;11(1):1–14. https://doi.org/10.1038/s41598-020-80205-4.
Acknowledgements
This research was funded by Estonian Research Council from National Programme for Addressing Socio-Economic Challenges through R&D (RITA1/01-60), which is supported by the Estonian Government and European Regional Development Fund. The authors would like to thank Silvia East for her help in measuring the sulfur and nitrogen contents of the shale oil fractions.
Author information
Authors and Affiliations
Contributions
All authors made substantial contribution to design of the study as well as the analysis and interpretation of the obtained results; Conceptualization was contributed by SM, OJ, Methodology was contributed by SM, OJ, ZSB, Data curation was contributed by ZSB, SM, Formal analysis and investigation were contributed by SM, OJ, ZSB, Writing—original draft preparation, was contributed by SM, Writing—review and editing, was contributed by OJ, ZSB, Supervision was contributed by OJ, ZSB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mozaffari, S., Baird, Z.S. & Järvik, O. Sulfur in kukersite shale oil: its distribution in shale oil fractions and the effect of gaseous environment. J Therm Anal Calorim 147, 11601–11610 (2022). https://doi.org/10.1007/s10973-022-11359-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11359-8