Abstract
The use of alcohol with traditional diesel fuel in diesel engines reduces environmental damage. When the ternary mixtures obtained by adding biodiesel to diesel-alcohol fuel mixtures are used without making any changes in the compression-ignition (CI) engine, there is no significant problem in terms of performance and emissions. This research dealt energetic, exergetic, and environmental evaluation for a CI engine fueled with blends created using diesel/biodiesel/n-octanol at a constant speed of 1500 rpm and different loads (25, 50, 75, and 100%). Performance and emission values were recorded in the tests. Economic and environmental analyses were realized by using the data obtained in these tests in thermodynamic relations. The losses and efficiency of the engine were computed in the energy analysis. The highest thermal efficiency was found to be 40.6% in B20 and B20OCT5 at full load, while the lowest one was observed to be 15.77% when the engine fueled with B100 at 25% load. In the exergy analysis, exhaust exergy, exergy destroyed, and entropy generation were determined. Thermal and exergy efficiencies were parallel in all fuels depending on the load. The highest exergy efficiency was calculated to be 30.4% for B20 and B20OCT5 at full load. Lower exergy destruction was acquired for diesel fuel at full load in comparison with B20OCT20, B20OCT15 and B20OCT10. CO2 emission of fuels was used in exergy-based environmental analysis. The lowest environmental cost was determined as 3.85 $ month−1 at 25% load in B20OCT10. The highest power cost was achieved to be 10.61 $ MJ−1 at 25% load when the engine was run on B20OCT20. The cost of exergy losses at 25% load was computed to be 3.67 $ h−1 for B20OCT20. While the increase in alcohol content in the blends caused a decrease in harmful pollutants, it is not economical due to the expensive pump prices. To conclude, it is to be clearly indicated that due to systematic thermodynamic, economic, and environmental analyses and the usage of n-octanol as a long-chain alcohol in the CI engine with blending diesel and biodiesel, this paper goes beyond previous efforts in the literature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, factors such as industrialization, population growth, and increasing living standards increase the demand for energy. Fossil fuels meet most of the energy needs in the world. Internal combustion engines commonly used worldwide for transportation, industrial machinery, cogeneration, and agricultural purposes are among the main responsible for the depletion of fossil fuels [1, 2]. The reserves of fossil fuels, including petroleum, coal, and natural gas, are rapidly depleted. The reserves of fossil fuels, which are limited and finite, are concentrated more in particular regions of the world. In addition, since fossil-based fuels cause serious problems to the environment we live in and on human health, there are heavy limitations on the exhaust emissions of these fuels [3, 4]. The increasing need for energy and the awareness of environmental problems such as global warming in recent years encourage many researchers to conduct studies on the development of alternative energy resources that can be renewed, sustained, and produced from domestic resources instead of fossil fuels [5, 6].
Biodiesel fuel, which is the methyl ester of vegetable oils, is the most preferred alternative fuel among renewable and sustainable alternative fuels. Biodiesel is increasingly used in the market as a fuel in CI engines [7, 8]. Biodiesel is obtained from oilseed plants such as canola, soybean, sunflower, safflower, mustard, peanut, rubber, or animal oils such as beef tallow, fish, and chicken. Pure biodiesel and diesel–biodiesel blends in certain proportions can be utilized in any diesel engine without the need for a change in the engine or by making minor changes [9,10,11].
Alcohols have been used for many years either directly or as a blend as an alternative to CI engines. Alcohols are in the liquid phase. Alcohols have a lower cetane number than diesel fuel depending on the carbon number [12]. Alcohols have oxygen in their content. Alcohol fuels are widely used in internal combustion engines to improve fuel properties, combustion performance, and reduce harmful exhaust emissions [13, 14]. To use bioalcohol in CI engines, alcohol must be able to dissolve in diesel fuel at any rate. Currently, countries do not make enough investments in alcohol production and consumption for fuel purposes [15]. The pump price of diesel fuel is very low compared to alcohols. The amount of water in the alcohol content has a low corrosive effect on the fuel systems of diesel engines [16].
Octanol represents an organic compound with the molecular formula of CH3(CH2)7OH. Octanol is used in many areas such as cosmetics and solvent production [17]. The blending process of octanol and diesel used as fuel additives is not very complicated. They can be easily blended without any phase separation problem. As the amount of octanol in fuel blends increases, its viscosity increases but its calorific value decreases [18, 19]. The increase in viscosity of fuel causes ignition delay in diesel engines [20]. The following section has demonstrated a comprehensive background of the present study regarding the alternative fuels and its applications and thermodynamic evaluation.
The data necessary for thermodynamic analysis in internal combustion engines are obtained from engine experimental studies. The energy distribution and thermal efficiency in engines are found with the energy analysis based on the first law of thermodynamics [21]. Within the framework of the second law, the quality of energy in engines is also evaluated. The exergy analysis determines the losses due to the irreversibilities that occur during the combustion process of fuels in the engine [22].
Energy and exergy destroyed should be reduced for the more efficient operation of diesel engines. Therefore, the objective of the thermodynamic analysis is to determine energy and exergy destroyed [23, 24]. Fuel exergy is used to produce useful mechanical work in diesel engines. Exergy destroyed originates from the insufficient use of fuel exergy [25].
The operation of diesel engines with various fuel blends has been recently investigated in alternative fuel studies. In these studies, it has been observed that the chemical exergy of fuels with the same lower heating value, but different properties differs. Therefore, the exergic evaluation of fuels should be performed [26].
In addition to performance and emission values, economic evaluation is important in selecting an alternative fuel [27]. Economical fuels are used in engines due to the increased fuel costs. During the initial period, only performance and emission values were taken into consideration in alternative fuel studies in diesel engines. During the following periods, irreversibilities came to the forefront with energy and exergy analysis [28]. Exergy-based exergoeconomic analysis is currently used by researchers [29]. As of today, numerous researchers have investigated different types of alcoholic-based fuels in the CI engines in order to monitor the performance, combustion characteristics, and emission levels. Obviously, some of the aforementioned studies have been summarized underneath on account of underlying the importance of the current study.
Nanthagopal et al. [30] performed experimental studies by creating blends (Diesel, B100, B90P10, B80P20, B70P30, B90O10, B80O20, and B70O30) using biodiesel obtained from Calophyllum inophyllum oil, n-pentanol, n-octanol, and pure diesel fuels in different proportions in a four-stroke, single-cylinder, air-cooled, Kirloskar TAF-1 model CI DI engine. In experimental studies, brake thermal efficiency, BSFC, brake specific energy consumption, and harmful exhaust emission values were investigated at various engine loads (25%, 50%, 75%, and 100%) and a constant engine speed of 1500 rpm. This study compared the effects of blending different alcohols with biodiesel obtained from Calophyllum inophyllum oil to enhance performance and decrease NOX emissions. The study explained that blending n-pentanol and n-octanol alcohols with biodiesel reduced HC emissions by 8–22% and increased CO emissions by 16–50%. Chandra Sekar et al. [31] carried out experimental studies by blending biodiesel obtained from neem oil, n-octanol and pure diesel fuels in different proportions (Diesel, B100, B90OCT10, and B80OCT20) in a four-stroke, single-cylinder, water-cooled Kirloskar TV-1 model CI DI research engine. This research conducted experimental studies for five different engine powers (1.1 kW, 2.2 kW, 3.3 kW, 4.4 kW, and 5.5 kW) and at a constant engine speed of 1800 rpm using four different fuels and investigated brake thermal efficiency, BSFC, cylinder pressure, and harmful exhaust emission values. It was stated that brake thermal efficiency, HC, CO, NOX, and smoke emissions were lower in pure biodiesel and two different biodiesel/n-octanol blends compared to pure diesel fuel. Moreover, it was indicated that biodiesel/n-octanol fuel blends increased BSFC compared to pure diesel fuel while having lower brake thermal efficiency. Gopal et al. [32] blended diesel and n-octanol fuels in different proportions (OCT10, OCT20, and OCT30) in a four-stroke, single-cylinder, water-cooled Kirloskar brand TV-1 model CI DI engine and conducted experimental studies for different exhaust gas recirculation (EGR) rates and different injection timings. The study stated that injection timing had a very considerable impact on NOX emissions, and smoke opacity significantly affected the EGR rates. Pan et al. [33] blended n-octanol and pure diesel fuels in different proportions (Diesel, OCT20, and OCT40) for different EGR rates (at 5% intervals, 0% to 30%) and investigated their effects on combustion and harmful exhaust emissions in a four-stroke, four-cylinder, water-cooled, variable-geometry turbocharger, CI common-rail injection system DI engine. According to the results obtained, the researchers stated that the in-cylinder pressure curves of n-octanol/diesel fuel blends were almost the same under test conditions compared to pure diesel fuel. It was also explained that adding n-octanol to pure diesel fuel decreased CO, HC, and soot emissions and slightly increased NOX emissions. De Poures et al. [34] performed experimental studies using n-octanol (OCT30) and pure diesel fuels in a four-stroke, single-cylinder, air-cooled, Kirloskar brand TAF-1 model CI DI engine. In experimental studies conducted under full engine load and at a constant engine speed of 1500 rpm, the impacts of injection timing and EGR values on combustion, performance, and harmful exhaust emissions were examined. According to the results obtained, it was indicated that injection timing and EGR values reduced CO, HC, NOX, and smoke emissions. In another study carried out by De Poures et al. [35] in a Kirloskar brand TV-1 model engine, they performed experimental studies by creating blends using waste plastic oil (WPO), n-octanol, and ultra-low sulfur diesel fuels in different proportions (Diesel, WPO100, and WPO70OCT30). The effects of three different injection timings and three different EGR rates on combustion and performance characteristics, brake thermal efficiency, BSFC, and harmful exhaust emission values were investigated in experimental studies conducted under full engine load and at a constant engine speed of 1500 rpm. Both studies reported that blending pure diesel fuel with alcohols eliminated many deficiencies of diesel fuels. It was also stated that adding n-octanol into waste plastic oil worsened HC and NOX emissions, while significant improvements occurred in CO and smoke exhaust emissions.
Zhou et al. [36] experimentally investigated the effects of new fuel blends formed from diesel and n-octanol fuels in different ratios on harmful exhaust gas particles in a four-cylinder, four-stroke, water-cooled, turbocharged, and common-rail direct injection diesel engine. It was explained that the increase in the amount of n-octanol in the fuel blends increases the harmful exhaust gas particles. It was also stated that adding n-octanol creates a higher specific active surface that facilitates oxidation as well as reduces the exhaust particle diameters.
Wang et al. [37] experimentally investigated the effects of neat diesel, n-octanol, methylal, and dimethyl carbonate fuels on engine performance, exhaust emissions and combustion characteristics at different operating parameters in a turbocharged direct injection diesel engine. In this study, it was stated that the addition of n-octanol improved the heat release rate in terms of combustion performance, but the use of methylal and dimethyl carbonate reduced the heat release rate. According to the results obtained, it was reported that all three fuel blends containing oxygen increase brake specific fuel consumption and exhaust temperatures compared to pure diesel fuel. Also, it was explained that HC and NOX emissions increased as the amount of n-octanol increased, while CO and soot emissions decreased depending on the amount of n-octanol in the blends.
Tian et al. [38] using pure diesel and octanol fuels, created different fuel blends and investigated as experimentally and theoretically the injection characteristics of diesel fuel by adding octanol in diesel in different parameters. Experimental studies were carried out separately for five different injection pressures (60, 80, 100, 120, and 140 MPa) and three different ambient pressures (1, 2, 3 MPa) in a special experimental setup with a common-rail direct injection system they designed. Also, artificial neural network (ANN) analysis model was used in their theoretical studies. In order to train and test the artificial neural network model, analyses were made using some values obtained in experimental studies. In the next process, the experimental results that were not used in the training process and the prediction results obtained with the artificial neural network analysis model were compared. According to the results obtained, it was explained that the artificial neural network analysis model showed that the appropriate octanol ratio can improve the spraying properties of diesel fuel.
López et al. [39] used biodiesel obtained from olive–pomace oil and pure diesel fuels in a three-cylinder, water-cooled, Perkins AD 3-152 model CI direct-injection (DI) engine and experimentally examined the engine’s performance characteristics for full engine load and different engine speed conditions. Energy and exergy analyses were conducted using the performance and exhaust emission values acquired in experimental studies. In experimental studies, it was explained that when biodiesel (B100) fuel was utilized instead of pure diesel fuel, the maximum engine power decreased by approximately 5.6%, while the brake specific fuel consumption (BSFC) increased by approximately 7%. According to the exergy results, it was reported that the biodiesel obtained from olive–pomace oil and its blends with pure diesel fuel could be used instead of conventional diesel fuel in internal combustion engines without any increase in exergy costs. Magno et al. [40] used biodiesel obtained from rapeseed oil and pure diesel fuels in different proportions (B0, B50, and B100) in three-cylinder, water-cooled, Lombardini LDW 1003 model CI, common-rail injection system DI engine and experimentally examined the engine’s performance characteristics for different engine loads (medium and full) and different engine speed conditions. The mentioned research conducted energy and exergy analyses to examine the energy distribution of a small CI engine and to characterize the waste heat energy. According to the experimental results obtained, it was reported that biodiesel increased the combustion efficiency while creating lower exhaust emissions and was a more environmentally friendly and cleaner fuel compared to pure diesel fuel. Nemati et al. [41] carried out experimental studies using biodiesel obtained from waste cooking oils and pure diesel fuels in different proportions (B0, B5, B20, B50, and B100) under full engine load operating conditions in a single-cylinder, water-cooled, CI DI engine. Exergy analysis was carried out using the performance and exhaust emission values obtained in experimental studies. It was explained that as the biodiesel ratio in the blends increased, exergy efficiency also increased. According to the results of the exergy analysis, the researchers reported that B20 biodiesel blend was optimum since it exhibited the best combustion performance among the biodiesel blends used in the study. Khoobbakht et al. [42] conducted experimental studies by creating ternary blends using biodiesel obtained from soybean oil, ethanol and pure diesel fuels in different proportions in a four-stroke, four-cylinder, water-cooled, CI DI engine. In that study, energy and exergy analyses were conducted using performance and exhaust emission values. In addition to the operating parameters of engine load and engine speed, the effects of biodiesel and ethanol levels in the blends on exergy efficiency were examined. It was explained that as the biodiesel and ethanol ratios in the blends increased, the exergy efficiency also increased. Moreover, it was stated that with the increasing engine load and engine speed, exergy efficiency increased. According to the results obtained from the energy and exergy analyses, it was reported that 43.09% of the fuel exergy was destroyed and the average thermal efficiency was approximately 36.61%, and the exergetic efficiency was about 33.81%. Nabi et al. [43] performed experimental studies by creating blends using two different biodiesels obtained from macadamia integrifolia and waste cooking oils and pure diesel fuel in different proportions in a four-stroke, four-cylinder, water-cooled, naturally aspirated Kubota V3300 model CI DI engine. Experimental studies examined the impacts of these different fuel blends on engine performance characteristics, emissions, and combustion properties at two different engine loads (25% and 100%) and a constant engine speed of 1400 rpm. Additionally, energy and exergy analyses were conducted using the performance and exhaust emission values obtained from experimental studies. It was determined from experimental results that compared to pure diesel fuel, all diesel–biodiesel blends did not cause a significant change in engine performance but led to higher combustion efficiency. It was stated that carbon monoxide (CO) and hydrocarbon (HC) emissions were lower in two different biodiesel and diesel blends in comparison with pure diesel fuel. Nevertheless, an increase was reported in NOX emissions in accordance with biodiesel studies in the literature. According to the results obtained from the energy and exergy analysis using pure biodiesel and diesel/biodiesel blends utilized in the study, it was stated that it did not change at all compared to pure diesel fuel. Sarıkoç et al. [44] conducted experimental studies by creating ternary blends using biodiesel obtained from waste cooking oil, butanol and euro diesel fuels in different proportions (Diesel, B100, B20, B20But5, B20But10, and B20But20) in a four-stroke, single-cylinder, water-cooled, naturally aspirated CI DI engine. In the mentioned research, the effects on performance and harmful exhaust emissions were examined in experimental studies using ternary fuel blends at 1400 rpm and 2400 rpm engine speeds and under full engine load conditions. Furthermore, energy and exergy analyses were conducted using the performance and exhaust emission values obtained from experimental studies. In the said research, it was explained that the maximum energy-exergy efficiency and sustainability index (SI) values were obtained as 32.49%, 30.25%, and 1.434, respectively, using B100 fuel at an engine speed of 1400 rpm. According to the findings of the energy and exergy analysis, the researchers reported that this ternary fuel blend was optimum because B20But5 fuel blend exhibited the best performance characteristics among the biodiesel blends used in the study. In addition, there are studies in the literature in which exergoeconomic analyzes are performed on different power systems [45,46,47].
There are many studies in the literature on the performance and emissions of CI engines using diesel, biodiesel, and n-octanol fuel blends. However, studies in the literature in which the exergy and exergoeconomic analyses of ternary fuel blends from alcohol/biodiesel/n-octanol fuels are conducted are quite limited compared to performance and emission studies. In order for binary and ternary blends of diesel, n-octanol, and biodiesel to be preferred as an alternative to pure diesel fuel, it is necessary to evaluate exhaust emissions and engine performance in thermodynamic and economic analyses. The priority of the chosen the alcohol-based fuel was to consider the advantage of the excellent characterizations on account of enhance the poor properties of biodiesel having a high kinematic viscosity and conventional diesel fuel which is not contained any oxygen molecules in their chemical structure. With this viewpoint, the present research aimed to determine the potential of using diesel/biodiesel/n-octanol fuels in a CI engine without any significant structural change and without changing the fuel system and engine settings. Ternary fuel blends (Diesel, B20, B20OCT5, B20OCT10, B20OCT15, B20OCT20, and B100) were prepared using diesel/biodiesel/n-octanol fuels in different proportions, and experimental studies were carried out to determine their effects on power, torque, BSFC, and exhaust emissions at a constant engine speed of 1500 rpm for four different engine loads (25%, 50%, 75%, and 100%). Moreover, in this research; energy, exergy, exergoeconomic and environmental analyses were examined using the performance and emission values obtained in experimental studies.
The innovative approach of the present experimental research is to deliver a systematic perspective, assessment, and discussion of the chosen tested fuel samples in the same CI engine and working conditions. As submitted, the proportion of the biodiesel which was transesterified from rapeseed oil was kept to be constant in all the binary and ternary fuel samples because of the importance of the above-mentioned ratio. 20% is known as a common blending concentration since it exhibits a preferable balance of cost, harmful pollutants, low-temperature characteristics, the ability to act as a solvent, and compatibility with materials. For that reason, most biodiesel consumers have bought B20 or lower blends from the markets. In this direction, the authors selected the aforesaid biodiesel concentration in the present study to improve the emission behaviors and decrease the cost of the final fuel. However, as known, biodiesel has high density and viscosity specifications, and hence, engine performance and combustion characteristics were influenced in a negative way. Therefore, the long-chain alcohol (C8 type) was infused to the binary blend of biodiesel and neat diesel fuel to enhance the specifications of the test fuels because most of the researchers implied that poor properties of the biodiesel were eliminated blending with alcohol when it was operated in a diesel engine. On the other hand, it is important to consider the thermodynamic, environmental, and economic analysis in order to decide this situation. In this regard, the present study will be one of the initial attempts according to the aforementioned motivation as far as the authors know. As observed, the lower concentrations of alternative fuels were advised in the engine testes for the sake of a few backgrounds. These were summarized as follows: at first, a limited number of alternating biofuels can be blended with conventional diesel fuel so as not to apply a modification to the test engine. Namely, there is no need for paying an extra price on account of modifying the engines and their corresponding sectors. The other factor is the production cost of the higher-order alcohols, unfortunately, because the production cost of the alcohol-based fuels is higher than that of mineral diesel fuel. This case is explained to be the low production capacity of the alcohols commercially all over the world except for ethanol and methanol. In conclusion, the combustion, performance, and emission parameters of all the tested fuel samples were achieved under the operating conditions and compared with the unmodified diesel fuel and discussed in detail. In addition to this, the data obtained from the engine tests were used to analyze the thermodynamic, environmental, and economic points of view for the alternating fuel samples comprehensively in order to present a sustainable and economic blending ratio for the users.
In this study, performance and emission tests in a CI engine were carried out using fuel blends created by blending diesel, biodiesel and octanol in different proportions. For fuel blends, the energy and exergy distributions in the engine were determined by performing thermodynamic analyses on a single-cylinder, water-cooled, naturally aspirated diesel engine. Thence, the parameters for first law of thermodynamics such as fuel energy, exhaust energy, energy value transferred to the refrigerant, lost energy, effective power, effective efficiency, specific fuel consumption and the parameters for second law of thermodynamics such as fuel exergy, exhaust exergy, net exergetic power, exergy value of the cooling process, exergy value to the environment due to losses, exergy destruction, exergetic efficiency, unit exergy cost were calculated. In the obtained fuel types, the energy and exergy analyses data were compared with each other and evaluated according to different parameters and the most suitable fuel blend was detected.
Material and methods
Test fuels
In this work, the engine trials were carried out conducting traditional diesel fuel, canola oil biodiesel, and n-octanol. The conventional diesel fuel was purchased from a local petroleum station in Samsun, Turkey. The canola oil biodiesel was provided from a licensed biodiesel producer located in İzmir, Turkey, and this company has produced biodiesel which is met the EN 14,214 biodiesel standard. The fatty acid composition of the canola oil biodiesel was determined by Gas Chromatography/Mass Spectrometry (GC/MS) analysis using an Agilent GC/MS instrument (Model: 7890B GC-5977MSD) with a HP-5 ms column (30 m × 250 μm × 0.25 μm). The fatty acid composition for canola oil biodiesel is presented in Table 1. In addition, the GC/MS graph was also given in Appendix section (Fig. 21) at the end of the paper. As observed, the dominant fatty acids in the canola oil biodiesel are oleic acid methyl ester (55.11%) and linoleic acid methyl ester (29.75%).
Lastly, the n-octanol as an oxygenated next-generation fuel additive used in the present examination was bought from the Sigma-Aldrich Chemical Company (St. Louis, Missouri, USA). Within this framework, canola oil biodiesel was mixed with the diesel fuel at a ratio of 20% on a volume basis. Afterward, n-octanol as a sustainable alternating long-chain alcohol was blended the aforementioned mixture at the fractions of 5%, 10%, 15%, and 20% by volume implementing a splash blending technique keeping the biodiesel concentration as a constant. Thanks to a calibrated beaker that has a precision of ± 0.5 mL, accurate mixing proportions were supplied for each component of the blends. It is to be noted that seven test fuel samples were formed so as to employ in the engine experiments. The blending concentrations of the tested fuel samples and their abbreviations are exhibited in Table 1s of the supplementary data file. The above-stated fuel blends were volumetrically prepared with the help of a thermomagnetic mixer for 20 min at the speed of 1500 rpm. To conclude, the prepared test fuels were filled into the sealed glass bottles and rested in a dark environment on account of preventing contact from the air.
Firstly conventional diesel fuel was used in order to acquire the reference data and then the other test fuel samples have experimented in the same conditions. The considerable physical and chemical characteristics of the n-octanol, canola oil biodiesel, and diesel fuel used in this research are tabulated in Table 2. Besides that, some of the fundamental fuel characteristics for the binary blend (conventional diesel fuel + canola oil biodiesel) and all ternary blends (conventional diesel fuel + canola oil biodiesel + n-octanol) were estimated based on the suggested and most used techniques in recent literature and tabulated in Table 2 as well. In other words, Kay’s mixing rule-\(\varphi_{{{\text{mix}}}} = \mathop \sum \limits_{i}^{n} x_{{\text{i}}} .\varphi_{{\text{i}}}\) [48] and Arrhenius-type equation-\(\ln \left( {v_{{{\text{mix}}}} } \right) = X_{{{\text{biodiesel}}}} .\ln \left( {v_{{{\text{biodiesel}}}} } \right) + X_{{{\text{diesel}}}} .\ln \left( {v_{{{\text{diesel}}}} } \right) + X_{{{\text{alcohol}}}} .\ln \left( {v_{{{\text{alcohol}}}} } \right)\) [49] were employed in order to find the fuel properties of blends.
It is to be notified that the infusion of n-octanol into the diesel + biodiesel blends has caused to descend the lower calorific value as well as the density of all ternary blends. On the other hand, kinematic viscosities and oxygen content were increased. Enhancement in the oxygen content of the fuel blends has led to improve the combustion characteristics of the tested engine. This subject will be discussed in the next sections comprehensively. To conclude, all prepared fuel blends might be used to be as alternating test fuels in the CI engine applications. This fact can be validated because of the almost similar cetane numbers of ternary blends to the traditional diesel fuel.
By the way, there is no phase separation within a week before starting the experiments as a result of the authors’ observations. Prior to the beginning of the trials, even so, the test fuel specimens were re-mixed at laboratory conditions. It can be guaranteed that the homogeneous type fuel blends were provided in the tests.
Engine setup and experimental procedure
The engine tests were performed on a water-cooled, DI, naturally aspirated, single-cylinder, and four-stroke CI engine. It is to be highlighted that no modification applied on the experimental setup. The main specifications of the used CI engine in the present work was presented in Table 2s in the supplementary data file section. A Baturalp Taylan brand eddy current dynamometer was mounted on the test engine crankshaft with the aim of loading the engine in the experiments. All experiments were carried out at the fixed engine speed of 1500 rpm. The technical specifications of the other test equipment are tabulated in Table 3. To sum up, the schematic and pictorial views of the experimental apparatus used in this study is displayed in Fig. 1s and Fig. 2s in the supplementary data file section. A Bosch brand Bea 550 model exhaust gas analyzer was used to measure the harmful pollutants. This device is able to meter the CO, NO, CO2, O2, and smoke opacity values. With the intention of measuring the temperatures like exhaust gas temperature, cooling water inlet and outlet temperatures, etc. were metered using a K-type thermocouples that have an accuracy of 1 °C. The main technical specifications of the exhaust emission measurement device are given in Table 3s in the supplementary data file section.
To get the reference data such as engine performance and exhaust emissions, first of all, the tested CI engine was operated with the conventional diesel fuel. Afterward, the engine experiments were continued with B100, B20, B20OCT5, B20OCT10, B20OCT15, and B20OCT20 fuels. Just before commencing the tests, all pieces of the apparatus on the engine, exhaust emissions measurement device, and dynamometer were controlled. In an effort to provide a steady-state conditions for each fuel type, the test engine was initially operated for 30 min. Afterward, it was assessed whether the engine reached the requested operating status. In conclusion, the stabilization duration of the engine was recorded and the stabilization of the engine was ensured for all of the tested fuel samples. The diesel engine reached stable conditions after approximately 20 min on average even though the stabilization durations were monitored distinctly for all test duels and engine loads. As mentioned-above, the experimental works have been realized at a fixed engine speed of 1500 rpm for distinct loading conditions (from 25% to full load in steps of 25%) on the engine. By the way, prior to each engine test, the fuel tank was discharged and cleaned. After, the other test fuel was refilled. Moreover, the tested engine has been stopped with a focus on decreasing the temperature of the engine for a particular time before each experimental work so as to avoid the temperature errors. The flowchart of the present experimental study is depicted in Fig. 1.
Uncertainty analysis
During the appraisement of the engine performance and exhaust emissions findings acquired from the experiments, all tests have been performed a minimum of three times, and thereupon the average values were considered in order to avoid the errors. In order to prove the accuracy of the measured parameters, an uncertainty analysis was performed using the least squares method recommended by [55]. Table 4 presents the uncertainty values for the instruments used in the experiments.
The overall uncertainty analyses of the experiments could be calculated using the square root of [(0.6)2 + (1.0)2 + (0.2)2 + (0.8)2 + (0.9)2 + (0.6)2 + (0.5)2 + (1.0)2 + (1.0)2 + (0.7)2 + (0.6)2 + (0.6)2 + (0.9)2]. To conclude, the total percentage of uncertainty was found to be at ± 2.74%, which was an acceptable value for the experimental study.
Analysis
In the study, performance and exhaust emissions were determined using seven different fuel blends at different loads (25%–100%) at a constant speed of 1500 rpm in a CI engine. Energy and exergy analyses were conducted using these data. The following assumptions were used in the thermodynamic analysis.
-
In all calculations, the engine runs in a steady state.
-
It is assumed that the combustion air and exhaust gases are ideal gases.
-
Average values are taken for specific heat.
-
Changes in kinetic and potential exergy are neglected.
-
Ambient temperature is taken as 293 K and ambient pressure as 1 atm.
Energy analysis
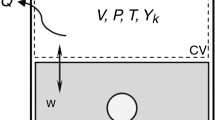
The total amount of mass in the control volume in a steady state does not change over time. According to the principle of conservation of mass, it is required that the total amount of mass that enters a control volume given in Fig. 2 be the same as the total mass leaving it [56].
In an internal combustion engine, the amount of energy that enters the control volume in all forms, such as heat rate, power, and mass flow rate, must be equal to the amount of energy rate released [57].
Since the source of energy in engines is fuel, the energy flow can be determined using the lower heating value (Hu) and the mass flow rate (\(\dot{m}_{{{\text{fuel}}}}\)) of the fuel [58].
The power obtained in an engine is equal to the difference between energy flow and thermal losses [59].
The energy flow of exhaust gases released from the engine to the atmosphere can be obtained using Eq. (5) [60].
Thermal efficiency is considered an important parameter in the evaluation of the energy analysis of an engine. The ratio of the useful energy exiting the engine to the energy entering the engine is known as thermal efficiency and can be calculated using Eq. (6) [61].
Exergy analysis
With the exergy analysis, the numerical evaluation of exergy destroyed and losses in the engine is made. For the control volume in Fig. 2, the equilibrium formula in Eq. (7) can be written. \({\dot{\text{E}}\text{x}}_{{{\text{air}}}} = 0\) is accepted since there is an air inlet to the test engine under atmospheric conditions. Exergetic power is equal to the useful power from the engine (\(\mathop {{\text{Ex}}_{{\text{w}}} }\limits^{ \cdot } = \dot{W}\)) [62].
The exergy factor \((\varphi\)) of fuel blends can be calculated using the ratios of hydrogen (h), carbon (c), sulfur (\(\alpha\)), and oxygen (o) in the fuel content [63].
Specific exergy (\(\varepsilon_{{{\text{fuel}}}} )\) and lower heating value are used while calculating fuel exergy \((\mathop {{\text{Ex}}}\limits^{ \cdot }_{{{\text{fuel}}}}\)). The lower heating value of fuel blends is an effective parameter on exergy flow [64, 65].
Before starting exhaust exergy calculations, the theoretical and actual combustion equations of fuels are written. The theoretical combustion equations of these fuels are given in Eq. (11). The emission data from the tests are used in Eq. (12) to obtain the actual combustion equation. In these equations, a, b, c, d, e, f, and g are constants [65].
Exhaust exergy contains physical (\(\varepsilon_{{{\text{phy}}}}\)) and chemical exergy (\(\varepsilon_{{{\text{chem}}}}\)). Physical exergy can be calculated using Eq. (14) and chemical exergy using Eq. (15). In these equations, (s) indicates specific entropy, (h) enthalpy, (\(\overline{R})\) gas constant, (T0) ambient temperature, and "0" indicates the dead state. In chemical exergy calculations, (\(y^{e} )\) the mole fraction of the components in the reference environment given in Table 4s in the supplementary data file was used [66].
The exergy accompanying the heat transfer consists of the exergy transitions with the radiation from the outer surface of the engine, by the flap wall on the outer edge of the engine, and during other heat transfers. Heat transfer and exergy losses \(~\left( {\dot{E}x_{{{\text{heat}}}} } \right)\) in the engine can be calculated using Eq. (16). Moreover, the heat released from the cooling water to the environment (Qcw) can be calculated using Eq. (17) [67].
Exergy is the part of the energy that is completely transformed into another form of energy and is used in the more efficient use of energy resources and is based on the second law of thermodynamics. In this case, the exergy efficiency can be calculated from Eq. (18) using exergetic power and exergy entering the system [68].
Exergy consumption is proportional to the entropy generated in the engine due to irreversibilities throughout the cycle. Exergy destroyed rate (\(\dot{E}x_{{{\text{dest}}}} )~\) can be found from Eq. (19), and entropy generation \(\left( {\dot{s}_{{{\text{gen}}}} } \right)\) from Eq. (20) [69].
Exergoeconomic analysis
In the study, the exergoeconomic analysis was conducted by assigning cost values to the exergy of the fuel entering the control volume and the output products. The selling prices of diesel, B100, and n-octanol fuels in Turkey are 0.93 $ L−1, 1.35 $ L−1, and 37.1 $ L−1, respectively. Mixing ratios were considered while determining the cost of fuel blends.
The exergoeconomic balance for the fuel blends used in the study is presented in Eq. (21). Since the combustion air entering the cylinder is taken from the atmosphere, its cost is zero. Here, (c) is the cost at the entry and exit to the control volume, and (\(\dot{Z}\)) is the investment cost ratio. The investment cost ratio is found using the engine initial investment cost (Z), the capital factor of the investment (\(E_{{\text{f}}}\)), maintenance factor (\(M_{{\text{f}}}\)), and the annual usage hours (\(t_{{{\text{year}}}}\)) [70].
A CI engine with the economic data which were engine cost: 6000 $, annual working hours: 3285 h, maintenance factor: 1.21, interest rate: 10%, and engine life time: 20 year was used in the study. The investment’s capital factor of this engine can be determined depending on the interest rate (i) and the engine’s life (n) [71].
The exergoeconomic balance for the engine can be written using the concept of fuel and product based in SPECO approach [72].
In the analysis, the fuel unit exergy cost is taken equal to the unit exergy cost of exhaust and thermal losses. Since the purpose of internal combustion engines is to obtain useful energy (power), the expenditures in all components are used for power. The unit cost for exergetic power can be calculated from Eq. (27) [73].
The unit cost of losses in the engine (\(\dot{C}_{{{\text{loss}}}}\)) can be calculated using Eq. (28) after the exhaust exergy, the exergy of thermal losses, and the exergy destroyed rate are determined [74].
Exergoeconomic factor (f) can be calculated using Eq. (29) by considering the initial investment cost of the engine and the losses during operation. Finally, relative cost difference for fuel blends can be calculated from Eq. (30) [75].
Environmental and exergoeconomic-environmental analyses
The exergoeconomic-environmental analyses include a cost for environmental damage of pollutant emission. The environmental impact is known as the total pollution damage ($ s−1). The cost of pollution damage is directly added to the expenditures according to ref [48]. CO2 emission in engines is defined as environmental impact. In this study, environmental evaluations were made by considering CO2 from the exhaust gases thrown into the atmosphere. In the exergoeconomic-environmental analysis, the mass of CO2 released to the environment in a particular period of time is calculated, while in the exergoeconomic-environmental analysis, the economic evaluation of environmental pollution in the same period is made [76]. CO2 emissions of fuel blends measured in engine tests were used for environmental analysis. Moreover, the operating hours were estimated. With environmental analysis, the CO2 emission “\(C_{{{\text{xCO}}_{2} }}\)” released at a certain time (10 h day−1) from Eq. (30) is determined using the exergy flow “\({\dot{\text{E}}\text{x}}_{{{\text{in}}}}\)” of the fuel blends entering the engine [77]. With environmental analysis, economic evaluation (\({\text{Ex}}C_{{{\text{CO}}_{2} }}\)) can be made with exergoeconomic-environmental analysis from Eq. (31) using the values of CO2 emission determined by mass. In this study, \(P_{{{\text{CO}}_{2} }}\) = 0.0145 $ kg−1 \({{\text{CO}}_{2}^{-1}}\) was used [78].
Results and discussion
Performance and exhaust emissions of fuel blends
The BSFC of fuel blends at different loads in the engine tests is presented in Fig. 3. The percentage change in consumption of other fuel blends compared to the reference fuel is given in Fig. 3b. BSFC decreased with the increasing engine load in all fuel blends. BSFC is directly related to the lower heating value of the tested fuel. As observed, Table 2 presents the energy content of the test fuels. In this context, unmodified diesel fuel has the highest energy content, whereas B20OCT20 has the lowest. In this context, the lowest BSFC is 0.202 kg/kWh in diesel fuel at an engine load of 100%. The lowest BSFC was obtained in the B20OCT5 fuel blend in ternary fuel blends. The highest BSFC occurred in B100 fuel with the minimum of lower heating value. In B100 and n-octanol blends, due to the fact that the lower heating value was lower than diesel fuel, additional BSFC occurred to maintain constant power output. In their study, Atabani et al. [79] observed that BSFC increased when waste cooking oil methyl ester, pentanol, and n-octanol were added to diesel fuel. In studies in the literature, BSFC increases when alcohols with low lower heating value are added in fuel blends [80,81,82,83]. It can be noted that the results do not have significant changes when the BSFCs of the tested fuels formed by the combustion of them in a diesel engine are taken into account in the uncertainties given in Table 4. In addition, this situation is caused by the fact that the fuel consumption and lower calorific values of the fuels are close to each other, as presented in Table 2, because the proportion of additives in fuel mixtures is very low. Therefore, it can be concluded that the BSFCs are very close to each other. This fact was similar in most outcomes of the CI engine.
The real cause of the formation of CO emissions is the lack of combustion because there is not sufficient oxygen. This lack of oxygen causes the carbon in the fuel to be released from the exhaust to the atmosphere as CO molecules due to oxygen deficiency in the cylinder. Since CI engines operate with excess air due to their operating systems, CO emission is generally low in all test fuels. CO emission variations of fuel blends at different loads in the engine tests is presented in Fig. 4. When Fig. 4b is examined, positive values show an increase, negative values show a decrease. This definition is also valid for other graphs in the study. Increasing engine load in all fuel blends reduces CO emission. The highest CO emission was 0.122% in diesel fuel at a load of 100%. The lowest CO emission in ternary fuel blends was 0.163% in B20OCT10 fuel blend at an engine load of 100%. Compared to the reference fuel, the highest increase in CO emissions was observed in B20OCT15 and B20OCT20 fuels at 100% load. While CO emissions of B100 and B20 fuels are higher at low engine loads, an improvement has been achieved with increasing engine load. In the tests performed by Alsok et al. [18], CO emission is constant at a change in the engine load between 25 and 75%. However, when the engine load was 100%, the CO emission increased seven times. In the studies in the literature [82, 84], the CO emission in fuel blends increased with the increasing engine load. Concerning the variation of CO emission for test fuels, it can be seen that n-octanol included fuels produced higher CO emissions than B100 and B20, generally. The reason for this can be attributed to the low cylinder temperature. The formation of CO emission also depends on in-cylinder temperature [85]. An increase in combustion temperature will accelerate the oxidation reaction of CO and hence less CO will be generated. However, in the case of using n-octanol blended fuels combustion temperature was reduced due to the high vaporization enthalpy and low calorific value of the n-octanol, in turn, higher CO emission compared to other fuels. From this observation, it can be concluded that the negative impact of low combustion temperature on CO formation is dominant than the positive effect of fuel's oxygen content on CO oxidation.
The fact that the fuel air ratio is higher than the fuel air ratio at low engine loads reduces CO2 emission, and the increase in the air quantity compared to low speeds with the increasing load increases the CO2 ratio. In the study, according to the test results presented in Fig. 5, the CO2 emissions of the fuel blends increased with the increasing engine load. The lowest CO2 emission was in B100 fuel at an engine load of 25%. B20OCT5 fuel emits more CO2 emissions than the reference fuel, while other blends act more environmentally. The highest CO2 emission was 3.31% in B20OCT5 fuel at 100% engine load. The oxygen content of biodiesel enhances the complete combustion process and increases the combustion quality. As a result, the fuel that can find sufficient oxygen can complete its combustion by igniting. In the study carried out by Devarajan et al. [86, 87], the CO2 emission of diesel fuel was lower than biodiesel blends containing alcohol at all engine loads.
NOX emissions occur as a result of nitrogen in the air reacting with oxygen at high temperatures. Upon examining Fig. 6, it is revealed that the amount of NOX increases with the increasing engine load. There was a decrease of 64.60% and 40.77%, respectively, in NOX amount at an engine load of 25% and 100% in B100 fuel compared to diesel fuel. The reason for this is thought to be the fact that temperatures at the end of combustion are lower since the lower heating value of B100 fuel is lower than diesel fuel. When Fig. 6b is examined, the NOX emission of other fuels is lower than the reference fuel, except for B20OCT5 fuel. As the combustion temperature of exhaust gases is reduced, the NOX formation is lower. As load increases, the air–fuel ratio is reduced and the combustion temperature and NOx emission increased [88, 89]. While the results obtained in this study are similar to some studies in the literature in terms of NOX emissions, some studies have obtained different results. The lowest NOX emission in ternary fuel blends was found to be 15.99% in B20OCT5 at an engine load of 100%. In the study conducted by Mahalingam et al. [90], NOX emissions were higher for mahua oil biodiesel and n-octanol with an increase in engine load. Biodiesel and n-octanol improve the combustion process and increase the temperature in comparison with diesel fuel because of their higher oxygen content [91,92,93].
In CI engines, oxygen that helps combustion is either completely exhausted at the end of the combustion, or the remaining oxygen is released. Oxygen, one of the exhaust emission products, consists of oxygen in the air and fuel. The change in O2 emissions of the fuel blends utilized in the study according to the engine load is shown in Fig. 7. In the beginning, the amount of oxygen was high due to the large amount of air taken in the cylinder at low engine loads. However, due to the decrease in the amount of air taken into the cylinder with the increasing engine load, the amount of oxygen released from the exhaust decreased.
Energetic evaluation
The energy input into the control volume is only the chemical energy of the fuel. In this case, the fuel energy flow is the function of the lower heating value of the fuel. The energy flow of the fuel blends used in the study is presented in Fig. 8. The increasing engine load increases the fuel flow rate and energy flow. B100 fuel’s lower heating value is the lowest among fuel blends. However, the highest energy flow occurred in B100 fuel at all engine loads. Since the lower heating values of the fuel blends obtained as a result of the combination of three different fuels (ternary blends) were close to each other, there was no noticeable difference in energy flow at all engine loads. The lowest energy flow in fuel blends at 100% engine load was calculated as 3.44 kW in B20OCT20 fuel. In fuel blends, there is no noticeable change at 25% engine load compared to the reference fuel. The highest change occurred at 50% and 75% engine load. Nabi et al. [43] showed that the fuel energy increased with the increase in engine power in a diesel engine using new series of non-edible biodiesels. In cases where the brake power value is 10 kW and 30 kW in diesel fuel, the fuel energy is 37 kW and 100 kW, respectively.
Some of the energy obtained with the combustion of the fuel passes to the cooling water and lubricating oil, while some of it is released through the exhaust [65, 94]. These losses are calculated from the difference between the fuel energy flow and the useful work obtained from the engine. Heat transfer losses occur predominantly through conduction, convection, and radiation through the cylinder walls and cylinder head. Similar to studies in the literature, thermal losses increased with the increasing engine load in this study [68, 95, 96]. Heat losses variations of fuel blends at different loads in the engine tests are presented in Fig. 3s in the supplementary data file section. The highest thermal loss was computed to be 5.14 kW in B20OCT15 fuel at an engine load of 100%. Thermal losses were higher in B100 fuel than diesel fuel at all engine loads.
Thermal efficiency is the rate at which the heat energy generated as a result of the combustion of the fuel is transformed into useful work by the engine. As seen in Fig. 9, the fuel blends used in the study increased their thermal efficiency with the increasing engine load. The maximum thermal efficiency was calculated as 40.65% in B20OCT5 fuel blend at an engine load of 100%. In this fuel blend, the lowest thermal loss was determined at an engine load of 100%. In studies in the literature, an increase in engine load increases thermal efficiency [67, 68, 94]. In the increase in engine speed, thermal efficiency increases up to a particular speed and then decreases [44, 94]. Doğan et al. [97] determined the thermal efficiency of diesel-heptanol fuel blends depending on engine load. The increase in the heptanol ratio in fuel blends decreases the thermal efficiency. In the case of 100% engine load, the thermal efficiency of HP0 and HP20 fuels is 41% and 38%, respectively. AghbaShlo et al. [98] determined the thermal efficiency of a diesel engine using polymer waste dissolved in biodiesel. In the study, the thermal efficiency of diesel B5P50 fuels at 1600 rpm engine speed and 50% engine load was 39% and 44%, respectively.
Exergetic evaluation
To determine engine losses, energy analysis and exergy analysis were performed in the research. Using the chemical exergy and lower heating value of the fuel blends used in the tests, exergy flows were calculated at different engine loads. When the test engine was switched from 25% load to 100% load, exergy flow increased by approximately 35% in all fuel blends. Exergy flow variations of fuel blends at different loads in the engine tests are presented in Fig. 10. The lowest exergy flow was computed to be 7.2 kW in B100 fuel at an engine load of 25%. Exergy flows were found in exergy studies in the literature, depending on the engine load and engine speed [48, 67]. The increasing engine load increased the exergy flow. Furthermore, the exergy flow of biodiesel is lower compared to diesel fuel [99, 100].
The combustion equations obtained by using the exhaust emission values measured during the tests were used. The exhaust exergy flow was found with the flow rates and enthalpy values of each combustion product and is given in Fig. 4s in the supplementary data file section. The lowest exhaust exergy was calculated in B20OCT20 and B20OCT10 fuels at an engine load of 25%. The highest exhaust exergy was determined as 1.845 kW in B20OCT5 fuel at an engine load of 100%. In the study of Şanlı [88], the exhaust exergies of diesel and microalgae (microalgae) biodiesel were calculated as 15.4 kW and 14.13 kW, respectively, at 1500 rpm engine speed. The increase in engine speed increased the exhaust exergies. In studies in the literature, exhaust and thermal losses increase significantly with the increasing engine load [101, 102].
A significant amount of the heat energy gained by the combustion of the fuel is transferred to the cooling water and lubricating oil, while some part of it is released into the atmosphere as hot exhaust gases. While calculating these losses, some part of fuel energy flow is also lost with heat transfer [101, 103]. The exergy resulting from thermal losses is given in Fig. 5s in the supplementary data file section. The decrease in the exergy of thermal losses affects fuel efficiency. The lowest heat exergy flow was found to be 0.246 kW in B20OCT5 fuel at an engine load of 25%. Aghbashlo et al. [98] calculated the exergy transfer rate to cooling water in a diesel engine using polymer waste dissolved in biodiesel. In this calculation, when the engine load is 100%, the exergy transfer rate to cooling water for diesel and B5P75 fuels was determined as 3.51 kW and 4.15 kW, respectively.
Some part of the fuel exergy is destroyed by the irreversibility of combustion and other irreversibilities in the engine. Upon examining Fig. 6s in the supplementary data file section, it is observed that the lowest exergy destroyed rate was calculated in B100 fuel at all engine loads. The highest exergy destroyed rate in ternary fuel blends was computed to be 5.67 kW in B20OCT5 fuel. Among all fuel blends, the highest exergy destroyed rate was determined as 5.88 kW in diesel fuel at an engine load of 100%. In studies in the literature, exergy flow, exhaust exergy, and exergy of thermal losses also increase with the increasing engine load. Şanli et al. [90] carried out the exergy analysis of pure diesel and biodiesel produced from palm oil and opium poppy oil. When the engine speed is 1600 rpm, diesel, OPB, and PB fuel exergies are 122.96 kW, 121.95 kW, and 113.80 kW, respectively. The exergy destructions under the same conditions are 60.59 kW and 62.94 kW and 56.90 kW, respectively. In this case, exergy destroyed rate changes in parallel with the engine load [97, 104]. Aghbashlo et al. [98] showed that exergy destruction decreased as the additive ratio increased in a diesel engine using polymer waste dissolved in biodiesel. In the case of 100% engine load, the exergy destruction of diesel and B5P75 fuels is 68 kW and 62 kW, respectively.
According to exergy analysis calculations, the useful part of energy decreases and an increase in entropy occurs. In the test engine in the study, entropy generation occurs as a result of irreversible processes. Entropy, a measure of molecular disorder, depends on the exergy destroyed rate of the engine and ambient temperature. When Fig. 11 is examined, entropy generation rate increases with the increase in engine load. The lowest entropy generation rate was 0.014 kW K−1 in B100 and B20OCT5 fuels at an engine load of 25%. In the literature, entropy generation rate was determined at different engine loads and speeds. Entropy generation rate decreases at different engine speeds up to a certain speed value and then increases [25, 97]. Since the increase in engine load increases exergy destroyed rate, entropy generation rate increases [99, 101]. Şanli et al. [105] determined entropy generation in diesel, hazelnut biodiesel and canola biodiesel. In the study, entropy generation in diesel and hazelnut biodiesel at 1800 rpm engine speed is 0.16 kW K−1. Under the same conditions, the entropy generation for canola biodiesel is 0.15 kW K−1.
In the tests, the part of the fuel exergy entering the combustion chamber that turned into work was calculated as exergy efficiency and is presented in Fig. 12 depending on the engine load. It was observed that changes in exergy efficiency were parallel to changes in the thermal efficiency curve. The increase in engine power obtained with the increasing engine load affects exergy efficiency positively. The highest exergy efficiency at all engine loads was achieved in B100 fuel. The highest exergy efficiency in ternary fuel blends was calculated as 30.49% in B20OCT5 fuel at an engine load of 100%. In the studies of Yeşilyurt and Arslan [68], the exergy efficiencies of diesel and biodiesel are 21.275% and 20.052%, respectively. Şanli et al. [105] calculated the exergy efficiencies of diesel, hazelnut biodiesel, and canola biodiesel as 36%, 35%, and 33%, respectively. Upon reviewing studies in the literature, it is determined that exergy efficiency increases with the increasing engine load [98, 101, 103].
Economic evaluation
In the exergy analysis, the exergy of all losses was calculated using the data obtained from engine tests. The costs of the data in exergy analysis were found with exergoeconomic analysis. While performing this analysis, firstly, the costs of the fuel blends entering the control volume were calculated depending on the engine load and shown in Fig. 13. Fuel flow rates and costs increase with the increasing engine load in all fuel blends. The pump price of n-octanol is very high compared to diesel and B100 fuels. Therefore, the cost increased significantly in fuel blends containing n-octanol. When the engine load was 100%, the cost was calculated as 9.19 $ h−1 in B20OCT20 fuel and 2.42 $ h−1 in B20OCT5 fuel. Changing the amount of n-octanol in the content of these two fuels significantly influences the cost. In studies in the literature, it was observed that fuel costs increased in case of adding diesel fuel, biodiesel, and alcohol-based fuels [98, 106, 107].
While calculating the cost of the useful work (power) obtained from engines, the exergy flow cost in the control volume of fuel blends and the cost of the exergy of products and losses are used in calculations. The engine power cost of each fuel blend used in the study was calculated and is presented in Fig. 14. The increase in engine load reduces the cost of engine power of fuel blends. The engine power cost was calculated as 10.61 $ MJ−1 at an engine load of 25% in B20OCT20 fuel with the highest n-octanol content. The lowest engine power cost was determined in diesel fuel at all loads. To reduce the cost of engine power in ternary blends, new production methods should be used to reduce the pump prices of n-octanol. In studies in the literature, biodiesel and alcohol-based fuels added to diesel fuel were observed to considerably increase the cost of engine power [97, 98, 106].
The cost of exergy losses in the engine is presented in Fig. 7s in the supplementary data file section. The increasing engine load reduces the cost of exergy losses. The cost of exergy losses was high in B20OCT20 fuel at all engine loads. Although the values of the lost exergy of diesel and B100 fuels were close to each other (Figs. 12 and 13), according to the exergoeconomic analysis data, the cost of B100 fuel was 35–43% higher at different engine loads than diesel fuel. This is explained by the higher pump price of B100 fuel compared to diesel fuel.
The cost rate for exergy destroyed calculated in the exergy analysis is presented in Fig. 8s in the supplementary data file section. Diesel fuel, with a low pump price, is the fuel with the lowest exergy destroyed cost at all engine powers. The exergy destroyed cost of B20OCT20 fuel is 7.78 $ h−1 at an engine load of 25%. The cost rate values of this fuel at all engine loads are significantly higher than other ternary blends. The cost of exergy destroyed depends on the pump prices of fuel blends, engine load and speed [104, 107].
The exergonomic factor is the evaluation of the initial investment cost of the engine and the total cost of exergy losses together [97, 103, 106]. The exergoeconomic factor of the fuel blends utilized in the test engine decreases depending on the increase in engine load, as presented in Fig. 15. Exergy losses in fuel blends increase as the engine load increases. The exergonomic factor also decreases depending on these losses. In ternary fuel blends, the exergonomic factor is lower than diesel, B100, and B20 fuels. The highest exergonomic factor at all engine loads was calculated in diesel fuel.
Figure 16 shows the effect of fuel blends on the relative cost difference at different engine loads. It will be easier to reduce the exergy cost in the fuel with a large relative cost difference. The relative cost difference value decreased with the increase in engine load. Relative cost difference in fuel blends varies between 1.69 and 5.35 depending on engine load. The highest relative cost difference value was calculated as 5.35 for diesel fuel at 25% engine load. Relative cost difference in dual and triple fuel blends is close to values.
Environmental evaluation
The CO2 emissions released into the atmosphere as a result of the combustion of fuel blends in the test engine were measured, and the cost of the damage to the environment was calculated with the exergy-based analysis. The results obtained from the exergoenvironmental and exergoenviroeconomic analysis are shown in Figs. 17 and 18. The highest environmental damage occurred in diesel fuel at all engine loads. In the exergoenvironmental analysis, the lowest emission value was found as 271.63 kg CO2 month−1 in B100 fuel at an engine load of 25%. In ternary fuel blends, the lowest CO2 emission occurred in B20OCT10 fuel at all engine loads.
According to the exergoenviroeconomic analysis, it will be more efficient to use diesel fuel with binary and ternary fuel blends to reduce the environmental cost. When Fig. 18 was examined, their values were calculated as 8.8 $ month−1, 8.6 $ month−1, and 8.4 $ month−1, respectively, in diesel, B20, and B20OCT20 fuels at an engine load of 100%. As the pure diesel fuel ratio in blends decreases, the cost of the damage caused by CO2 emissions to the environment decreases. Studies in the literature have investigated the impact of engine speed and load on the cost of CO2 emissions. The cost of CO2 emissions of diesel fuel is lower than that of biodiesel [108, 109].
The comparison of the results obtained in the study with the literature is tabulated in Table 5.
Combustion analysis results
The in-cylinder gas pressure (CP) of fuels at various engine loads is presented in Table 5s in the supplementary data file section. As can be seen in this table, the peak CP increased by rising engine load and B20OCT10 and B20OCT15 fuel presented higher CP values than the other blended fuels and pure biodiesel, generally. The variation of CP at full engine load is shown in Fig. 19. The highest CP values for fuels were recorded at the engine load of 100% since the engine was fueled with the highest quantity of fuel under this load. The peak pressure for diesel, B100, B20, B20OCT5, B20OCT10, B20OCT15, and B20OCT20 was measured as 54.67 bar, 53.58 bar, 52.60 bar, 52.58 bar, 54.94 bar, 54.68 bar, and 51.55 bar, respectively. It can be observed that the peak CP for B100, B20, B20OCT5, and B20OCT20 decreased compared to that of diesel. The main reason behind this could be the low lower heating value of biodiesel and octanol than that of diesel fuel. However, a slight increase in CP occurred by B20OCT10 and B20OCT15 fuel. This result can be ascribed to the fact that the kinematic viscosity of biodiesel and octanol is higher than diesel fuel. High viscosity hinders uniform fuel–air mixing causing a longer ignition delay. In this case, more fuel accumulated in the combustion chamber during the ignition delay which leads to more fuel burning in the premixed combustion phase in turn high CP. On the other hand, B20OCT20 decreased the peak CP concerning diesel fuel. This could be because the highest reduction in heating value has occurred by 40 vol.% of oxygenated fuels (octanol + biodiesel) fraction. These findings are matched with those of [53] and [110].
The variation of the heat release rate with the crank angle at full engine load for fuels is shown in Fig. 20. The peak heat release rate for diesel, B100, B20, B20OCT5, B20OCT10, B20OCT15, and B20OCT20 is determined as 31.57 J deg−1, 29.80 J deg−1, 26.08 J deg−1, 28.78 J deg−1, 31.57 J deg−1, 31.98 J deg−1, and 25.07 J deg−1, respectively. It can be noticed that HRR increased for 10% and 15% n-octanol blended fuel compared to others. This may be occurred due to a longer ignition delay that causes excess fuel combustion in the premixed combustion phase. As submitted in Table 2, the cetane number of the n-octanol is lesser than that of diesel fuel. For that reason, the ignition delay getting increased by n-octanol blends. Since the calorific value of biodiesel and octanol is lower than that of diesel, B20OCT20 yielded the lowest heat release rate among the test fuels. This finding is consistent with the result concluded by [111]. A noticeable peak in the heat release rate at a crank angle of about 425 deg for the B20 fuel was observed. This may be because more B20 fuel droplets get in contact with air molecules and burned during the expansion stroke. In that case, the heat release rate increased, which, in turn, stronger diffusion combustion compared to other test fuels.
The rate of pressure rise versus the crank angle for fuels is depicted in Fig. 9s in the supplementary data file section. The highest rate of pressure rise value was observed at full engine load for all tested fuels. At this operating condition, the peak rate of pressure rise for diesel, B100, B20, B20OCT5, B20OCT10, B20OCT15, and B20OCT20 was calculated as 3.65 bar deg−1, 3.54 bar deg−1, 3.07 bar deg−1, 2.94 bar deg−1, 3.65 bar deg−1, 3.38 bar deg−1, 2.88 bar deg−1, respectively. Also as seen in Table 5s, the rate of pressure rise for all test fuels increased as ascending the engine load since the combustion rate increases by enhancing the engine load. Besides, the addition of octanol by the amount of 10% and 15% caused a slight increase in the rate of the pressure rise compared to other blend fuels because of a higher fraction of premixed combustion. The same trends for these fuels were also obtained from the net heat release rate analysis.
The variation in cumulative heat release versus the crank angle for fuels at full engine load condition is illustrated in Fig. 10s in the supplementary data file section. The cumulative heat release rate at full load operation for diesel, B100, B20, B20OCT5, B20OCT10, B20OCT15, and B20OCT20 was calculated as 1150 J, 905 J, 1435 J, 1260 J, 1040 J, 965 J, and 970 J, respectively. It is observed that the cumulative heat release of B100 is significantly lower than the other fuels. This may be due to the lower energy density and high viscosity of the biodiesel, resulting in poor combustion and hence less heat release. Also, due to the high water content of the ternary blends, the heating value of fuels further reduces, and a higher heat of vaporization is absorbed and therefore cumulative heat release might descend [53].
The crank angle wherein 50% of the total heat release rate being released is termed as CA50, and it has a great effect on the combustion quality and fuel conversion efficiency [112]. Also, CA50 is an indicator for the midpoint of combustion and the value of the CA50 should be fall in 5–25 CA after the top dead center on account of the high thermal efficiency [113]. Figure 11s in the supplementary data file section illustrates the location of CA50 for tested fuels. The CA50 angle at full engine load operation for diesel, B100, B20, B20OCT5, B20OCT10, B20OCT15, and B20OCT20 was determined as 375 °CA, 375 °CA, 389 °CA, 377 °CA, 376 °CA, 374 °CA, and 373 °CA, respectively. It is noticed that the CA50 location for D80B20 was found far away from the optimum point. However, other fuels offered a closer CA50 value compared to diesel. The CA50 angle was highly greater for B20 than that of diesel. The reason was considered to be that biodiesel has a higher kinematic viscosity and it contains heavy molecular components. In addition, the CA50 value for B100 was fairly lower compared to B20. This could be attributed to the higher oxygen mass fraction in B100, which could enhance the diffusion combustion rate [114].
The combustion duration of the fuels at full engine load is presented in Fig. 12s in the supplementary data file section. The combustion duration in terms of crank angle for diesel, B100, B20, B20OCT5, B20OCT10, B20OCT15, and B20OCT20 was obtained as 101 °CA, 74 °CA, 115°CA, 109 °CA, 78 °CA, 77 °CA, and 74 °CA, respectively. It is observed that the combustion duration was decreased when the increased octanol fraction. The reason for the shorter combustion duration could be due to the in-built oxygen content of the alcohol fuel, which accelerates the combustion process [98]. Additionally, this could be because more fuel is burned in the premixed combustion phase due to the low cetane number of octanol [19].
Conclusions
Performance and emission tests were carried out with seven different fuel blends obtained using diesel, biodiesel, and n-octanol in a CI engine at a constant speed of 1500 rpm at four various loads. Energy, exergy, exergoeconomic, exergoenvironment, and exergoenviroeconomic analyses were performed using the data obtained from these tests.
Since the lower heating values of the fuel blends were close to each other, there was no considerable difference in BSFC. There was an increase in CO emissions in the fuel blends to which n-octanol was added. While CO emission was 0.31% in B20OCT15 fuel at an engine load of 25%, it was 0.29% in B20OCT5 fuel. Increasing n-octanol content in fuel blends adversely affected CO emissions. CO2 emissions released into the atmosphere from diesel fuel are higher compared to other fuels. In the exergoenvironment and exergoenviroeconomic analysis, the highest environmental cost was calculated as 607.17 kg CO2 month−1 and 8.8 $ month−1, respectively, in diesel fuel at an engine load of 100%.
When the combustion analyses results were examined, the peak in-cylinder pressures for the tested fuel samples were found between 51.55 and 54.94 bar at full load. The maximum HRR was increased in general when the octanol was infused to the fuel blend due to the long ignition delay period that lead to excess fuel combustion in the premixed combustion phase. The ignition delay period of the tested fuel was decreased when the engine load was increased. Moreover, the ID period was generally prolonged when the octanol was added into the binary blend because of the cetane number of the alcohol which was lower than the conventional diesel fuel.
The highest thermal efficiency and exergy efficiency were determined to be 40.65% and 30.49%, respectively, in B20OCT5 fuel. According to the exergoeconomic analysis, the highest cost of the power taken from the engine was found to be 10.61 $ MJ−1 in B20OCT20 fuel at an engine load of 25%. This high cost originates from the high pump price of n-octanol. It is thought that this fuel will be used as an alternative fuel in diesel fuel blends as a result of increasing n-octanol production and reducing pump prices.
While determining the fuel blend that will be an alternative to the traditional diesel fuel, the alcohol ratio in the fuel mixtures should be increased in order to reduce the harmful pollutants generated from the engine thrown into the atmosphere. However, due to the high pump prices of alcohol fuels, the increase in alcohol content in fuel blends is not economically viable. Biodiesel is economically and environmentally similar to conventional diesel. However, the use of agricultural lands for fuel production has been a problem in recent years. Therefore, the most suitable fuel mixture depends on alcohol prices and biodiesel production. Since fossil fuels will be depleted, the number of alcohol production facilities that can be fuel in power generation systems should be increased. If subsidies are given for agricultural materials used in alcohol production, alcohol pump prices will decrease. Thus, alcohols as fuel can be used for commercial purposes.
Data availability
The data used and/or analyzed throughout the present study are available from the authors on reasonable request.
Abbreviations
- c :
-
Specific exergy cost ($ MJ−1)
- C :
-
Cost flow rate ($ h−1)
- \(C_{{{\text{co}}_{{2}} }}\) :
-
CO2 emission (kg CO2 time−1)
- CRF:
-
Capital recovery factor (–)
- Cp:
-
Specific heat capacity (kJ kg−1 K−1)
- \(E_{{{\text{co}}_{{2}} }}\) :
-
Envireconomic parameter ($ time−1)
- \({\text{Ex}}_{{{\text{co}}_{{2}} }}\) :
-
Exergoenvireconomic parameter ($ time−1)
- E fuel :
-
Energy of fuel (kW)
- \(\mathop {{\text{Ex}}}\limits^{ \cdot }\) :
-
Exergy rate (kW)
- f :
-
Exergoeconomic factor (%)
- Hu:
-
Heat value of fuel (kJ kg−1)
- h :
-
Specific enthalpy (kJ)
- i :
-
Interest rate (%)
- M f :
-
Maintenance factor (–)
- \(\dot{m}\) :
-
Mass flow rate (kg s−1)
- n :
-
Engine speed (rpm)
- N :
-
System lifetime (year)
- \(N_{{{\text{co}}_{{2}} }}\) :
-
CO2 emission value (kg CO2 kW−1 h−1)
- P :
-
Pressure (kPa)
- \(P_{{{\text{co}}_{{2}} }}\) :
-
CO2 emission value ($ kW−1 CO2−1)
- P 0 :
-
Pressure of the environment (kPa)
- \(\dot{Q}\) :
-
Heat transfer rate (kW)
- R :
-
Gas constant (kJ kg−1 K−1)
- \({\overline{\text{R}}}\) :
-
Universal gas constant (8.314 J mol K−1)
- T :
-
Temperature (K)
- T 0 :
-
Temperature of the environment (K)
- T :
-
Torque (Nm)
- t year :
-
Annual working hours (h)
- rpm:
-
Revolutions per minute
- s:
-
Specific entropy (kJ kg−1 K−1)
- S gen :
-
Entropy generation rate (kW K−1)
- y e :
-
Component mole fraction (%)
- \(\dot{W}\) :
-
Power (kW)
- Z :
-
Engine cost ($)
- \(\dot{Z}\) :
-
Capital investment cost rate ($ h−1
- η :
-
Thermal efficiency
- µ :
-
Gas viscosity
- φ :
-
Fuel exergy factor
- ε :
-
Flow exergy
- a:
-
Air
- chem:
-
Chemical
- cw:
-
Cooling water
- dest:
-
Destroyed
- ex:
-
Exhaust
- heat:
-
Heat transfer
- in:
-
Inlet
- k:
-
Kinetic
- out:
-
Outlet
- p:
-
Potential
- phy:
-
Physical
- ref:
-
Reference
- s:
-
Source
- w:
-
Work
- 0:
-
Environmental conditions
- ABDC:
-
After the bottom dead center
- ATDC:
-
After the top dead centre
- BBDC:
-
Before the bottom dead centre
- BTDC:
-
Before the top dead centre
- BTE:
-
Brake thermal efficiency
- B0:
-
100% Diesel
- B5:
-
95% Diesel and 5% biodiesel
- B10:
-
90% Diesel and 10% biodiesel
- B15:
-
85% Diesel and 15% biodiesel
- B20:
-
80% Diesel and 20% biodiesel
- B25:
-
75% Diesel and 25% biodiesel
- B30:
-
70% Diesel and 30% biodiesel
- B65:
-
65% Diesel and 35% biodiesel
- B70:
-
30% Diesel and 70% biodiesel
- B100:
-
100% Biodiesel
- BSFC:
-
Brake specific fuel consumption
- B90P10:
-
90% Biodiesel and10% n-pentanol
- B80P20:
-
80% Biodiesel and 20% n-pentanol
- B70P30:
-
70% Biodiesel and 30% n-pentanol
- B90O10:
-
90% Biodiesel and 10% n-octanol
- B80O20:
-
80% Biodiesel and 20% n-octanol
- B70O30:
-
70% Biodiesel and 30% n-octanol
- BBU10:
-
90% Biodiesel and 10% n-butanol
- BBU20:
-
80% Biodiesel and 20% n-butanol
- BBU40:
-
60% Biodiesel and 40% n-butanol
- C15H25 :
-
Diesel
- C21H28O2 :
-
Biodiesel
- C8H18O:
-
N-octanol
- CI:
-
Compression-ignition
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- B20OCT5:
-
75% Diesel, 20% biodiesel and 5% n-octanol
- B20OCT10:
-
70% Diesel, 20% biodiesel and 10% n-octanol
- B20OCT15:
-
65% Diesel, 20% biodiesel and 15% n-octanol
- B20OCT20:
-
60% Diesel, 20% biodiesel and 20% n-octanol
- E5B50:
-
45% Diesel, 5% ethanol and 50% biodiesel
- E10B50:
-
40% Diesel, 10% ethanol and 50% biodiesel
- E15B50:
-
35% Diesel, 15% ethanol and 50% biodiesel
- E20B50:
-
30% Diesel, 20% ethanol and 50% biodiesel
- DI:
-
Direct-injection
- EGR:
-
Exhaust gas recirculation
- EXEN:
-
Exergoenvironment
- EXENEC:
-
Exergoenviroeconomic
- HC:
-
Unburned hydrocarbon
- HCCI:
-
Homogeneous charge compression-ignition
- H2O:
-
Water
- KME5:
-
95% Diesel and 5% karanja biodiesel
- KME10:
-
90% Diesel and 10% karanja biodiesel
- KME20:
-
80% Diesel and 20% karanja biodiesel
- KME30:
-
70% Diesel and 30% karanja biodiesel
- KME100:
-
100% Karanja biodiesel
- N:
-
Nitrogen
- N2 :
-
Nitrogen
- NOX :
-
Nitrogen oxides
- OCT30:
-
70% Diesel and 30% n-octanol
- O2 :
-
Oxygen
- PM:
-
Particulate matter
- SAE:
-
Society of automotive engineers
- SI:
-
Sustainability index
- SO2 :
-
Sulfur dioxide
- WPO:
-
Waste plastic oil
References
Said Z, Ghodbane M, Tiwari AK, Ali HM, Boumeddane B, Ali ZM. 4E (Energy, Exergy, Economic, and Environment) examination of a small LFR solar water heater: an experimental and numerical study. Case Stud Therm Eng. 2017;27:101277.
Hassan F, Jamil F, Hussain A, Ali HM, Janjua MM, Khushnood S, Farhan M, Altaf K, Said Z, Li C. Recent advancements in latent heat phase change materials and their applications for thermal energy storage and buildings: a state of the art review. Sustain Energy Technol Assess. 2022;49:101646.
Abedin MJ, Masjuki HH, Kalam MA, Sanjid A, Rahman SA, Masum BM. Energy balance of internal combustion engines using alternative fuels. Renew Sustain Energy Rev. 2013;26:20–33.
Ejaz A, Jamil F, Ali HM. A novel thermal regulation of photovoltaic panels through phase change materials with metallic foam-based system and a concise comparison: an experimental study. Sustain Energy Technol Assess. 2022;49:101726.
Othman MF, Adam A, Najafi G, Mamat R. Green fuel as alternative fuel for diesel engine: a review. Renew Sustain Energy Rev. 2017;80:694–709.
Sultan H, Muhammad HA, Bhatti UH, Min GH, Baek IH, Baik YJ, Nam SC. Reducing the efficiency penalty of carbon dioxide capture and compression process in a natural gas combined cycle power plant by process modification and liquefied natural gas cold energy integration. Energy Convers Manag. 2021;244:114495.
Janaun J, Ellis N. Perspectives on biodiesel as a sustainable fuel. Renew Sustain Energy Rev. 2010;14(4):1312–20.
Khan MM, Sharma RP, Kadian AK, Hasnain SM. An assessment of alcohol inclusion in various combinations of biodiesel-diesel on the performance and exhaust emission of modern-day compression ignition engines-a review. Mater Sci Energy Technol. 2022;5:81–98.
Verma P, Sharma MP. Review of process parameters for biodiesel production from different feedstocks. Renew Sustain Energy Rev. 2016;62:1063–71.
Huang D, Zhou H, Lin L. Biodiesel: an alternative to conventional fuel. Energy Procedia. 2012;16:1874–85.
Yildiz I, Caliskan H, Mori K. Assessment of biofuels from waste cooking oils for diesel engines in terms of waste-to-energy perspectives. Sustain Energy Technol Assess. 2022;50:101839.
Agarwal AK. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog Energy Combust Sci. 2007;33(3):233–71.
Razak NH, Hashim H, Yunus NA, Klemeš JJ. Reducing diesel exhaust emissions by optimisation of alcohol oxygenates blend with diesel/biodiesel. J Clean Prod. 2021;316:128090.
Rahiman MK, Santhoshkumar S, Subramaniam D, Avinash A, Pugazhendhi A. Effects of oxygenated fuel pertaining to fuel analysis on diesel engine combustion and emission characteristics. Energy. 2022;239:122373.
Zakaria Z, Kamarudin SK, Abd Wahid KA, Hassan SHA. The progress of fuel cell for Malaysian residential consumption: Energy status and prospects to introduction as a renewable power generation system. Renew Sustain Energy Rev. 2021;144:110984.
Kumar S, Cho JH, Park J, Moon I. Advances in diesel–alcohol blends and their effects on the performance and emissions of diesel engines. Renew Sustain Energy Rev. 2013;22:46–72.
Zhang K, Pereira AS, Martin JW. Estimates of octanol–water partitioning for thousands of dissolved organic species in oil sands process-affected water. Environ Sci Technol. 2015;49(14):8907–13.
Ashok B, Nanthagopal K, Anand V, Aravind KM, Jeevanantham AK, Balusamy S. Effects of n-octanol as a fuel blend with biodiesel on diesel engine characteristics. Fuel. 2019;235:363–73.
Nour M, Attia AM, Nada SA. Combustion, performance and emission analysis of diesel engine fuelled by higher alcohols (butanol, octanol and heptanol)/diesel blends. Energy Convers Manag. 2019;185:313–29.
Górski K, Przedlacki M. Evaluation of the influence of diethyl ether (DEE) addition on selected physicochemical properties of diesel oil and ignition delay period. Energy Fuels. 2014;28(4):2608–16.
Li Y, Jia M, Chang Y, Kokjohn SL, Reitz RD. Thermodynamic energy and exergy analysis of three different engine combustion regimes. Appl Energy. 2016;180:849–58.
Chintala V, Subramanian KA. Assessment of maximum available work of a hydrogen fueled compression ignition engine using exergy analysis. Energy. 2014;67:162–75.
Caliskan H, Tat ME, Hepbasli A, Van Gerpen JH. Exergy analysis of engines fuelled with biodiesel from high oleic soybeans based on experimental values. Int J Exergy. 2010;7(1):20–36.
Arshad A, Ali HM, Habib A, Bashir MA, Jabbal M, Yan Y. Energy and exergy analysis of fuel cells: a review. Therm Sci Eng Prog. 2019;9:308–21.
Jena J, Misra RD. Effect of fuel oxygen on the energetic and exergetic efficiency of a compression ignition engine fuelled separately with palm and karanja biodiesels. Energy. 2014;68:411–9.
Zaharin MSM, Abdullah NR, Najafi G, Sharudin H, Yusaf T. Effects of physicochemical properties of biodiesel fuel blends with alcohol on diesel engine performance and exhaust emissions: a review. Renew Sustain Energy Rev. 2017;79:475–93.
Panithasan MS, Gopalakichenin D, Venkadesan G, Veeraraagavan S. Impact of rice husk nanoparticle on the performance and emission aspects of a diesel engine running on blends of pine oil-diesel. Environ Sci Pollut Res. 2019;26(1):282–91.
Hasan MM, Rahman MM. Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: a review. Renew Sustain Energy Rev. 2017;74:938–48.
Rajak U, Chaurasiya PK, Nashine P, Verma M, Kota TR, Verma TN. Financial assessment, performance and emission analysis of Moringa oleifera and Jatropha curcas methyl ester fuel blends in a single-cylinder diesel engine. Energy Convers Manag. 2020;224:113362.
Nanthagopal K, Ashok B, Saravanan B, Korah SM, Chandra S. Effect of next generation higher alcohols and Calophyllum inophyllum methyl ester blends in diesel engine. J Clean Prod. 2018;180:50–63.
Chandra Sekar MS, Ananthan VR, Baskaran N, Suresh Kumar HK, Arumugam R. Combustion, performance, and emission study on the octanol-neem biodiesel blends fueled diesel engine. Energy Sources Part A 2020;1–13 (in print).
Gopal K, Sathiyagnanam AP, Kumar BR, Saravanan S, Rana D, Sethuramasamyraja B. Prediction of emissions and performance of a diesel engine fueled with n-octanol/diesel blends using response surface methodology. J Clean Prod. 2018;184:423–39.
Pan M, Zheng Z, Huang R, Zhou X, Huang H, Pan J, Chen Z. Reduction in PM and NOX of a diesel engine integrated with n-octanol fuel addition and exhaust gas recirculation. Energy. 2019;187:115946.
De Poures MV, Siva AP, Rana D, Babu RK, Subramani S, Sethuramasamyraja B. Using renewable n-octanol in a non-road diesel engine with some modifications. Energy Sources Part A. 2019;41(10):1194–208.
Damodharan D, Gopal K, Sathiyagnanam AP, Rajesh Kumar B, Depoures MV, Mukilarasan N. Performance and emission study of a single cylinder diesel engine fuelled with n-octanol/WPO with some modifications. Int J Ambient Energy. 2021;42(7):779–88.
Zhou Q, Wang Y, Wang X, Bai Y. Experimental investigation into the oxidation reactivity, morphology and graphitization of soot particles from diesel/n-octanol mixtures. J Environ Sci. 2022;112:218–30.
Wang Q, Ni J, Huang R. The potential of oxygenated fuels (n-octanol, methylal, and dimethyl carbonate) as an alternative fuel for compression ignition engines with different load conditions. Fuel. 2022;6(309):122129.
Tian J, Liu Y, Bi H, Li F, Bao L, Han K, Zhou W, Ni Z, Lin Q. Experimental study on the spray characteristics of octanol diesel and prediction of spray tip penetration by ANN model. Energy. 2022;239:121920.
López I, Quintana CE, Ruiz JJ, Cruz-Peragón F, Dorado MP. Effect of the use of olive–pomace oil biodiesel/diesel fuel blends in a compression ignition engine: preliminary exergy analysis. Energy Convers Manag. 2014;85:227–33.
Magno A, Mancaruso E, Vaglieco BM. Effects of both blended and pure biodiesel on waste heat recovery potentiality and exhaust emissions of a small CI (compression ignition) engine. Energy. 2015;86:661–71.
Nemati P, Jafarmadar S, Taghavifar H. Exergy analysis of biodiesel combustion in a direct injection compression ignition (CI) engine using quasi-dimensional multi-zone model. Energy. 2016;115:528–38.
Khoobbakht G, Akram A, Karimi M, Najafi G. Exergy and energy analysis of combustion of blended levels of biodiesel, ethanol and diesel fuel in a DI diesel engine. Appl Therm Eng. 2016;99:720–9.
Nabi MN, Rasul MG, Anwar M, Mullins BJ. Energy, exergy, performance, emission and combustion characteristics of diesel engine using new series of non-edible biodiesels. Renew Energy. 2019;140:647–57.
Sarıkoç S, Örs İ, Ünalan S. An experimental study on energy-exergy analysis and sustainability index in a diesel engine with direct injection diesel-biodiesel-butanol fuel blends. Fuel. 2020;268:117321.
Hosseini SE, Barzegaravval H, Ganjehkaviri A, Wahid MA, Jaafar MM. Modelling and exergoeconomic-environmental analysis of combined cycle power generation system using flameless burner for steam generation. Energy Convers Manag. 2017;135:362–72.
Cavalcanti EJC. Exergy, exergoeconomic and environmental analysis of diesel engine operating with EGR rate. Int J Exergy. 2019;29(1):22–42.
Ehyaei MA, Mozafari A. Energy, economic and environmental (3E) analysis of a micro gas turbine employed for on-site combined heat and power production. Energy Build. 2010;42(2):259–64.
Krishna SM, Salam PA, Tongroon M, Chollacoop N. Performance and emission assessment of optimally blended biodiesel-diesel-ethanol in diesel engine generator. Appl Therm Eng. 2019;155:525–33.
Gülüm M, Bilgin A. A comprehensive study on measurement and prediction of viscosity of biodiesel-diesel-alcohol ternary blends. Energy. 2018;148:341–61.
Kumar BR, Saravanan S. Use of higher alcohol biofuels in diesel engines: a review. Renew Sustain Energy Rev. 2016;60:84–115.
Kumar BR, Saravanan S, Rana D, Anish V, Nagendran A. Effect of a sustainable biofuel–n-octanol–on the combustion, performance and emissions of a DI diesel engine under naturally aspirated and exhaust gas recirculation (EGR) modes. Energy Convers Manag. 2016;118:275–86.
Nour M, Attia AM, Nada SA. Improvement of CI engine combustion and performance running on ternary blends of higher alcohol (Pentanol and Octanol)/hydrous ethanol/diesel. Fuel. 2019;251:10–22.
Atabani AE, Badruddin IA, Masjuki HH, Chong WT, Lee KT. Pangium edule Reinw: a promising non-edible oil feedstock for biodiesel production. Arabian J Sci Eng. 2015;40(2):583–94.
Yeşilyurt MK, Cesur C. Biodiesel synthesis from Styrax officinalis L. seed oil as a novel and potential non-edible feedstock: a parametric optimization study through the Taguchi technique. Fuel. 2020;265:117025.
Holman P. Experimental methods for engineers. 8th ed. New York, USA: McGraw-Hill; 2012.
Tian Z, Zeng W, Gu B, Zhang Y, Yuan X. Energy, exergy, and economic (3E) analysis of an organic Rankine cycle using zeotropic mixtures based on marine engine waste heat and LNG cold energy. Energy Convers Manag. 2021;228:113657.
Yu X, Li D, Sun P, Li G, Yang S, Yao C. Energy and exergy analysis of a combined injection engine using gasoline port injection coupled with gasoline or hydrogen direct injection under lean-burn conditions. Int J Hydrog Energy. 2021;46(11):8253–68.
Cavalcanti EJ. Energy, exergy and exergoenvironmental analyses on gas-diesel fuel marine engine used for trigeneration system. Appl Therm Eng. 2021;184:116211.
Nabi MN, Rasul MG, Arefin MA, Akram MW, Islam MT, Chowdhury MW. Investigation of major factors that cause diesel NOx formation and assessment of energy and exergy parameters using e-diesel blends. Fuel. 2021;292:120298.
Rai RK, Sahoo RR. Impact of different shape based hybrid nano additives in emulsion fuel for exergetic, energetic, and sustainability analysis of diesel engine. Energy. 2021;214:119086.
Karthickeyan V, Thiyagarajan S, Ashok B, Geo VE, Azad AK. Experimental investigation of pomegranate oil methyl ester in ceramic coated engine at different operating condition in direct injection diesel engine with energy and exergy analysis. Energy Convers Manag. 2020;205:112334.
Yildiz I, Caliskan H, Mori K. Energy, exergy and environmental assessments of biodiesel and diesel fuels for an internal combustion engine using silicon carbide particulate filter. J Therm Anal Calorim. 2020;145:739–750.
Caliskan H. Energy, exergy, environmental, enviroeconomic, exergoenvironmental (EXEN) and exergoenviroeconomic (EXENEC) analyses of solar collectors. Renew Sustain Energy Rev. 2017;69:488–92.
Özcan H, Çakmak A. Comparative exergy analysis of fuel additives containing oxygen and HC based in a Spark-Ignition (SI) engine. Int J Automot Eng Technol. 2018;7(3):124–33.
Moran MJ, Shapiro HN, Boettner DD, Bailey MB. Fundamentals of engineering thermodynamics. Hoboken: John Wiley & Sons; 2010.
Yaman H, Doğan B, Yeşilyurt MK, Erol D. Application of higher-order alcohols (1-Hexanol-C6 and 1-Heptanol-C7) in a spark-ignition engine: analysis and assessment. Arabian J Sci Eng. 2021;46(12):11937–61.
Doğan B, Yesilyurt MK, Erol D. Çakmak AA study toward analyzing the energy, exergy and sustainability index based on performance and exhaust emission characteristics of a spark-ignition engine fuelled with the binary blends of gasoline and methanol or ethanol. Int J Eng Res Dev. 2020;12(2):529–48.
Yesilyurt MK, Arslan M. Analysis of the fuel injection pressure effects on energy and exergy efficiencies of a diesel engine operating with biodiesel. Biofuels. 2019;10(5):643–55.
Yesilyurt MK. The examination of a compression-ignition engine powered by peanut oil biodiesel and diesel fuel in terms of energetic and exergetic performance parameters. Fuel. 2020;278:118319.
Doğan B, Erol D, Yaman H, Kodanli E. The effect of ethanol-gasoline blends on performance and exhaust emissions of a spark ignition engine through exergy analysis. Appl Therm Eng. 2017;120:433–43.
Aghbashlo M, Tabatabaei M, Soltanian S, Ghanavati H, Dadak A. Comprehensive exergoeconomic analysis of a municipal solid waste digestion plant equipped with a biogas genset. Waste Manag. 2019;87:485–98.
Lazzaretto A, Tsatsaronis G. SPECO: a systematic and general methodology for calculating efficiencies and costs in thermal systems. Energy. 2006;31(8–9):1257–89.
Khounani Z, Hosseinzadeh-Bandbafha H, Nazemi F, Shaeifi M, Karimi K, Tabatabaei M, Aghbashlo M, Lam SS. Exergy analysis of a whole-crop safflower biorefinery: a step towards reducing agricultural wastes in a sustainable manner. J Environ Manag. 2021;279:111822.
Amid S, Aghbashlo M, Tabatabaei M, Karimi K, Nizami AS, Rehan M, Hosseinzadeh-Bandbafha H, Soufiyan MM, Peng W, Lam SS. Exergetic, exergoeconomic, and exergoenvironmental aspects of an industrial-scale molasses-based ethanol production plant. Energy Convers Manag. 2021;227:113637.
Caliskan H. Environmental and enviroeconomic researches on diesel engines with diesel and biodiesel fuels. J Clean Prod. 2017;154:125–9.
Meyer L, Tsatsaronis G, Buchgeister J, Schebek L. Exergoenvironmental analysis for evaluation of the environmental impact of energy conversion systems. Energy. 2009;34(1):75–89.
Caliskan H, Mori K. Environmental, enviroeconomic and enhanced thermodynamic analyses of a diesel engine with diesel oxidation catalyst (DOC) and diesel particulate filter (DPF) after treatment systems. Energy. 2017;128:128–44.
Yesilyurt MK. The evaluation of a direct injection diesel engine operating with waste cooking oil biodiesel in point of the environmental and enviroeconomic aspects. Energy Sources Part A. 2018;40(6):654–61.
Atabani AE, Kulthoom SA. Spectral, thermoanalytical characterizations, properties, engine and emission performance of complementary biodiesel-diesel-pentanol/octanol blends. Fuel. 2020;282:118849.
Atabani AE, Mekaoussi M, Uguz G, Arpa O, Ayanoglu A, Shobana S. Evaluation, characterization, and engine performance of complementary fuel blends of butanol-biodiesel-diesel from Aleurites moluccanus as potential alternative fuels for CI engines. Energy Environ. 2020;31(5):755–84.
Azad AK, Adhikari J, Halder P, Rasul MG, Hassan N, Khan MM, Naqvi SR, Viswanathan K. Performance, emission and combustion characteristics of a diesel engine powered by macadamia and grapeseed biodiesels. Energies. 2020;13(11):2748.
Nanthagopal K, Kishna RS, Atabani AE, Ala’a H, Kumar G, Ashok B. A compressive review on the effects of alcohols and nanoparticles as an oxygenated enhancer in compression ignition engine. Energy Convers Manag. 2020;203:112244.
Mathimani T, Kumar TS, Chandrasekar M, Uma L, Prabaharan D. Assessment of fuel properties, engine performance and emission characteristics of outdoor grown marine Chlorella vulgaris BDUG 91771 biodiesel. Renew Energy. 2017;105:637–46.
Devarajan Y, Babu Munuswamy D, Nagappan B. Emissions analysis on diesel engine fuelled with cashew nut shell biodiesel and pentanol blends. Environ Sci Pollut Res. 2017;24(14):13136–41.
Çakmak A, Kapusuz M, Özcan H. Experimental research on ethyl acetate as novel oxygenated fuel in the spark-ignition (SI) engine. Energy Sources Part A 2020;1–16 (in print).
Devarajan Y, Munuswamy DB, Mahalingam A, Nagappan B. Performance, combustion, and emission analysis of neat palm oil biodiesel and higher alcohol blends in a diesel engine. Energy Fuels. 2017;31(12):13796–801.
Devarajan Y, Munuswamy D, Nagappan B, Subbiah G. Experimental assessment of performance and exhaust emission characteristics of a diesel engine fuelled with Punnai biodiesel/butanol fuel blends. Pet Sci. 2019;16(6):1471–8.
Bermúdez V, Lujan JM, Pla B, Linares WG. Effects of low pressure exhaust gas recirculation on regulated and unregulated gaseous emissions during NEDC in a light-duty diesel engine. Energy. 2011;36(9):5655–65.
De Serio D, de Oliveira A, Sodré JR. Effects of EGR rate on performance and emissions of a diesel power generator fueled by B7. J Braz Soc Mech Sci Eng. 2017;39(6):1919–27.
Mahalingam A, Devarajan Y, Radhakrishnan S, Vellaiyan S, Nagappan B. Emissions analysis on mahua oil biodiesel and higher alcohol blends in diesel engine. Alex Eng J. 2018;57(4):2627–31.
Yuvarajan D. Investigation on effect of magnetite nanofluid on performance and emission patterns of methyl esters of bio diesel. J Environ Eng Landsc Manag. 2016;24(2):90–6.
Elbayoumi M, Ramli NA, Yusof NFFM. Development and comparison of regression models and feedforward backpropagation neural network models to predict seasonal indoor PM2. 5–10 and PM2. 5 concentrations in naturally ventilated schools. Atmos Pollut Res. 2015;6(6):1013–23.
Deep A, Kumar N, Karnwal A, Gupta D, Vibhanshu V, Sharma A, Patel JS. Assessment of the performance and emission characteristics of 1-octanol/diesel fuel blends in a water cooled compression ignition engine. SAE Tech Paper. No. 2014-01-2830, 2014.
Sayin Kul B, Kahraman A. Energy and exergy analyses of a diesel engine fuelled with biodiesel-diesel blends containing 5% bioethanol. Entropy. 2016;18(11):387.
Hoseinpour M, Sadrnia H, Tabasizadeh M, Ghobadian B. Energy and exergy analyses of a diesel engine fueled with diesel, biodiesel-diesel blend and gasoline fumigation. Energy. 2017;141:2408–20.
Babu V, Murthy M. Butanol and pentanol: the promising biofuels for CI engines-a review. Renew Sustain Energy Rev. 2017;78:1068–88.
Doğan B, Cakmak A, Yesilyurt MK, Erol D. Investigation on 1-heptanol as an oxygenated additive with diesel fuel for compression-ignition engine applications: an approach in terms of energy, exergy, exergoeconomic, enviroeconomic, and sustainability analyses. Fuel. 2020;275:117973.
Aghbashlo M, Tabatabaei M, Mohammadi P, Pourvosoughi N, Nikbakht AM, Goli SAH. Improving exergetic and sustainability parameters of a DI diesel engine using polymer waste dissolved in biodiesel as a novel diesel additive. Energy Convers Manag. 2015;105:328–37.
Aghbashlo M, Tabatabaei M, Mohammadi P, Mirzajanzadeh M, Ardjmand M, Rashidi A. Effect of an emission-reducing soluble hybrid nanocatalyst in diesel/biodiesel blends on exergetic performance of a DI diesel engine. Renew Energy. 2016;93:353–68.
Şanli BG. Energetic and exergetic performance of a diesel engine fueled with diesel and microalgae biodiesel. Energy Sources Part A. 2019;41(20):2519–33.
Aghbashlo M, Tabatabaei M, Khalife E, Najafi B, Mirsalim SM, Gharehghani A, Mohammadi P, Shojaei TR, Khounani Z. A novel emulsion fuel containing aqueous nano cerium oxide additive in diesel–biodiesel blends to improve diesel engines performance and reduce exhaust emissions: Part II–Exergetic analysis. Fuel. 2017;205:262–71.
Şanli BG, Uludamar E, Özcanli M. Evaluation of energetic-exergetic and sustainability parameters of biodiesel fuels produced from palm oil and opium poppy oil as alternative fuels in diesel engines. Fuel. 2019;258:116116.
Aghbashlo M, Tabatabaei M, Mohammadi P, Khoshnevisan B, Rajaeifar MA, Pakzad M. Neat diesel beats waste-oriented biodiesel from the exergoeconomic and exergoenvironmental point of views. Energy Convers Manag. 2017;148:1–15.
Çakmak A, Bilgin A. Exergy and energy analysis with economic aspects of a diesel engine running on biodiesel-diesel fuel blends. Int J Exergy. 2017;24(2–4):151–72.
Şanli BG, Uludamar E. Energy and exergy analysis of a diesel engine fuelled with diesel and biodiesel fuels at various engine speeds. Energy Sources Part A. 2020;42(11):1299–313.
Aghbashlo M, Tabatabaei M, Khalife E, Shojaei TR, Dadak A. Exergoeconomic analysis of a DI diesel engine fueled with diesel/biodiesel (B5) emulsions containing aqueous nano cerium oxide. Energy. 2018;149:967–78.
Karagoz M, Uysal C, Agbulut U, Saridemir S. Exergetic and exergoeconomic analyses of a CI engine fueled with diesel-biodiesel blends containing various metal-oxide nanoparticles. Energy. 2021;214:118830.
Cavalcanti EJ, Carvalho M, Ochoa AA. Exergoeconomic and exergoenvironmental comparison of diesel-biodiesel blends in a direct injection engine at variable loads. Energy Convers Manag. 2019;183:450–61.
Yildiz I, Açıkkalp E, Caliskan H, Mori K. Environmental pollution cost analyses of biodiesel and diesel fuels for a diesel engine. J Environ Manag. 2019;243:218–26.
Devarajan Y, Munuswamy DB, Nagappan B, Pandian AK. Performance, combustion and emission analysis of mustard oil biodiesel and octanol blends in diesel engine. Heat Mass Trans. 2018;54(6):1803–11.
E-Seesy AI, Waly MS, He Z, El-Batsh HM, Nasser A, El-Zoheiry RM. Influence of quaternary combinations of biodiesel/methanol/n-octanol/diethyl ether from waste cooking oil on combustion, emission, and stability aspects of a diesel engine. Energy Convers Manag. 2021;240:114268.
Çalam A. Effects of the fusel oil usage in HCCI engine on combustion, performance and emission. Fuel. 2020;262:116503.
Li B, Li Y, Liu H, Liu F, Wang Z, Wang J. Combustion and emission characteristics of diesel engine fueled with biodiesel/PODE blends. Appl Energy. 2017;206:425–31.
Chen Z, He J, Chen H, Geng L, Zhang P. Comparative study on the combustion and emissions of dual-fuel common rail engines fueled with diesel/methanol, diesel/ethanol, and diesel/n-butanol. Fuel. 2021;304:121360.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors acknowledge that no financial interest or benefit has been raised from the direct applications of their research.
Ethical approval
Ethical approval for this study was not sought.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Rights and permissions
About this article
Cite this article
Çakmak, A., Yeşilyurt, M.K., Erol, D. et al. The experimental investigation on the impact of n-octanol in the compression-ignition engine operating with biodiesel/diesel fuel blends: exergy, exergoeconomic, environmental analyses. J Therm Anal Calorim 147, 11231–11259 (2022). https://doi.org/10.1007/s10973-022-11357-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11357-w