Abstract
In the last decades, one of the most ordinary challenges that countries of Southern Europe face is the management of wastes and by-products of olive oil production activities which are disposed into the environment and contribute to the enhancement of major ecological issues due to their organic loads. Exploitation of these olive residues wastes by thermochemical treatment has been proved a very popular way to generate a wide range of valuable products. In this concept, this work studies the thermal decomposition process of olive stone samples using thermogravimetric analysis (TGA) data obtained under inert atmosphere at various heating rates (5–20 °C) and their kinetic analysis conducted via model-free and model fitting methods. The changes in the crystal structure for various pyrolysis temperatures of the sample were examined by X-ray diffraction analysis (XRD), while the morphological characteristics were examined by scanning electron microscopy (SEM). Results indicated that the thermal behavior of olive stone sample was a typical one for a lignocellulosic material with the first mass loss being attributed to moisture removal while the following stages were assigned to lignocellulosic degradation substances. Vyazovkin (VYA) isoconversional method was performed to estimate effective activation energy (Ea) and the pre-exponential factor, while using a model fitting method the best fitting results were obtained by a three independent parallel reactions model, obeying the nth order with Fn code and described by the f(α) = (1 − α)n equation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide 900 million olive trees are cultivated over 10 million hectares with the 98% of them are estimated to be located in the Mediterranean region. Spain is the biggest olive-oil-producing country with an average annual output of 9.8 million tons of olive while Greece is the third country in the world in the production of olive oil after Italy. Olive oil production is an activity particularly developed in Greece for many centuries, but even today it maintains a leading role in the economy of the whole country [1, 2]. Greek production originates by the approximately 130 million cultivated trees and accounts about 15% of the world production. Each olive tree produces 15 to 40 kg of olives/year depending on the climate conditions [3]. It is therefore understood that large quantities of wastes generated after the procedure of pruning trees and harvesting fruit remain practically unused, and this is unsolved issue with significant environmental impact [4, 5]. In particular, the by-products created by this activity can be classified into solid wastes (untreated olive stone, olive flesh), known as “olive cake,” obtained after extracting the oil from the fruit and liquid wastes (mill effluent). The degradation of these by-products is a difficult procedure due to their contents which is abundance in phenolic components [6].
In the last decades, biomass materials have attracted research interest due to their composition and physicochemical characteristics, and for this reason, they have being thoroughly studied. In general, biomass can be classified into three main categories, namely energy crops, agricultural wastes and forestry residues which consist an alternative source of heat and power production through many conversion processes [7]. This energy source “provide” a potential to minimize fossil fuel dependence becoming a promising solution in sustainability issues worldwide [8]. In addition, considering the advantages of biomass such as being renewable, cheap, not adding new CO2 in the atmosphere, feasibility and high availability around the word set its use as it highly competitive to fossil fuels [9,10,11]
Combustion, gasification and pyrolysis are some biomass thermochemical conversion processes that suggest a viable option in the reduction of pollutants by wastes disposal and for energy production [12]. Among these processes, pyrolysis has attracted much interest since it succeeds to convert biomass into bio-oil, biogas and carbonaceous char products oriented for several applications. Pyrolysis occurs in the absence of oxygen and includes complex reactions defined by several parameters such as heating rate, particle size composition of biomass and temperature [8, 13]. Pyrolysis kinetics can be studied by several methods. One of the most common techniques used to study the degradation behavior of biomass during pyrolysis process is thermogravimetric analysis (TGA) which monitors mass loss data by either isothermal or non-isothermal pyrolysis processes [14]. Dynamic solid state kinetic analysis can be obtained either by model-free or by model fitting methods. The model fitting methods are considered to be approximate, while they are based on the assumption that all kinetic parameters are constant during the reaction progress for every individual step and the thermoanalytical signal is the sum of the signals of the single reaction step [15]. In the model-free methods, multiple TGA experiments at different heating rates are performed and the kinetic parameters are calculated in progressive degree of conversion (α) without evaluating the reaction model f(α).
Determination of the pyrolysis kinetic mechanism is a critical task to fulfill in the effort to guide the process toward favorable products, increase reaction efficiency, promote feasibility and, thus, assist the design and optimization of large-scale reactors. Regarding available literature works, the majority of olive stone pyrolysis studies, approach the process (mainly) by model-free methods. Aboulkas et al. studied olive residue decomposition via Ozawa–Flynn–Wall (OFW) and Vyazovkin (VYA) model-free methods determining the Ea at 148–154 kJ mol−1 and 160–166 kJ mol−1 for hemicellulose and at 200–211 kJ mol−1 and at 210-219 kJ mol−1 for cellulose, respectively [16]. Alrawashdeh et al. determined the Ea with the Kissinger method at 130 kJ mol−1 and compared the results with the isoconversional OFW model-free and the Freeman–Carol model fitting methods, estimating their Ea at 26–214 kJ mol−1 and at 123 kJ mol−1, respectively, for the whole process. Moreover, they reported that the OFW isoconversional model-free method revealed the “not one-step” mechanism of reaction that occurs during the slow pyrolysis process compared to the Kissinger model-free and model fitting methods [17]. Guida et al. determined the Ea,Mean at 176 kJ mol−1, 167 kJ mol−1, 150 kJ mol−1 for hemicellulose component and at 235 kJ mol−1, 210 kJ mol−1, 212 kJ mol−1 for cellulose component by Friedman (FR), OFW and VYA isoconversional model-free methods for the entire conversion range [13]. It is therefore understood that since the study of the kinetics of the thermal degradation of olive residue has been mainly approached by model-free methods, the thermal decomposition mechanisms has not been thoroughly studied.
Another part be mentioned is dealing with the factors affecting the biomass pyrolysis process. Many studies have been carried out to investigate the effect of these processing parameters aiming to succeed optimum product yield. Biomass composition is a major factor with a remarkable effect in pyrolysis performance since it has been reported that for high bio-oil yield it is preferable to use biomass containing cellulose and hemicellulose while to achieve a high biochar yield recommended with higher lignin content is recommended. On the contrary, moisture content negatively affects the stability, viscosity and corrosiveness and is preferable to be removed. Particle size of biomass is a parameter of fundamental importance. For larger particle size, char formation is favored as a result of the large temperature gradient inside particle transmitting poor heat transfer while smaller particle size is suitable for a high bio-oil yield. Pyrolysis of biomass can be classified in three main categories, with respect to the heating rate along with the reaction temperature, namely slow pyrolysis, fast pyrolysis and ultra-fast or flash pyrolysis. Slow pyrolysis is typically used to modify the solid material, minimizing the oil produced while fast pyrolysis and ultra-fast pyrolysis maximize the gases and oil produced. Finally, it should be considered the residence time and the reaction atmosphere parameters which also affect the performance of the process and the product yield [18,19,20,21].
This work aims to study the pyrolysis process of olive stone with TGA, determine the effective activation Ea by an isoconversional model-free method (VYA) and suggest the degradation mechanisms of olive stone decomposition by a model fitting method. Additionally, to highlight the structural modifications, several pyrolysis temperatures were chosen to spot the final temperature of degradation process, which were employed by XRD analysis. The morphological characteristics of the pyrolyzed samples were examined by (SEM) analysis.
Materials and methods
Olive stones samples were supplied by Pharmagnose S.A. which is a company based in Athens specialized in natural ingredients research. The olive stones were selected from crops in the Central Greece region after the oil extraction, and they were milled and sieved to succeed a particle size ≤ 200 μm. In addition, the olive stones were air dried at 100 °C for 3 h.
Thermogravimetric analysis (TGA)
Thermogravimetric measurements were taken using a TG–DTA Setaram Setsys 16/18 instrument. Approximately 3–4 mg of each sample was placed in alumina crucibles while an empty alumina crucible was used as reference. Then the samples were heated from 25 to 800 °C in N2 atmosphere at heating rates of 5, 10, 15, 20 °C min-1. Τhermographs of mass loss versus temperature and derivative thermogravimetric curves were obtained indicating the decomposition steps and the maximum mass loss of each sample. The kinetic study was carried out via the NETZSCH Kinetics NEO software.
X-ray diffraction analysis (XRD)
Monitoring of structural phase modifications at specific temperature intervals in the region of mass loss occurrence due to pyrolysis was carried out by X-ray diffraction (XRD) measurements. Proper sampling was performed by collecting the residual of TG experiment at the corresponding temperature 260–420 °C. The X-ray diffraction patterns were obtained using a water-cooled Rigaku Ultima + instrument operating with CuKa (λ = 1.5406 A) radiation a step size of 0.05° and a step time of 5 s, operating at 40 kV and 30 mA.
Scanning electron microscopy (SEM)
The morphological characteristics of the samples were studied with SEM analysis using a JEOL JSM-7610F Plus scanning microscope, equipped with an AZTEC ENERGY ADVANCED X-act EDS Oxford analytical system. The samples were used in powder form and they were placed on a double-sided adhesive tape, attached to the holder, whereas carbon coating the samples reassured an efficient conductivity of the electron beam. The operating conditions were accelerating voltage 20 kV, probe current 45 nA and counting time 60 s.
Model-free analysis
Theoretical background
The fundamental rate equation used in all kinetic studies is generally described as
where k is the rate constant and f(α) a function that describes the reaction model and depends on the actual reaction mechanism. Equation (1) expresses the rate of conversion, dα/dt, at a constant temperature as a function of the reactant mass loss and rate constant.
The conversion rate α is defined as:
where mt, mo and mf are the mass of the sample at the time t, the initial and the final condition, respectively. The rate constant k is generally given by the Arrhenius equation:
where E is the apparent activation energy (kJ mol−1), R is the gas constant (8.314 J K−1 mol−1), A is the pre-exponential factor (min) and T is the absolute temperature (K). The combination of Eq. (1) and (3) gives the following relationship:
For a dynamic TGA process, introducing the heating rate, β = dT/dt, into Eq. (4), Eq. (5) is obtained as:
Equations (4) and (5) are the fundamental expressions of analytical methods expressions of analytical methods to calculate kinetic parameters based on TGA data.
Vyazovkin (VYA) model
Vyazovkin has also developed an advanced integral isoconversional method using the integral form of Eq. 1 divided by the heating rate β, where k is given by Eq. (3). This method refers to the kinetics that occur under arbitrary variation in the temperature [22, 23]. For a series of n experiments executed under different temperature programs, Ti(t), the effective activation Ea is calculated at any specific value of α by finding the Ea, value which minimizes the function:
where
The subscript α indicates the values at a given degree of conversion; in Eq. (7), α varies from ∆α to 1–∆α with a step of ∆α = m–1, where m is the number of the chosen integrals. The minimization procedure is repeated for each value of α to obtain the effective’s Ea dependence on the degree of conversion.
Results and discussion
Structural modification
The XRD of olive stone samples at 260, 290, 320, 350, 390 and 420 °C pyrolysis temperatures is presented in Fig. 1. The selection of temperature range between 260 and 420 °C was based in the preliminary TG experiments which revealed that all thermal degradation effects and consequent structural modifications take place this temperature range. It is observed that that the samples pyrolyzed up to 360 °C revealed broaden diffraction peaks at 2θ = 16°, 22° and 34° which correspond to the diffraction of (110), (200) and (004) planes of the cellulose I structure since this is the only lignocellulosic component in comparison with hemicellulose and lignin that “provides” crystallinity [24]. So, it may be concluded that the pyrolysis of olive stone did occur below 320 °C. For pyrolysis temperatures higher than 350 °C, the broaden diffraction peaks almost seem to disappear, especially for the sample pyrolyzed at 420 °C, which can be characterized as completely amorphous. This structural conversion is attributed to the thermal decomposition of cellulose substance which takes place in the 320–400 °C temperature range.
Thermogravimetric analysis (TGA)
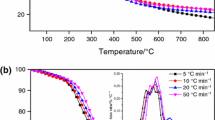
Figure 2 illustrates the thermogravimetric (TGA) and derivative thermogravimetric curves (DTG) of the residual biomass of olive stone sample. It was confirmed that the thermal decomposition of the olive stone achieved in three areas of mass loss: moisture evaporation, main devolatilization and slight devolatilization. The first occurs up to 107 °C corresponding to the elimination of the “remaining” moisture and is accompanied by a mass loss of 3.8 mass%. With further heating, follows a second mass loss in the temperature range from 200 °C to the end of the process in which take place the decomposition of the lignocellulosic materials: cellulose, hemicellulose and lignin takes place. Initially the hemicellulose component degradation is observed followed by that of cellulose with the decomposition of those components occurring partially simultaneously, contributing to the overlapping peaks at DTG curves (Fig. 2) [25]. The decomposition range of lignin is not as narrow as that of cellulose and hemicellulose estimated to initiate with hemicellulose’s decomposition and extending up to the end of the process [26, 27]. Indicatively for a rate of 10 °C min−1, which is normally used in the literature, the decomposition of the residual biomass of olive stone samples is represented by two peaks, with maximum mass loss rates at 362 °C as depicted by the DTG curve of Fig. 2. The formation of the peaks in the DTG curve is a result of the sum of the contributions by all three constituents assigned to the three lignocellulosic components. It can be assumed that the hemicellulose degradation takes place at 170–360 °C and that of cellulose at 240–380 °C while lignin decomposes over a broader temperature range almost simultaneously with hemicellulose expanding up to the end of the process. The referred temperature ranges for these effects are in a good agreement with the literature [28].
One of the most crucial factors that affect the process is the heating rate. It is obvious that higher heating rates cause a shift the "less pronounced peak" to higher maximum decomposition temperatures resulting in higher peak overlapping (Fig. 3). This change in the maximum pyrolysis rate can be assigned to the heterogeneous structure of biomass and to various substances of biomass which give decomposition peaks in specific temperature ranges. This phenomenon is dominant at higher heating rates where some of the components decomposed simultaneously, and several adjacent peaks were merged to form overlapped broader and higher peaks [29].
Kinetic analysis
The TGA profile for the rate 10 °C min−1 in Fig. 2 indicates that the pyrolysis process has not be completed up to 450 °C proceeding slowly up to 800 °C. It should be clarified that the first mass loss, region I, related to moisture removal was not taken into account and was not included in the data to be processed as this stage has no particular value in kinetic analysis. Nevertheless, for the kinetic study of olive stone degradation the applied model was restricted in region II, overlooking the almost linear part of the curve above 450 °C (region III) which interferes the accuracy of the fitting.
Model-free analysis
The kinetic parameters and the estimation of the reaction mechanism function for both model-free and model fitting methods were determined via NETZSCH Kinetics NEO software. The model-free methods were applied to experimental data aiming to determine Ea and log A for each degree of conversion, corresponding to the decomposition process of olive stone. It should be mentioned that the Ozawa–Flynn–Wall (OZW) approach was not applicable for the case of variable activation energy, and for this reason, it is not presented for comparison [30]. Figure 4 illustrates the Ea and log A as a function of the degree of conversion (α), using the VYA isoconversional method.
From the observation of Fig. 4, the presence of several regions in both kinetic parameters’ variation for the olive stone decomposition sample is clearly visible. The first region corresponds to the degradation of hemicellulose component, which is the least stable component and undergoing rapid thermal decomposition and is characterized by an increase in Ea values up to 139 kJ mol−1 while the log A value estimated up to 9.7 according to VYA method for conversion up to 0.5. In the next step, higher values of Ea are obtained, ranging between 140 and 168 kJ mol−1, for conversion levels extended from 0.5 to 0.85 which probably related to the degradation of cellulose component. The log A values in this conversion range calculated at 9.5–10.5. For conversion values above 0.85, a sudden increase in Ea and log A values is visible, as a result of the increasing aromatic character of the lignin-derived biochar when higher temperatures are reached [11]. In addition, Fig. 4 indicates a higher standard deviation (error bars) in both kinetic parameters which further increases at initial of the analysis for conversion beyond 0.85. According to the literature, these increased standard deviations in effective Ea and log A values may attribute to the methods themselves which are sensitive to baseline subtract. Here the variation in Ea at different conversion values reveals that pyrolysis of olive stone is a complex process, and probably could be described by more than one-step mechanism. Comparing the results of VYA method with those have mention in the introduction is obvious that the values which have been estimated by the former are lower. Particularly Aboulkas et al. estimated the Ea of olive residue decomposition via Vyazovkin (VYA) model-free methods Ea at 160–166 kJ mol−1 for hemicellulose and at 210-219 kJ mol−1 for cellulose.
Model fitting analysis
Model fitting methods involve fitting of conversion–temperature curves by different models and simultaneous determination of the effective Ea and the log A [31]. Model fitting methods assume that the reaction has several steps, each of which has its own kinetic equation while the kinetic parameters of each step are constant values. Determination of the kinetic triplet ((Ea, A, f(a)) in biomass pyrolysis using model fitting methods is in general a difficult “process” because the required information about the interaction of steps is not directly presented in the experimental data [15].
In our case, for the determination of the kinetic triplet ((Ea, A, f(a)) in olive stone pyrolysis process various reaction models were considered for the fitting to the experimental data at different heating rates. Taking into consideration that the biomass degradation is a complex process and results of the VYA method revealed that the pyrolysis should be described by more than one mechanism, two different models were tested for the mechanism determination. Figure 5 illustrates the mass loss–T data and the corresponding fitting curves of olive stone sample at four different heating rates for a two independent parallel reaction mechanism and a three independent parallel reaction one, both obeying nth order with Fn reaction models (Fn code). Although the quality of the two independent parallel reaction mechanisms is considered satisfactory (R2 = 0.99974), there are divergences, particularly obvious at the end of the process, and for rate 20 °C min−1 on the fitting model data, thus, the assumption of the two independent parallel reaction mechanism was not qualified appropriate for describing the process. The corresponding parameters obtained by the aforementioned model are summarized in Table 1.
The reaction model that describes better the experimental data of olive stone sample can be expressed by three independent parallel reactions, obeying the nth order with Fn code and described by the f(α) = (1 − α)n equation. In particular, the established three independent parallel reaction models analyze only the decomposition of the main components of olive stone, cellulose, hemicellulose and lignin. The results of model fitting analysis, which concludes the best selected model (Fn) and three independent reaction mechanisms, are presented in Table 2.
Hemicellulose is constituted by sugar monomers and short-chain heteropolysaccharides and presents an amorphous and branched structure, such as xylan while the shorter chain formation of this component results in lower thermal stability. Cellulose is characterized as polycrystal component with β 1,4-glycosidic bonds and with higher thermal stability [32]. On the other hand lignin is the most stable component due to the aromatic rings constituents and covers a range from 170 °C to the end of the process [27, 33]. In accordance with the aforementioned information, the first reaction (A→B) with effective Ea = 118.9 kJ mol−1, log A = 8.96 and contribution 0.32 is associated with the degradation of hemicellulose component, while the second reaction (C→D) with effective Ea = 143.7 kJ mol−1, log A = 9.94 and contribution 0.45 is attributed to the degradation of cellulose. The effective Ea and the contribution of the third reaction (Ε→F) correspond to Ea = 217.6 kJ mol−1, log A = 4.67 and 0.22, respectively, suggesting that this reaction is related to the lignin degradation. In this way, the Fn model simplified the complex nature of the olive stone degradation.
The rate equations with respect to each reaction, considering the Fn kinetic model for three reaction mechanisms, can be presented as:
Step: A → B
Step: C → D
Step: E → F
The a, c and e elements represent the concentration of reactants, a→concentration of A, c→concentration of C and e→concentration of E that they could simulate/match with the decomposition of the main components: cellulose, hemicellulose and lignin.
Scanning electron microscopy—morphological characterization
The morphological characteristics of pyrolyzed samples were examined by scanning electron microscopy (Fig. 6). Suggestively, observations involved four samples for which thermal degradation were stopped at different temperatures in the range 260–800 °C. Figure 6a depicts olive stone powder pyrolyzed up to 260 °C for which the surface appears to be inhomogeneous and rough with the presence of irregular shapes. SEM micrographs of the resulting char obtained by olive stones at 320 °C and 420 °C (Fig. 6b, c) reveals the presence of cavities and holes at various geometries implying to improved porosity. The appearance of such morphology is enhanced with the increase in the pyrolysis temperature, and for the sample pyrolyzed at 800 °C (Fig. 6d), a high density of pores is observed, suggesting the formation of a and extended porous structure.
Conclusions
Better understanding of the pyrolysis kinetics is essential for the thermochemical treatment of the olive biomass as useful information for the proper design pyrolysis system, and the operation conditions are conducted. Understanding in depth the process involved in biomass pyrolysis and analyzing all the degradation stages can help to optimize biomass pyrolysis systems resulting in increased efficiency. In this study, dynamic thermogravimetric analysis was performed under nitrogen atmosphere to examine the thermal degradation process of olive stone samples while the kinetic analysis was done by comparing model-free and model fitting methods through NETZSCH Kinetics NEO software. In this work, we attempted to propose the degradation mechanism of olive stone via simple TGA experiments followed by proper fitting using model-based methods. This is an advance to the existing literature which approached the same problem based exclusively on model-free methods. The thermal behavior of the studied biomass was typical of a lignocellulosic material with the first mass loss assigned to the moisture release, following the thermal degradation of cellulose, hemicellulose and lignin substances. The effective Ea obtained by the VYA isoconversional model-free method presented same tendency, presenting a slight increase till α = 0.85 while for conversion range α > 0.85, a sudden increase was confirmed. Changes in Ea values evidence that more than one reaction occurs during decomposition process. Model fitting approach was applied in order to propose the most appropriate mechanism for the pyrolysis process description of olive stone which is described by three independent parallel reactions, governed by the nth order with Fn code and described by the f(α) = (1 − α)n equation. Additionally, DTG curves reveal that the process consists of at least two degradation mechanisms; thus, the selection of the aforementioned kinetic model is based on these two assumptions. The first reaction (A→B) with effective Ea = 118.9 kJ mol−1 is associated with the degradation of hemicellulose, while the second reaction (C→D) with effective Ea = 143.7 kJ mol−1 is attributed to the degradation of cellulose in a good agreement with effective Ea values of model-free methods, especially to Friedman’s. The third reaction (Ε→F), which is related to the lignin degradation, corresponds to effective Ea = 217.6 kJ mol−1.
References
Koçer O, Acemioğlu B. Adsorption of Basic green 4 from aqueous solution by olive pomace and commercial activated carbon: process design, isotherm, kinetic and thermodynamic studies. Desalin Water Treat. 2016;57:16653–69.
Braadbaart F, Marinova E, Sarpaki A. Charred olive stones: experimental and archaeological evidence for recognizing olive processing residues used as fuel. Veg Hist Archaeobot. Springer Berlin Heidelberg 2016;25:415–30.
Dermeche S, Nadour M, Larroche C, Moulti-Mati F, Michaud P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem [Internet]. Elsevier Ltd 2013;48:1532–52. Available from https://doi.org/10.1016/j.procbio.2013.07.010.
Ghanem MTM, Tawfik WA, Mahdy E-SM, Abdelgawad ME, Abdel-Azim NS, El-Missiry MM. Chemical and biological evaluation of olive leaves as a waste by-product of olive oil industry. Egypt Pharm J. 2019;18:172–7.
Sánchez-Gutiérrez M, Espinosa E, Bascón-Villegas I, Pérez-Rodríguez F, Carrasco E, Rodríguez A. Production of cellulose nanofibers from olive tree harvest—a residue with wide applications. Agronomy. 2020;10:1–15.
Dardouri S, Jalila S. A comparative study of adsorption and regeneration with different agricultural wastes as adsorbents for the removal of methylene blue from aqueous solution. Chinese J Chem Eng [Internet]. Elsevier B.V. 2017;25:1282–7. Available from https://doi.org/10.1016/j.cjche.2017.01.012.
Ghouma I, Jeguirim M, Guizani C, Ouederni A, Limousy L. Pyrolysis of Olive Pomace: degradation kinetics, gaseous analysis and char characterization. Waste Biomass Valorization. Springer Netherlands 2017;8:1689–97.
Ceylan S. Kinetic analysis on the non-isothermal degradation of plum stone waste by thermogravimetric analysis and integral Master-Plots method. Waste Manag Res. 2015;33:345–52.
Guida MY, Bouaik H, El Mouden M, Moubarik A, Aboulkas A, El harfi K, Hannioui A. Utilization of Starink Approach and Avrami Theory to evaluate the kinetic parameters of the pyrolysis of olive mill solid waste and olive mill wastewater. J Adv Chem Eng. 2017;07:1–8.
Hu X, Gholizadeh M. Biomass pyrolysis: a review of the process development and challenges from initial researches up to the commercialisation stage. J Energy Chem [Internet]. Elsevier B.V. and Science Press 2019;39:109–43. Available from https://doi.org/10.1016/j.jechem.2019.01.024.
Manić N, Janković B, Dodevski V. Model-free and model-based kinetic analysis of Poplar fluff (Populus alba) pyrolysis process under dynamic conditions. J Therm Anal Calorim [Internet]. Springer International Publishing; 2020; Available from https://doi.org/10.1007/s10973-020-09675-y.
Sobek S, Werle S. Kinetic modelling of waste wood devolatilization during pyrolysis based on thermogravimetric data and solar pyrolysis reactor performance. Fuel. Elsevier; 2020;261.
Guida MY, Bouaik H, Tabal A, Hannioui A, Solhy A, Barakat A, et al. Thermochemical treatment of olive mill solid waste and olive mill wastewater: Pyrolysis kinetics. J Therm Anal Calorim. Springer Netherlands 2016;123:1657–66.
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour Technol [Internet]. Elsevier Ltd 2011;102:6230–8. Available from https://doi.org/10.1016/j.biortech.2011.02.060.
Moukhina E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J Therm Anal Calorim. 2012;109:1203–14.
Brachi P, Miccio F, Miccio M, Ruoppolo G. Isoconversional kinetic analysis of olive pomace decomposition under torrefaction operating conditions. Fuel Process Technol [Internet]. Elsevier B.V. 2015;130:147–54. Available from https://doi.org/10.1016/j.fuproc.2014.09.043.
Ounas A, Aboulkas A, El harfi K, Bacaoui A, Yaacoubi A. Pyrolysis of olive residue and sugar cane bagasse: Non-isothermal thermogravimetric kinetic analysis. Bioresour Technol [Internet]. Elsevier Ltd 2011;102:11234–8. Available from https://doi.org/10.1016/j.biortech.2011.09.010.
Gao Y, Yang Y, Qin Z, Sun Y. Factors affecting the yield of bio-oil from the pyrolysis of coconut shell. Springerplus. Springer International Publishing 2016;5.
Samolada MC, Stoicos T, Vasalos IA. An investigation of the factors controlling the pyrolysis product yield of Greek wood biomass in a fluidized bed. J Anal Appl Pyrolysis. 1990;18:127–41.
Prakash Bamboriya O, Singh Thakur L, Parmar H, Kumar Varma A, Hinge VK. A review on mechanism and factors affecting pyrolysis of biomass. Int J Res Advent Technol [Internet]. 2019;7:1014–24. Available from www.ijrat.org.
Leng L, Huang H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour Technol [Internet]. Elsevier Ltd; 2018;270:627–42. Available from https://doi.org/10.1016/j.biortech.2018.09.030.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18:393–402.
Alshammari BA, Alotaibi MD, Alothman OY, Sanjay MR, Kian LK, Almutairi Z, et al. A new study on characterization and properties of natural fibers obtained from Olive Tree (Olea europaea L.) residues. J Polym Environ [Internet]. Springer US 2019;27:2334–40. Available from https://doi.org/10.1007/s10924-019-01526-8.
Elbir M, Moubarik A, Rakib EM, Grimi N, Amhoud A, Miguel G, et al. Valorization of moroccan olive stones by using it in particleboard panels. Maderas Cienc y Tecnol. 2012;14:361–71.
Gomez-Martin A, Chacartegui R, Ramirez-Rico J, Martinez-Fernandez J. Performance improvement in olive stone’s combustion from a previous carbonization transformation. Fuel [Internet]. Elsevier 2018;228:254–62. Available from https://doi.org/10.1016/j.fuel.2018.04.127.
Al-Farraji A, Marsh R, Steer J. A comparison of the pyrolysis of olive kernel biomass in fluidised and fixed bed conditions. Waste Biomass Valorization. Springer Netherlands 2017;8:1273–84.
Luz Yolanda Toro Suarez. No Biomass Gasification and Pyrolysi. 2015.
Gai C, Dong Y, Zhang T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour Technol [Internet]. Elsevier Ltd 2013;127:298–305. Available from https://doi.org/10.1016/j.biortech.2012.09.089.
Šimon P, Thomas P, Dubaj T, Cibulková Z, Peller A, Veverka M. The mathematical incorrectness of the integral isoconversional methods in case of variable activation energy and the consequences. J Therm Anal Calorim. 2014;115:853–9.
Chrissafis K. Kinetics of thermal degradation of polymers. J Therm Anal Calorim. 2009;95.
Sobek S, Werle S. Kinetic modelling of waste wood devolatilization during pyrolysis based on thermogravimetric data and solar pyrolysis reactor performance. Fuel [Internet]. Elsevier 2020;261:116459. Available from https://doi.org/10.1016/j.fuel.2019.116459.
Koutsomitopoulou AF, Bénézet JC, Bergeret A, Papanicolaou GC. Preparation and characterization of olive pit powder as a filler to PLA-matrix bio-composites. Powder Technol [Internet]. Elsevier B.V. 2014;255:10–6. Available from https://doi.org/10.1016/j.powtec.2013.10.047.
Khalideh Al bkoor Alrawashdeh, Katarzyna Slopiecka, Abdullah A. Alshorman, Pietro Bartocci, Francesco Fantozzi. Pyrolytic Degradation of Olive Waste Residue (OWR) by TGA: thermal decomposition behavior and kinetic study. J Energy Power Eng. 2017;11.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Acknowledgements
The author would like to thank Pharmagnose S.A. (Athens) for collaboration and supply of olive stone samples. Funding was provided by GSRI (Grant No. Project Code: T6YBΠ- 00161).
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call Special Actions AQUACULTURE-INDUSTRIAL MATERIALS-OPEN INNOVATION IN CULTURE (project code: T6YBΠ- 00161).
Author information
Authors and Affiliations
Contributions
ΤΑ performed the experiments, material characterization measurements and evaluation. KC and TA wrote the paper. KC supervised the research study.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asimakidou, T., Chrissafis, K. Thermal behavior and pyrolysis kinetics of olive stone residue. J Therm Anal Calorim 147, 9045–9054 (2022). https://doi.org/10.1007/s10973-021-11163-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11163-w