Abstract
The chemical modification of potato starch by cyclohexyl methacrylate monomer in the presence of potassium persulfate as an initiator and some of physico–chemical properties of the resulted copolymers have been presented. On a basis of the received results, the maximum of the grafting (45.3% ± 0.2) was found using the following conditions: potato starch to cyclohexyl methacrylate ratio 1:3, an initiator concentration 1.5 mass%, the reaction temperature 70 °C and the reaction time 90 min. The obtained copolymers showed ca. 1.9–5.5 times lower polar solvent swelling, ca. 2.2–9.6 times higher non-polar swelling, ca. 1.6–3.6 times higher moisture resistance and ca. 1.9–6.3 times higher chemical resistance as compared to these properties of unmodified potato starch. Moreover, as it was stated based on the TG/DTG analyses, the novel materials were thermally stable up to the temperature of ca. 180 °C. Their pyrolysis process has included at least four stages where the emission of different structure volatiles was detected by a FTIR analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyclohexyl methacrylate (CHMA) monomer is a methacrylic acid ester commonly applied to the preparation of homopolymer, copolymers, composites or blends. The application of CHMA to the synthesis of PCHMA particles and nano PCHMA particles through emulsion polymerization and modified microemulsion polymerization is reported [1, 2]. CHMA monomer was also used to the preparation of copolymers, diblock and triblock copolymers with a wide variety of unsaturated compounds, for example with iso-butyl acrylate, di(ethylene glycol) methyl ether methacrylate, polyacrylamide, di[(methylamine)ethyl methacrylate, tetra(ethylene glycol) dimethacrylate, methyl methacrylate, 2-hydroxyethyl methacrylate, butyl methacrylate, 2-vinylpyridine, styrene, poly(caprolactone)dimethacrylate, etc. [3,4,5,6,7,8,9,10,11,12,13,14,15]. The graft copolymerization of cyclohexyl methacrylate onto chloroprene rubber, 1,2-polybutadiene, natural rubber, ethylene-propylene-diene (EPDM), polyethylene, carbon nanotubes and styrene-block-butadiene is also reported [16,17,18,19].
In turn, the influence of the addition of poly(cyclohexyl methacrylate) (PCHMA) on the properties and morphology of PCHMA/poly(vinyl acetate) blends, PCHMA/poly(α-methylstyrene) blends, PCHMA/poly(methyl methacrylate)/poly(α-methylstyrene)/poly(p-methylstyrene) blends, PCHMA/polypropylene, etc. has been evaluated [20,21,22,23,24,25,26,27]. Also, the preparation of PCHMA/poly(2-hydroxyethyl methacrylate)/montmorillonite nanocomposites obtained by combination atom transfer radical polymerization and photoinduced crosslinking is known [28].
CHMA monomer is also used as a feedstock for syntheses due to it tendency to addition reactions with organic and inorganic compounds. The presence of cyclohexyl methacrylate in the structure of the polymers, copolymers or blends improves their hardness, hydrolytic stability, hydrophobicity, chemical resistance or scratch resistance [29].
In the present studies, cyclohexyl methacrylate (CHMA) monomer was applied as a comonomer to the preparation of the graft copolymers with potato starch which can be applied as more environmentally friendly stabilizers, compatibilizers, fillers, resistant materials, modifiers, plastics or excipients for a drug delivery systems. The free radical graft copolymerization process was carried out by applying variable reaction parameters (comonomer ratios, temperature and time, initiator concentration) to find optimal graft conditions. The reaction was initiated by water-soluble initiator: potassium persulfate. The spectroscopic measurements (FTIR and 13C CP/MAS NMR) were used to affirm the structure of the received graft copolymers. The effect of the grafting percent on the changes in the selected physico–chemical properties such as moisture resistance, chemical stability, solvent resistance, glass transition temperature and thermal stability was discussed. The decomposition mechanism of starch-g-poly(cyclohexyl methacrylate) copolymers was evaluated involving the TG-FTIR coupled technique.
Experimental
Materials
Cyclohexyl methacrylate (CMA) (≥ 97%) was purchased with Sigma-Aldrich. Potato flour (Melvit S.A., Poland) was purified in order to extract potato starch according to the procedure developed by Lim et al. [30]. A radical initiator: potassium persulfate (≥ 99%) was from Fluka, Buchs, Switzerland. The following solvents: tetrahydrofurane, dichloromethane, ethanol, chloroform, hexane, toluene, butanol and silica gel were delivered by Merck, Germany. However, sodium carbonate, magnesium sulfate, sodium hydroxide, carbon tetrachloride, hydrochloric acid, magnesium sulfate and buffers solutions (pH 5,7,9) were from POCh, Gliwice, Poland.
Methods
The 13C CP/MAS NMR tests were done at the resonance frequency of 75.5 MHz using a Bruker Avance 300 MSL apparatus.
The FTIR spectra were gathered over the region 600 cm−1–4000 cm−1 and 4 cm−1 resolution and using KBr disk technique on a FTIR Tensor 27, Bruker instrument.
The gelation properties of the obtained copolymers with water were studied using the thermal analysis (DSC Phoenix 204, Netzsch, Germany). The samples prepared by mixing ca. 3 mg of the copolymer and ca. 6 mg of distilled water were heated between the temperatures 20 °C and 100 °C, after their equilibration (one day at room temperature).
The swelling studies of starch-g-poly(cyclohexyl methacrylate) copolymers were carried out in the following solvents: water, ethanol, butanol, toluene, hexane and carbon tetrachloride using a centrifugation method. The swellability coefficients (B) were determined from the equation [31]:
V0—the initial volume of the sample, V1—the final volume of the sample.
The moisture resistance tests were performed using a desiccator where an adequate amount of the copolymer’s sample was placed and exposed to the water vapor. The exposition was carried out at 25 °C for two days. The moisture absorption: M (%) was calculated based on the equation given below [32]:
m0—the initial mass of the sample (ca 100 mg), m1—the final mass of the sample.
The chemical resistance tests were performed using the following chemical environments: 1 M solution of sodium hydroxide, the buffer solutions with pH 5, 7 and 9 and 1 M solution of hydrochloric acid. An adequate number of the sample was immersed in the above solutions and being held until the constant mass of the sample was obtained. After the tests, each sample was carefully washed with distilled water, dried and weighed. The mass loss (WL) of the studied copolymers was evaluated from the equation given in Ref. [33]:
m1—the initial mass of the sample (ca. 100 mg), m2—the final mass of the sample.
The DSC studies were performed using a DSC 204 Jupiter F1 manufactured by Netzsch, Germany apparatus. Firstly, the samples (ca. 10 mg) were preheated to 100 °C to remove the absorbed moisture. Then, the dried samples were heated from 20 to 500 °C with the heating rate 10 °C min−1 in the presence of argon atmosphere (a flow rate 20 mL min−1) in Al crucibles with a pierced lid. From the received DSC curves, the glass transition temperatures (Tg) and the temperature range where the decomposition stages were happened with the maximum decomposition temperatures (Tmax) were assigned.
The TG/DTG tests were made using a STA 449 Jupiter F1 instrument produced by Netzsch, Germany. The sample (ca. 10 mg) was put into Al2O3 crucible and heated between the temperatures 40 °C and 1000 °C with the heating rate 10 °C min−1 in the presence of helium atmosphere (a flow rate 40 mL min−1). On the basis of the obtained TG/DTG curves, the temperature ranges characteristic for the decompostion stages of the copolymers, maximum decomposition temperatures (Tmax), the mass losses (Δm) achieved in each decomposition stage and the residual mass at 1000 °C (rm) were evaluated.
The type of the volatiles created under the heating of the studied materials was identified using the simultaneous TG-FTIR analysis. The FTIR spectra were recorded on a FTIR TGA 585 analyzer delivered by Bruker, Germany over the range of 600–4000 cm−1 and with a resolution 4 cm−1.
The preparation of starch-g-poly(cyclohexyl methacrylate) copolymers
The copolymers have been prepared under the free radical graft polymerization using potassium persulfate as a thermal initiator. Adequate amounts of cyclohexyl methacrylate and potato starch were used, Tables 1–4. The different reaction conditions have been applied in order to evaluate the most favoring terms for the graft process, Tables 1–4. The graft copolymerization process was performed according to the method described in Refs. [34, 35] After the graft reaction has been completed, the raw products were precipitated in methanol, dried and purified under the Soxhlet extraction method using dichloromethane and tetrahydrofurane as solvents. The grafting parameters have been counted appropriate to the following equations:
where: %G—the grafting percent, %GE—the grafting efficiency, %H—the percent of homopolymer, m0—the mass of cyclohexyl methacrylate, m1—the mass of potato starch, m2—the mass of poly(cyclohexyl methacrylate), m3—the mass of the grafted poly(cyclohexyl methacrylate) [36].
Results and discussion
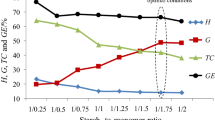
According to the results given in Table 1, as starch to monomer ratio increases, growth of the GE and G values is observed. However, the drop in the H values is visible. This indicates that for higher concentration of cyclohexyl methacrylate monomer, copolymerization process is more favorably than homopolymerization of methacrylate monomer. In addition, it is found that the maximum of the GE (ca. 85%) and G (ca. 45%) values and the minimum of the H (ca. 2.6%) value for starch to monomer ratio 1:3 are obtained. Above this ratio, the grafting parameters (GE and G) significantly fall but the H value is increasing. That suggests that above the optimal concentration of monomer, it homopolymerization process is more favorable.
The dependence of the grafting parameters on initiator concentration is shown in Table 2. For initiator concentration from 0.5 mass% to 1.5 mass%, poly(cyclohexyl methacrylate) formation is very low (2.6%–3.3%), the grafting efficiency stands at almost constant (ca. 80–85%) and the G values hesitate between ca. 34 and 45%. Meanwhile, an increase in initiator concentration more than 1.5 mass% leads to the significant reduction of the GE values (from ca. 85 to ca. 23%) and G values (from ca. 45 to ca. 20%). But a big increase in the H values is noticed (from ca. 2.6 to ca. 22%). The data indicate that the rate of the copolymerization process is higher for lower initiator concentration, but the rate of homopolymerization is faster for higher initiator concentration.
The effect of the reaction temperature on the course of the graft process of potato starch with cyclohexyl methacrylate monomer is presented in Table 3. The maximum of the grafting is received for the reaction temperature 70 °C. Below this temperature, the GE, G and H take their values in the range of ca. 70–72%, ca. 22–26% and ca. 3–3.2%, respectively. On the other hands, the reaction temperature higher than 70 °C causes the reduction in the GE and G values and the increase of the H values (to the value of 10–15%) as compared to these values obtained at the optimal reaction temperature. As the graft reaction temperature increases, the amount of radicals formed from an initiator increases, and thus, the creation of higher number of active sites onto starch backbone is expected. However, at temperature higher than the optimum (70 °C), one can suspect the higher rate of termination reactions of growing graft chains. It is a result of an excess of radical creation and chain transfer reactions leading to grow of homopolymer amount.
In accordance with the data collected in Table 4, the reaction time least affects the course of the graft process of potato starch with cyclohexyl methacrylate than any other parameters. The prolongation of the reaction time from 30 to 90 min makes the growth of G values from ca. 21 to ca. 45%. However, the further extension of the reaction time does not affect greatly on the reaction course. Only minor drop in the GE and G values and little rise of the H values are noticed. This little effect of the extended reaction time on the grafting parameters may be explained by loss of available active sites onto starch, the reduction of monomer concentration, the reduction of initiator concentration or high viscosity of the reaction medium which hinders the diffusion between the reactants.
From the data collected, it can be summarized that the optimal reaction conditions for the studied grafting process are as follows: starch to cyclohexyl methacrylate ratio of 1:3, an initiator concentration 1.5 mass%, the reaction temperature 70 °C and the reaction time 90 min. This point to the rate of the grafting process is faster than the rate of monomer homopolymerization. Presumably, at these reaction conditions, the maximum concentration of active sites on starch macromolecule is created, and the viscosity of the reaction medium is appropriate to ensure adequately rapid migration between the reactants.
The confirmation of the copolymer’s structure
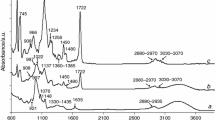
The structure of the obtained starch-g-poly(cyclohexyl methacrylate) copolymers was affirmed using the spectroscopic methods, Tables 5 and 6. The characteristic absorption bands read from the FTIR spectra and their assignment are gathered in Table 5. The stretching vibrations for the carbonyl group in esters are visible at 1718 cm−1. The bands which are ranged from 997 to 1245 cm−1 are characteristic for the stretching vibrations of C–O bonds coming from starch and the graft polymer. The absorption signals centered at 2855–2856 cm−1 and at 2930–2932 cm−1 are the result of the stretching vibrations of C–H. However, the two bands at 1361–1362 cm−1 and at 1448–1449 cm−1 are due to the deformation vibrations of C–H. Moreover, on the FTIR spectra, the existence of the stretching vibrations of the residual OH groups (3300–3352 cm−1) in the structure of the copolymers is confirmed [37].
The 13C solid-state NMR spectra for the studied copolymers show the presence of several signals originating in the carbons existing in the structure of the studied polymers, Table 6. The signal at 18.1–18.3 ppm is characteristic for the carbons in α-methyl (CH3) group from poly(cyclohexyl methacrylate) chains. The methylene carbon (–C–) is observed at 45.2–45.3 ppm. The ring carbons present in the structure of poly(cyclohexyl methacrylate) chains appear at 23.7–23.9 ppm, 26.1–26.4 ppm, 31.5–31.7 ppm and at 72.6–72.8 ppm (C–O). Meanwhile, the ester carbonyl (C=O) is noticed at 177.2–177.4 ppm. However, the signals responsible for the chemical shifts of the carbons in the structure of starch are visible at the following ranges: 61.1–61.4 ppm (CH2–O), 71.7–71.9 ppm (C–O) and 82.1–100.9 ppm (CH–O).
The physico–chemical properties
In order to study some of the physico–chemical properties of novel materials, the copolymers with the following G values: 24.5% ± 0.4 (copolymer 1), 30.5% ± 0.3 (copolymer 2), 36.8% ± 0.3 (copolymer 3) and 45.3% ± 0.2 (copolymer 4) have been selected. In addition, the results received for unmodified potato starch were also placed in Tables 7, 8 to compare the data. The chemical modification of potato starch causes the significant drop of the swelling of the obtained materials in polar solvents and the growth of their swelling in non-polar solvents as compared to unmodified potato starch. In addition, as it is well visible, the properties of the copolymers are directly dependent on their G values. Among all the studied starch-g-poly(cyclohexyl methacrylate) copolymers, the copolymer 4 exhibits the smallest swelling in polar type solvents and the highest swelling in non-polar type solvents. The highest swelling of the copolymers in toluene and CCl4 is noticed. It was probably due to the chemical character of the graft polymer chains.
Moreover, the moisture resistance of the novel materials is increased as the G values increase. The moisture resistance for all the studied copolymers is greater from ca. 1.6 times to ca. 3.6 times as compared to the moisture resistance observed for unmodified starch. This behavior is typical due to the replacing of the part of highly polar, hydroxyl groups of carbohydrate polymer by hydrophobic type poly(cyclohexyl methacrylate) chains.
The prepared starch-g-poly(cyclohexyl methacrylate) copolymers were characterized by a considerable higher chemical stability under acidic, buffers and basic environments as compared to potato starch. As the G values increase, the chemical stability of the copolymers is improving in all the used solutions. The chemical resistance of the copolymers is from ca. 1.9 to 6.3 times higher than this observed for potato starch. The copolymers showed the greatest chemical resistance in neutral and acidic environments. The chemical stability of the copolymer 4 is ca. 5 times higher in neutral conditions, ca. 5.7 times higher in buffer solution (pH 4) and ca. 6.3 times higher in acidic conditions (1 M HCl) as compared to potato starch. On the contrary, the worst chemical resistance of the copolymers was noticed in basic environments. The mass loss under acidic and neutral conditions does not exceed 13% for the copolymer 4 (G = 45.3% ± 0.2). Meanwhile, the mass loss determined for the copolymer 4 under basic conditions was lower than 21%. In comparison, the mass loss marked for potato starch in various environments was 81.4% ± 0.5 (1 M HCl), 65.5% ± 0.3 (pH = 5), 62.7% ± 0.5 (pH = 7), 86.7% ± 0.4 (pH = 9) and 100% (1 M NaOH), respectively.
In addition, DSC studies confirm that the graft copolymers are not able to gelation with water which suggest that OH amylose groups were completely blocked by poly(cyclohexyl methacrylate) chains [38, 39].
It can be summarized that the graft process of cyclohexyl methacrylate onto starch macromolecule significantly modifies the properties of starch.
The thermal properties
The DSC tests
The obtained DSC data and the DSC curves were shown in Table 9 and Fig. 1, respectively. Under the used thermal conditions, it was found that the glass transition temperature (Tg) for the obtained starch-g-poly(cyclohexyl methacrylate) copolymers was ca. 117–118 °C. It was in accordance with the Tg values for poly(cyclohexyl methacrylate) homopolymer given in the literature data [40, 41].
The heating of the copolymers above the temperature of 180–190 °C caused the appearance on the thermal curves, only the endothermic signals which were connected with the degradation processes of the studied materials were visible, Table 9, Fig. 1.
The TG/DTG curves and the data received on a basis of the TG/DTG curves are presented in Table 10 and Fig. 2. The TG analysis confirms the data received from the DSC analysis. The pyrolysis process of starch-g-poly(cyclohexyl methacrylate) copolymers happens at least four stages and it is connected with the emission of some gaseous decomposition products, Fig. 3. The first decomposition stage composed of at least two steps is detected between the temperatures 190 °C and 320 °C. The mass loss amounts to ca. 33–38%. Within this temperature range, the most characteristic FTIR spectra showing the maximum emission of the volatiles become apparent at Tmax1 and Tmax1a, Fig. 3. The gaseous spectra collected at Tmax1 show the absorption bands at the wavelengths as follows: low-intensity signal at 3090 cm−1 (the stretching vibrations for = C–H), the signals at the range 2773–2945 cm−1 (the stretching vibrations for C–H), the signal at 1734 cm−1 (the stretching vibrations for C=O), the signal at 1630 cm−1 (the stretching vibrations for C=C), two signals at 1400 cm−1 and 1450 cm−1 (the deformation vibrations for C–H), the signals at the range of 1012–1311 cm−1 (the stretching vibrations for C–O) and the signals at the range of 808–950 cm−1 (the out-of-plane deformation vibrations for = C–H). It indicates the emission of some cyclohexyl methacrylate as a result of depolymerization process initiated on the end graft chains connected with the cleavage of O–C bonds.
Meanwhile, the gaseous FTIR spectra gathered at Tmax1a have completely different appearances that suggests the emission of the volatiles with different structures than those visible at Tmax1. The beginning of the emission of inorganic gaseous decomposition products such as water (the range 3400–3700 cm−1), carbon dioxide (2310–2360 cm−1) and carbon monoxide (two characteristic bands at 2080 cm−1 and 2170 cm−1) is clearly confirmed. Apart from the emission of inorganic volatiles, the creation of some organic fragments is detected based on the characteristic vibrations for the functional groups. The stretching vibrations of C–H bands at the range of 2720–2990 cm−1 and the deformation vibrations of C–H bands at 1370 cm−1 and 1460 cm−1 have indicated on the emission of alkane fragments. However, the presence of the band at 2720 cm−1 (the characteristic band for the stretching vibrations for C–H in aldehyde groups) combined with the presence of the band at 1745 cm−1 (the stretching vibrations for C=O) and the band at 1105 cm−1 (the stretching vibrations for C–O) suggests the creation of aldehyde fragments. The bands at the range of 740–990 cm−1 responsible for the out-of-plane deformation vibrations of = C–H, the small intensity bands at ca. 1640 cm−1 (the stretching vibrations for C=C) and at ca. 3100 cm−1 (the stretching vibrations for = C–H) confirm the formation of alkene fragments. In addition, the clear occurrence of the bands at 1510–1580 cm−1 (the stretching vibrations for ArC=C) points to the emission of some furane fragments. On the current FTIR spectrum, the appearance of the stretching bands for C–O at the range of 1210–1320 cm−1, and the band at 1795 cm−1 responsible for the stretching vibrations of C=O confirm the creation of an acid fragments. It cannot be excluded the formation of alcohol fragments due to the presence of the following bands: at 1020 cm−1 and above 3500 cm−1. The appearance of these FTIR spectra and the DSC signal located at the temperature range from 240 to 300 °C testifies to the pyrolysis processes of potato starch from the copolymers under this decomposition stage. These processes cover the cleavage of glycoside bonds, strong bonds in the structure of starch and thermal dehydration processes of starch.
The second decomposition stage occurs between the temperatures 320 °C and 385 °C with the maximum decomposition temperature (Tmax2) located at the same temperature (349 °C). The mass loss softly grows as the G values increase. It is from 20.7% (copolymer 1) to 32.1% (copolymer 4). When you look at the FTIR spectrum collected at Tmax2, you can see the presence of the same absorption bands that were observed from the FTIR spectrum gathered at Tmax1. However, the intensity of the bands at Tmax2 is considerably higher than those observed at Tmax1a. The results received from this FTIR spectrum indicate the depolymerization process of poly(cyclohexyl methacrylate) chains as a result of random-main chain scissors (C–C) in the structure of grafted polymer and the emission of cyclohexyl methacrylate monomer as main decomposition, gaseous product.
The heating of the previously formed residues of more than 385 °C leads to their further decomposition processes. Between the temperatures 385 °C and 540 °C, the next mass loss is clearly shown. The maximum of the DTG signal (Tmax3) occurs at constant temperature (434–435 °C). The mass loss is within 18.3% and 26.1% and it is increased with the growing G values. The FTIR spectrum at Tmax3 shows the creation of inorganic gases such as H2O, CO2 and CO as well as organic volatiles. Among organic gaseous decomposition products, it can be mentioned the formation of unsaturated fragments due to the presence on the gaseous FTIR spectrum collected at Tmax3, the following absorption bands at 3070 cm−1 (the stretching vibrations for = C–H), at 1630 cm−1 (the stretching vibrations for C=C) and at the range of 790–930 cm−1 (the out-of-plane deformation vibrations for = C–H). In turn, the occurrence of the absorption bands at 2870–2950 cm−1 (the stretching vibrations for C–H), two bands at 1375 cm−1 and 1450 cm−1 (the deformation vibrations for C–H) may indicate on the creation of saturated fragments. Additionally, the appearance on the FTIR spectrum, the bands at 2720 cm−1 (the stretching vibrations for C–H in aldehyde group), at 1724–1760 cm−1 (the stretching vibrations for C=O) and at the range of 1006–1300 cm−1 (the stretching vibrations for C–O) prove the emission of oxygen-rich fragments. It indicates the secondary reactions between the decomposition products and/or formed residues.
Finally, the heating of the residues above the temperature 540 °C results in slow, minimal mass loss (ca. 1.2–4.3%) up to the temperature of 1000 °C and mainly the emission of some amounts of CO2, CO, H2O and a minor number of simple in structure, organic fragments, Fig. 3, Scheme 1.
Conclusions
Cyclohexyl methacrylate monomer was applied to the chemical modification of potato starch in the presence of potassium persulfate as a radical initiator. The optimum reaction conditions have been found to be the following: starch to monomer ratio 1:3, 70 °C, 90 min and 1.5 mass% of an initiator. At these conditions, the higher rate of the copolymerization process than the rate of homopolymerization due to the favoring terms (appropriate viscosity of the reaction medium, initiator concentration, the concentration of the formed active sites, the monomer concentration) led to obtain the copolymers with the maximum of the grafting percent (45.3% ± 0.2).
The physico–chemical properties of the received copolymers differ considerably as compared to unmodified potato starch which was caused by the presence of hydrophobic graft poly(cyclohexyl methacrylate) chains attached to hydrophilic starch backbone. The chemical modification of potato starch by cyclohexyl methacrylate monomer had resulted in ca. 3.6 times higher moisture resistance, ca. 5 times higher chemical stability in neutral conditions, ca. 5.7–6.3 times higher stability in acidic conditions and ca. 4.5–4.8 times higher stability in basic conditions of the copolymer with the maximum of grafting as compared to the chemical stability of potato starch. Moreover, the performed swelling tests have shown ca. 3.9–5.5 times lower swelling in polar solvents and ca. 2.2–9.6 times higher swelling in non-polar solvents of the copolymer with the maximum of grafting than the swelling of potato starch.
The copolymers were thermally stable up to the temperature of ca. 180 °C and they decomposed at least four stages connected with the emission of various structure volatiles at each decomposition stage. Generally, the TG-FTIR studies confirmed the creation of cyclohexyl methacrylate monomer, starch decomposition products (alkane, alkene, aldehyde, alcohol, furane fragments and H2O) and formed residues decomposition products (unsaturated, saturated, rich-oxygen fragments and CO2and CO) as a result of depolymerization, dehydration and complex pyrolysis processes of the prepared copolymers.
References
Chu HH, Gau JH. Characteristics of emulsion polymerization of cyclohexyl methacrylate. Polym Bull. 1995;34:433–40.
Tang R, Xue Y, Wang W, Zha L, Fu S. Rich-syndiotacticity of poly(cyclohexyl methacrylate) prepared by modified microemulsion polymerization. J Macrom Sci Part A. 2007;44:569–75.
Munoz-Bonilla A, Cerrada ML, Fernandez-Garcıa M. Physical properties of poly(cyclohexyl methacrylate)-b-poly(iso-butylacrylate)-b-poly(cyclohexyl methacrylate) triblock copolymers synthesized by controlled radical polymerization. Polymer. 2007;48:5581–9.
Munoz-Bonilla A, Fernandez-Garcia M, Cerrada ML, Mantovani G, Haddleton DM. Aggregation and solubilization of organic solvents andpetrol/gasoline in water mediated by block copolymers. Eur Polym J. 2007;43:4583–92.
Munoz-Bonilla A, Haddleton DM, Cerrada ML, Fernandez-Garcia M. Synthesis of poly(di[methylamine]ethyl methacrylate)-b-poly(cyclohexyl methacrylate)-b-poly(di[methylamine]ethyl methacrylate) amphiphilic triblock copolymers by ATRP: condensed-phase and solution properties. J Polym Sci Part A Polym Chem. 2008;46:85–92.
Fasla A, Kada SO, Seghier Z, Perichaud A. Cyclohexyl methacrylate with tetra (ethylene glycol) dimethacrylate in tertahydrofuran radical copolymerization. Mater Sci Forum. 2009;609:63–7.
Lospa C, Rusu M, Vouéa M, Adaoa MH, Sucklinga P, De Conincka J. Optical and surface properties of acrylic copolymers for crystalline lens implants. J Optoelectron Adv Mater. 2005;7:2831–4.
Min KE, Lee DH, Jung HM. Miscibility of polycarbonate with poly(methyl methacrylate-co-cyclohexyl methacrylate). Polym Bull. 1990;24:221–6.
Hill DJT, Moss NG, Pomery PJ, Whittaker AK. Copolymer hydrogels of 2-hydroxyethyl methacrylate with n-butyl methacrylate and cyclohexyl methacrylate: synthesis, characterization and uptake of water. Polymer. 2000;41:1287–96.
Xu P, Ji X, Abetz V, Jiang S. Uniformly gold nanoparticles derived from P2VP-b-PCHMA block copolymer templates with different reduction methods. J Nanosci Nanotechnol. 2011;11:6973–8.
Serrano B, Baselga J, Piérola IF. Fluorescence lifetime distributions of labeled amorphous polymers in bulk. Polym J. 2002;34:905–10.
Riess G. Micellization of block copolymers. Prog Polym Sci. 2003;28:1107–70.
Chun X. Studies on graft copolymerization of cyclohexyl methacrylate onto chloroprene rubber. J Appl Polym Sci. 1997;64:1733–7.
Barim G, Yayla MG, Degirmenci M. Copolymerization of cyclohexene-3-yl methyl methacrylate with styrene: synthesis, characterization, monomer reactivity ratios, and thermal properties. Des Monomers Polym. 2014;17:610–6.
Liaw DJ, Lin LL. Studies on graft copolymerization of cyclohexyl methacrylate onto 1,2-polybutadiene. J Appl Polym Sci. 1989;37:1993–2006.
Braun D, Fisher M, Hellmann GP. Block-graft copolymers as compatibilizers in polymer blends. Polymer. 1996;37:3871–7.
Brennan AB, Arnold JJ, Zamora MP. Surface modification of ultra-high molecular weight polyethylene fibers by γ-radiation-induced grafting. J Adhes Sci Technol. 1995;9:1031–48.
Dafader NC, Haque ME, Akhtar F, Ahmad MU. Study on grafting of different types of acrylic monomers onto natural rubber by γ-rays. Radiat Phys Chem. 2006;75:168–72.
Hayashida K. Dielectric properties of polymethacrylate-grafted carbon nanotube composites. RSC Adv. 2013;3:221–7.
Serrano B, Baselga J, Esteban I, Sesé LM, Piérola IF. Morphology of phase separated blends of poly(cyclohexyl methacrylate) with poly(vinyl acetate). J Appl Polym Sci. 2003;89:1284–90.
Kalogeras IM, Brostow W. Glass transition temperatures in binary polymer blends. J Polym Sci Part B Polym Phys. 2009;47:80–95.
Roland CM, Casalini R. Dynamics of poly(cyclohexyl methacrylate): neat and in blends with poly(R-methylstyrene). Macromolecules. 2007;40:3631–9.
Ling CL, Woo EA. Miscibility, morphology, and thermal characterization of an acrylic/styrenic blend system. Poly(cyclohexyl methacrylate) and poly(α-methyl styrene). Polym J. 2001;33:13–7.
Mugica A, Barral M, Pomposo J, Cortazar M. Effect of monomer architecture on segmental interaction parameters of binary blends involving copolymers of cyclohexyl methacrylate, methyl methacrylate and styrene derivatives. Acta Polym. 1999;50:304–11.
Charef H, Omonov T, Groeninckx G, Moldenaers P. Phase morphology development and stabilization in polycyclohexylmethacrylate/polypropylene blends: uncompatibilized and reactively compatibilized blends using two reactive precursors. Polymer. 2004;45:8115–26.
Adedeji A, Lyu S, Macosko CW. Block copolymers in homopolymer blends: interface vs micelles. Macromolecules. 2001;34:8663–8.
Kim JH, Park DS, Kim CK. Characterization of the interaction energies for polystyrene blends with various methacrylate polymers. J Polym Sci Part B Polym Phys. 2000;38:2666–77.
Yoshikawa Y, Ciftci M, Aydin M, Narusawa M, Karatsu T, Yagci Y. Synthesis, characterization and photoinduced cross-linking of functionalized poly(cyclohexyl methacrylate) copolymer/clay nanocomposite as negative image pattering material. J Photopolym Sci Technol. 2015;28:769–74.
BASF Group technical information TI/CP 1983e. October 2015. http://www.specialty-monomers.basf.com. Accessed 15 Dec 2019.
Lim ST, Lee JH, Shin DH, Lim HS. Comparision of protein extraction solutions for rice starch isolation and effects of residual protein content on starch pasting properties. Starch. 1999;51:410–5.
Tuncel K, Ecevit K, Kenesci K, Piskin E. Nonswellable and swellable ethylene glycol dimethacrylate-acrylic acid copolymer microspheres. J Polym Sci Part A Polym Chem. 1996;34:45–55.
Pathania D, Sharma R. Synthesis and characterization of graft copolymers of methacrylic acid onto gelatinized potato starch using chromic acid initiator in presence of air. Adv Mater Lett. 2012;3:136–42.
Kaith BS, Singha AS, Grupa SK. Graft copolymerization of flax fibres with binary vinyl monomer mixtures and evaluation of swelling, moisture absorbance and thermal behavior of the grafted fibres. J Polym Mater. 2003;20:195–9.
Worzakowska M, Grochowicz M. Effect of some parameters on the synthesis and the physico-chemical properties of new amphiphilic starch-g-copolymers. Carbohydr Polym. 2015;130:344–52.
Worzakowska M. The effect of starch-g-copolymers structure on the oxidative behavior studied by the TG/DSC/FTIR-coupled method. J Therm Anal Calorim. 2017;129:367–76.
Fares MM, El-Faqeeh AAS, Osman ME. Graft copolymerization onto starch. I. Synthesis and optimization of starch grafted with N-tert-butylacrylamide copolymer and its hydrogels. J Polym Res. 2003;10:119–25.
Sokrates G. Infrared and Raman characteristic group frequencies, tablets and charts. New York: Wiley; 2001.
Odenigbo AM, Ngadi N, Ejebe C, Nwankpa C, Danbaba N, Ndindeng S, Manful J. Study on the gelatization properties and amylose content of rise varieties from Nigeria and Cameroun. Int J Food Sci Nutr. 2013;2:181–5.
Ott M, Hester EE. Gel formation as related to concentration of amylose and degree of starch swelling. Cereal Chem. 1965;42:476–84.
Kunal K, Robertson CG, Pawlus S, Hahn SF, Sokolov AP. Role of chemical structure in fragility of polymers: a qualitative picture. Macromolecules. 2008;41:7232–8.
Friedrich C, Schwarzwälder C, Riemann RE. Rheological and thermodynamic study of the miscible blend polystyrene/poly(cyclohexyl methacrylate). Polymer. 1996;37:2499–507.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Worzakowska, M. Starch-g-PCHMA copolymers: preparation and properties. J Therm Anal Calorim 147, 1225–1235 (2022). https://doi.org/10.1007/s10973-020-10451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10451-1