Abstract
Cupuassu (Theobroma grandiflorum Schum.) is a typical Amazonian fruit, whose seed is used as raw material to produce cupulate. The by-product of its seeds, cupuassu seed by-product (CSB), is often discarded by the cosmetic and food industry, but studies showed that it has a high content of phenolic compounds with exploitable antioxidant activity. This study aimed to identify the classes of chemical compounds present in the extract of CSB by HPTLC, to confirm them by RMN 1H spectroscopy and to analyze the extract thermal analytical behavior in different processing stages. After elution of the extract in an isocratic system, TLC plates were developed with selective reagents for several classes of chemical compounds and antioxidant activity by the DPPH· method. Butter, CSB and crude extract presented mass losses characteristic of lipid decomposition reactions (92.63, 14.80, and 15.42%, respectively). Thermal degradation of bioactive and nutritional compounds (mainly polyunsaturated fatty acids) present in both CSB and crude extract has occurred at temperatures above 189 °C. Microencapsulation of the CBS extract by spray drying (MECoC) using maltodextrin as coating agent was shown to effectively protect the phenolic compounds. RMN 1H spectra confirmed HPTLC and FTIR results, which allowed us to conclude that the crude extract of the cupuassu by-product was mainly composed of terpenes, steroids and phenolic compounds, mainly flavonoids. These analytical techniques have proven to be effective tools for assessing the bioactive compounds incorporation into the polymeric matrix expected in the pre-formulation stage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cupuassu (Theobroma grandiflorum Schum.) is a typical fruit of the Amazon rainforest, which is very appreciated by the local population for its exotic flavor as well as acidic and intense aroma [1]. The use of cupuassu pulp to produce juice and sweets generates a large quantity of a by-products mainly made up of seeds. The crude oil extracted from seeds, which solidifies at a temperature of − 5 °C resulting in a butter, has great commercial value for the cosmetic sector due to its emollient potential. The extraction of butter/oil occurs by natural cold pressing, which is more advantageous than the traditional solvent extraction, for it produces more stable butter and is more environmentally friendly.
The residual material, i.e., the cupuassu seed by-product, is wasted by the cosmetic and food industries, being exploited only as raw material in handicrafts. However, studies showed that its crude extract still has a potent antioxidant activity due to the presence of phenolic compounds [2,3,4]. Cupuassu seeds contain approximately 45% of fixed oil that has a complex composition, due to the presence of different triglycerides, polyphenols and phytosterols [2, 3]. Moreover, since cold pressing hardly involves the production of other wastes, it can also be used as natural fertiliser.
Plant extracts containing a variety of bioactive compounds are obtained by different extraction techniques. However, due to their complex composition, they are easily oxidized and/or degraded during either processing or storage, which leads to a reduction in their content of natural bioactive components and, consequently, in their therapeutic properties. Drying by atomization, also called spray drying, is a mechanical method used especially in the pharmaceutical and food industries to effectively encapsulate heat-sensitive components (e.g., polyphenols) [3,4,5,6]. It has been successfully applied to preserve the antioxidant properties or even increase the stability of bioactive compounds present in tropical fruit by-products [5, 7], including that deriving from cupuassu seeds [3]. On this line, spray drying has been used in this study using maltodextrin as a coating agent to microencapsulate the extract of cupuassu seed by-product.
Moreover, the quality control of the raw plant material is a fundamental tool in the pre-formulation phase, through which it is possible to help in the relevant choices to incorporate active ingredients. It can be carried out investigating the thermal profile of formulation components by means of thermal analysis, which is a set of techniques that allow (a) obtaining information on the stability, degradation and reaction of substances, including those of plant origin [8,9,10,11,12], (b) quantifying one or more compounds, and (c) shedding light on the effect of adding coating agents on active ingredients during microencapsulation.
Based on these considerations, this study aimed to assess the thermal behavior of the cupuassu seed by-product in different processing stages, using thermoanalytical techniques (TGA and DSC), as well as to identify its main classes of compounds by high-performance thin-layer chromatography, RMN 1H and FTIR.
Experimental
Material
The cupuassu butter was acquired from Amazon Oil Indústria e Comércio (Ananindeua, PA, Brazil) (batch: AMO003-005/2016), while the cupuassu seed by-product was provided by Natura Cosméticos S/A (São Paulo, SP, Brazil).
The following chemicals were acquired from Sigma-Aldrich (Saint Louis, MO, USA) and used in chromatographic analyses: rutin (CAS Number: 207671-50-9), β-amyrin (CAS Number: 559-70-6), gallic acid (CAS Number: 149-91-7), ascorbic acid (CAS Number: 50-81-7), brucin (CAS Number: 357-57-3), esculin (CAS Number: 531-75-9), 2,2-diphenyl-1-picrylhydrazyl (DPPH), maltodextrin (equivalent dextrose 16.5-19.5), vanillin, diphenylboryloxyethylamine, Dragendorff reagent.
Methods
Preparation of the cupuassu seed by-product
Cupuassu seeds were underwent a segregation process at 65 °C for 45 min, after which they were cold pressed to remove the oil to be industrially exploited [9]. The resulting by-product, designated as cupuassu seed by-product (CSB), was dried in an oven with air circulation at 40 ± 2 °C up to constant mass. After dehydration, it was pulverized in a blender, model 650 W (Mondial, São Paulo, SP, Brazil).
Preparation of the crude extract
Dried and pulverized CSB was extracted with a 70% (w/v) ethanol solution in water according to the percolation process [13]. To obtain the crude extract (CE), the resulting solution was concentrated in a rotary evaporator, model Laborota 4000 (Heidolph, Schwabach, Germany), under low pressure and controlled temperature (40 ± 5 °C). CE obtained in this way had a dry mass content of 78.85 ± 0.50% [9].
Characterization of the raw extract
Chemical profile by HPTLC
Chromatographic analyses were conducted in a robotic system of high efficiency thin-layer chromatography (HPTLC) composed of application modules (Automatic TLC Sampler 4 – ATS4), densitometer (TLC Scanner 4) and photodocumentator (TLC Visualizer) (CAMAG, Muttenz, Switzerland), and the WinCats 1.4.6. software was used for data processing. Aliquots of 30 µg spot−1 of bio-oil and 0.1 µg spot−1 of standards, namely β-amyrin, gallic acid, rutin, esculin, brucine and ascorbic acid, were applied in silica gel TLC plates (Aluminum F-254 60 Å, SiliCycle, Quebec, Canada). The TLC plates were eluted in the glass chamber (CAMAG) in an isocratic system of 67.57:17.57:7.43:7.43 (v/v) ethyl acetate/H2O/formic acid/acetic acid (AAAA), with a chromatographic path of 80 mm. TLC plates were derivatized with selective reagents for terpenes (VAS), phenolic compounds (FBS), flavonoids (NP/PEG), coumarins (KOH), alkaloids (Dragendorff) and antioxidant activity by the DPPH· method.
RMN 1H spectra
Nuclear Magnetic Resonance (RMN 1H) spectra were acquired with a spectrometer, model Ascend (Bruker, Rheinstetten, Germany), operating at 400 MHz. For data control and treatment, the software TopSpin 3.6.0 was used, and displacements were recorded in dimensionless values δ (ppm), with the internal reference to the solvent (CD3OD) signal [14,15,16].
Preparation of microparticles containing cupuassu crude extract
The microencapsulated crude extract of cupuassu seed by-product (MECoC) was obtained in a spray dryer, model B-290 (Büchi, Flawil, Switzerland), with 0.5 mm diameter atomizing nozzle, 32.5 m3 h−1 drying airflow and 80% suction rate, under previously determined optimal microencapsulation conditions [3], namely 170 °C inlet temperature, 5.0 mL min−1 feed flow rate, 5.0% (w/v) maltodextrin concentration and 50% cupuassu extract. The same conditions were adopted to obtain empty microparticles without extract.
Thermoanalytical profile (TG and DSC)
The thermal profile of samples (butter, CSB, CE, MECoC, microparticles without extract and rutin) was assessed by thermogravimetry (TG/DTG) and differential scanning calorimetry (DSC), using a thermal analyzer, model DTG-60 (Shimadzu, Kyoto and Japan). Samples were weighed (5.0–10.0 mg) in aluminum crucible and analyzed under nitrogen atmosphere and flow rate of 50 mL min−1. Experiments were conducted in the temperature range from 25 to 600 °C at a heating rate of 10 °C min−1 [17].
After weighing samples (2.0–6.0 mg) in aluminum crucible, DSC analyses were performed in a DSC-60 Plus equipment (Shimadzu) in the temperature range from 25 to 300 °C under the same conditions described for TGA. Mass losses recorded in the thermoscale allowed us to calculate the corresponding heat and were expressed in J g−1.
Spectroscopic profile in the infrared region (FTIR)
The Fourier-Transform Infrared Spectroscopy (FTIR) profiles of the same samples were recorded by a spectroscope, model IRPrestige-21 (Shimadzu). For this purpose, approximately 1.0 mg of sample was compressed in KBr pellets and scanned in the wavenumber range of 4000 to 400 cm−1, with a resolution of 2.0 cm−1 and scanning number of 20 scans [17].
Results and discussion
Characterization by HPTLC
Chromatographic analysis by HPTLC allowed characterizing the crude extract (CE) in terms of complexity and chemical composition. With the use of selective developing reagents, it was possible to detect the presence and distribution of different classes of compounds by TLC plating (Fig. 1, Table 1). When the VAS solution was used, the formation of characteristic purple bands of terpenes, steroids and fatty acids derivatives was evidenced by comparison with the patterns of β-amyrin selected as a standard and CE (Fig. 1a), which suggests the presence of low polarity constituents of the Theobroma grandiflorum lipophilic fraction, such as triglycerides and steroids [18]. When TLC plates were derivatized with the FBS solution, comparison between gallic acid (standard) and CE showed the formation of the typical brown bands of phenolic compounds (Fig. 1b). In addition, these phenolic compounds, when derivatized with the NP/PEG solution and visualized under ultraviolet radiation at 366 nm, showed the typical yellow bands of flavonoids, as revealed by the rutin and CE patterns (Fig. 1c). A previous study by Silva da Costa et al. [4] described the presence of high-value flavonoids such as quercetin and kaempferol in the cupuassu residue, whose presence confirmed its economic potential. On the other hand, the use of KOH (Fig. 1d) and Dragendorff reagent (Fig. 1e) for staining did not allow detecting the presence of coumarins and alkaloids. Resuming, the phytochemical study of the T. grandiflorum agro-industrial residue allowed detecting the presence of compounds belonging to important phytochemical classes such as terpenes, steroids, phenolic compounds and mainly flavonoids, which, based on a comparison with previous studies on this and other vegetable residues [4, 18, 19], highlights its economic potential and viability.
Chemical profile of crude extract (CE) by HPTLC. Legend: Plates derivatized with selective solutions for terpenes—purple color (a), phenolic compounds (b), flavonoids—yellow (c), coumarines—blue (d), alkaloids—orange (e) and antioxidant compounds (f). β-amyrin (b-A); crude extract (CE); gallic acid (A.G); rutin (Ru); esculin (Sc); brucin (Br) and ascorbic acid (A.A). (Color figure online)
Antioxidant tests on TLC allow in situ evaluation of the ability of the components of a mixture to scavenge or reduce reddish 2,2-diphenyl-1-picryl-hydrazine free radicals (DPPH·) into yellow-colored stable molecules of DPPH. Tests on CE indicated a strong antioxidant activity due to high-polarity components compared to the positive control (ascorbic acid) (Fig. 1f), which can be ascribed to the presence of phenolic compounds, mainly flavonoids, that have a well-known antioxidant action.
Characterization by RMN 1H
The RMN 1H spectrum was acquired with the aim of (a) confirming the HPTLC results, (b) carrying out a quantitative analysis by standardizing the integration of proton signals from functional groups of CE components, and then (c) expanding the information about the phytochemical classes present (Fig. 2).
The percent abundance of each signal was determined from the chemical deviation in the spectrum (Table 2). The region from 0.5 to 1.5 ppm, which corresponds to the nuclei of methyl (–CH3), methylene (–CH2) and methine (–CH) groups typically present in the aliphatic chains of terpenes, steroids and fatty acids, was the one with the highest relative abundance (36.02% of the total signal area), consistently with the CE lipophilic nature. Comparable abundance (34.07%) was noticed in the 3.0 to 4.5 ppm region, which is characteristic of hydrogens on carbons directly linked or close to heteroatoms (O and N) like the oxymethine ones of sugar units. Less abundances were detected for hydrogens on side carbons linked to sp2 carbons, oxygenated or nitrogenated carbons (21.01%), and mainly for olefinic hydrogens (6.13%), which is consistent with the results obtained by HPTLC and confirms the presence of terpenes and steroids. Another set of important signals, even though with lower intensity and relative abundance (2.75%), are those typical of hydrogen nuclei directly linked to aromatic carbons in the 6.0–9.5 ppm region, which is characteristic of aromatic hydrogens of A and B rings of flavonoids and other phenolic compounds such as those identified por Silva da Costa et al. in T. grandiflorum [4]. These findings agree with those of HPTLC, which allowed us to conclude that the ethanolic CE of CBS was composed predominantly of terpenes, steroids and phenolic compounds, mainly flavonoids (Fig. 2).
Thermoanalytical profile
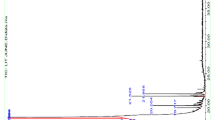
There is a huge number of studies in the literature on the use of the thermal analysis to (a) characterize materials of plant origin, (b) determine their thermal stability and (c) evaluate factors related to quality control during pre-formulation, formulation and storage steps of product to maintain its quality, effectiveness and safety. For this purpose, the TG/DTG profiles of butter, CSB, CE, microencapsulated crude extract of CBS (MECoC) and microparticles without extract (maltodextrin) are illustrated in Fig. 3.
TG/DTG curves obtained in the temperature range of 25 to 600 °C, at 10 °C min−1 under N2 atmosphere, and flow rate of 50 mL min−1. a butter, b cupuassu seed by-product, c crude extract, d microencapsulated crude extract of cupuassu seed by-product, e microparticles without extract (maltodextrin), f rutin
Figure 3a shows a single mass loss event in butter (92.63%) (Table 3) like that observed for cocoa butter [8], which was likely due to decomposition of fatty acids (mainly oleic, linoleic, palmitic and stearic acids). It should be remembered, in fact, that a significant percentage of crude oil remains in the residue after cold pressing [3]. Similar thermal behavior was found in cocoa seeds oil [10], oily Amazonian plant matrices such as Brazil nuts (Bertholletia excelsa) oil [11], pracaxi [Pentaclethra macroloba (Willd.) Kuntze] oil and murumuru (Astrocaryum murumuru Mart.) butter [12].
Figure 3b and c shows five mass loss events in the TG/DTG curves of both CSB and CE (Table 3). The first event, between Ton set = 51.35 and 58.94 °C and Tpeak = 89.30 and 80.01 °C, respectively, presented Δm = 5.92% and Δm = 14.44%, respectively, as the likely result of evaporation of water and other volatile compounds. These percentages are close to those of thermal decomposition of samples submitted to drying and standardization treatment. For instance, the CSB mass loss is close to that obtained by the gravimetric method (7.6 ± 0.4%) and to the moisture content reported for Arrabidaea chica [17]. However, the CBS decomposition temperature was lower than that of cocoa oil (Ton set and Tpeak of 77 and 168 °C, respectively), whose mass loss was about 4.8% [10]. In addition, both CSB and CE mass losses are in accordance with the recommended moisture content in crude materials of plant origin [13].
A second event occurred in both CBS and CE between Ton set of 223.52 °C and 103.04 °C and Tpeak of 260.13 °C and 120.82 °C, with Δm = 14.80 and Δm = 15.42%, respectively, which was followed by a third event with mass loss of 21.20% and 25.97%, respectively (Table 3). These major events can be related to progressive pyrolytic decomposition of organic compounds, i.e., carbohydrates, fibers, proteins and 8.0–25.0% of residual oil [9]. It implied the largest mass loss (36.0%) and resulted in a large endothermic Tpeak (21.83–39.38 °C) (Fig. 3b). CSB and CE also showed a fourth and fifth thermal events with Δm = 22.21 and 18.08% and Δm = 19.25 and 6.44%, respectively (Table 3).
Figure 3d and e, which depicts the thermoanalytical curves of MECoC and maltodextrin, show four and three events of mass loss, respectively. The MECoC TG curve highlights an initial mass loss (3.97%) ascribable to loss of volatile compounds and dehydration of microcapsules, which was like that of maltodextrin. The second and third events caused an intense overall mass loss (39.71% as a whole), which can be attributed to simultaneous decomposition reactions corresponding to the overlap of MECoC and maltodextrin degradation (Fig. 3d). Even so, this loss was less than the corresponding one of maltodextrin (64.96%).
The last MECoC mass loss event (21.43%) was lower compared to that of maltodextrin (27.21%). Intense endothermic peaks followed by exothermic peaks were observed in the DTA curves of both MECoC (Fig. 3d) and maltodextrin (Fig. 3e), with their respective endothermic Tpeak appearing at 220 °C and 300 °C, respectively. Since maltodextrin was able to preserve the physicochemical properties of CE phenolic compounds (Fig. 3c), these results are in line with the high efficiency previously reported either for the same extract (88.4 ± 5.3%) [3] or for other plant extracts [10,11,12, 17], hence confirming the effectiveness of maltodextrin as wall agent to microencapsulate the CBS extract.
The thermal behavior of the microencapsulated extract of cupuassu with maltodextrin is similar to that observed by Sampaio et al. [17] for the dry extract of A. chica using the same coating agent. These authors found that the use of maltodextrin did not qualitatively change the nature of thermal events. Thus, the exothermic reactions observed in the DTG curve (Fig. 3d) are recurrent in the microencapsulated extract, although to a lesser extent, probably due to the interaction between its compounds with maltodextrin. In another study, a significant interaction between the dry extract of Myracrodruon urundeuva and maltodextrin was observed as the result of overlapping their degradation [20].
The TG curve of maltodextrin (Fig. 3e) shows a three-step mass loss similar to that of starch, from which this substance was obtained. The first decomposition step was due to the dehydration process, followed by stability up Tpeak to 189 °C. Once dehydrated, decomposition and oxidation of organic matter occurred in two consecutive reactions [21]. A second more pronounced thermal event occurred in the Ton set–Tpeak of 250–310 °C with a Δm = 64.96%. Similar results were obtained by Saavedra-Leos et al. [22], who attributed the mass loss to thermal decomposition of long molecular chains, polymerization processes and isomerization reactions associated with dehydration.
Figure 3f shows the degradation profile of rutin, which exhibited four events of mass loss like those observed in Fig. 3b and c for CSB and CE, respectively. Comparing the TG/DTG curves, degradation of cupuassu phenolic compounds was observed in the two first events of mass loss, which occurred in the Ton set = 96.52 °C and Tpeak = 140.59 °C (7.74%) and Ton set = 261.26 and Tpeak = 297.90 °C (30.41%). However, the latter led to a higher mass loss due to decomposition of rutin carbon chain, which can be explained by the presence of sugar in its structure. The last decomposition stage can be ascribed to carbonization of the material (Table 3). These results corroborate the work of da Costa et al. [21], who demonstrated, through the stoichiometry of rutin decomposition reaction, that the highest percentage of Δm = 30.5% was the result of decomposition of rutin’s sugar moiety.
Figure 4 shows the results of differential scanning calorimetry (DSC) of butter, CSB, CE, MECoC, microparticles without extract and rutin. It is possible to observe an endothermic Tpeak probably corresponding to cupuassu triglycerides melting, which occurred at 38.21 °C for butter (Fig. 4a), 39.38 °C for CSB (Fig. 4b), 75.17 °C for CE (Fig. 4c) and 65.46 °C for MECoC (Fig. 4d). The profile was quite similar among the samples, because the by-product still contained a significant percentage of lipids (8.0–25.0%), in agreement with the TG results (Fig. 3). However, relevant endothermic Ton set to Tpeak events occurred in CE (Fig. 4c) and MECoC (Fig. 4c) at 173.79–225.91 °C and 183.05–216.14 °C, respectively, due to the presence of a complex mixture of several other organic components of the extract, but mainly to the carbohydrate composition of maltodextrin.
MECoC (Fig. 4d) displayed a main endothermic Tpeak at 49 °C due to dehydration followed by a second endothermic event that happened simultaneously with decomposition, already evidenced by TG/DTG (Table 3), thereby corroborating a previous study that reported two endothermic transitions at 79 and 139 °C for cocoa extract [23]. However, these results differ from those of the microencapsulated extract of cocoa by-product, which showed three endothermic events at 71.5, 157.0 and 258.5 °C [10].
The DSC profile of maltodextrin showed a wide endothermic Tpeak up to 123 °C, which can be attributed to the presence of hydroxyls (Fig. 4e). The use of maltodextrin as a coating agent for the extract of CSB was necessary in view of the presence of non-hydrolyzable sugars [9], which give it hygroscopic characteristics. Da Costa et al. [3] and Freitas et al. [24] found that the higher the concentration of maltodextrin used in the drying process, the lower the hygroscopicity of the microencapsulated extract of the cupuassu by-product and the atomized ‘Pérola’ pineapple by-product, respectively. Looking at Fig. 4d and e it is possible to infer that maltodextrin did not promote any physicochemical interaction with the extract of CSB that could harm its chemical composition. Therefore, the favorable chemical properties of maltodextrin justify its choice as an ideal drying aid for extracts derived from fruit by-products, able to preserve their physicochemical properties.
The rutin DSC curve (Fig. 4f) showed three endothermic events, the first probably related to dehydration up to Tpeak = 130.69 °C (ΔH = − 168.35 J g−1), the second to melting at Tpeak = 197.19 °C (ΔH = − 38.76 J g−1), and the third one to thermal decomposition of rutin (Tpeak = 281.53 °C; ΔH = − 14.70 J g−1) (Table 3). These events, which confirm those evidenced by the TG/DTG curves (Fig. 3f), agree with the observations of da Costa et al. [21], who attributed the two initial events of rutin decomposition to the loss of two water molecules, making it anhydrous and allotropic. The endothermic Tpeak referring to rutin melting (Fig. 4f) was maintained in the DSC curves of CE (Tpeak = 225.91 °C; ΔH = − 30.36 J g−1) (Fig. 4c) and MECoC (Tpeak = 216.14 °C; ΔH = − 40.49 J g−1) (Fig. 4d), which is a strong indication of rutin presence and preservation in the different stages of CSB processing.
Spectroscopic profile by FTIR
Butter, CSB, CE, MECoC and maltodextrin, which showed significant interactions among constituents according to thermal analysis (Fig. 3), were submitted to infrared spectroscopy analysis (FTIR) to infer their chemical composition (Fig. 5).
The spectroscopic profile of butter in the IV region of FTIR (Fig. 5a) showed a typical carbonyl elongation band at 1743 cm−1, corresponding to the stretching vibration of lipid ester carbonyls, while the C–H stretching may have been responsible for that observed at 1464 cm−1, confirming the results of HPTLC (Fig. 1a). Whereas the bands in the 700–1500 cm−1 region suggest an interaction between cis-monounsaturated and saturated fatty acids, those in the 1110–1155 cm−1 one are characteristic of C=O vibration of aliphatic esters. These results, which confirm that butter was mainly made up of triacylglycerols, are consistent with those reported for buriti [12] and cocoa [10] oils along with the presence of palmitic, stearic, oleic and linoleic acids as the main constituents of cupuassu by-product [9] and cocoa butter [8] extracts.
The spectra of CSB (Fig. 5b), CE (Fig. 5c) and MECoC (Fig. 5e) showed an aggregate absorption band with stretches that occurred at 2931–2846 cm−1 and 3304 cm−1, 2918–2850 cm−1 and 3342–3358 cm−1, respectively, which can be attributed to vibration of axial deformation of OH, mainly due to carbohydrates, lignin phenols, aromatic aminoacids or residual water [17]. On the other hand, stretches at approximately 2850 and 2364 cm−1 in the CE spectrum are indicative of axial deformation of accumulated double bonds and triple bonds [25], while those at 1668, 1120 and 1037 cm−1 of vibrations of esters, alcohols and sulfonic acids, respectively. The additional absorption band close to 725 cm−1 highlights the presence of aromatic compounds. Finally, the CE spectrum showed an increase in the band signal intensity compared to CSB, suggesting that extraction with the ethanolic solution led to concentration of chemical compounds.
In all cupuassu by-product-containing samples, a strong absorption band was observed at 2860 cm−1 and in the range 2929–2919 cm−1, which can be attributed to symmetric and asymmetric stretching vibrations of methyl groups, respectively, while bands at 2337 and 2344 cm−1 to vibrations of axial deformation of non-accumulated double bonds, corresponding to –CH stretching.
The absorption band around 1008 cm−1 corresponds to vibrations of esters and sulfonics, alcohols and acids. A 10 to 20 cm−1 displacement of this band toward lower frequency due to conjugation can be ascribed to the presence of aromatic rings [25].
Lastly, the bands appearing in the MECoC spectrum (Fig. 5e) at 1626 and 1031 cm−1, like those observed for microparticles without extract (Fig. 5d), are likely to correspond to the C=O stretching of maltodextrin bond. Instead, those appearing at 3372, 2924 and 1597 cm−1, like those observed for CE (Fig. 5c), can be ascribed to the phenolic group and the aromatic ring [26]. These results prove the effectiveness of the FTIR analysis as a tool to check the incorporation of active principles by the polymer matrix, which was hoped for during the pré-formulation phase [27].
Conclusions
The ethanolic extract of cupuassu seed by-product (CSB) obtained by cold pressing of the fruit was submitted to RMN 1H spectroscopy and HPTLC, which revealed that it was mainly made up of terpenes, steroids and phenolic compounds, especially flavonoids. On the other hand, thermoanalytical techniques were successful in exploring the thermal behavior of butter, CSB, crude extract (CE) and microparticles either containing the extract (MECoC) or not (maltodextrin), proving to be an efficient tool to check the stability of chemical components present in the samples. Butter, CSB and CE spectra showed mass loss events characteristic of decomposition reactions of lipids, still present in the pressed material, along with the thermal stability of bioactive compounds up to 189 °C. On the other hand, the MECoC mass loss events confirmed the excellent features of maltodextrin as a wall agent to microencapsulate the cupuassu extract by spray drying. FTIR spectra of CSB, CE and MECoC in the IV region showed an aggregate absorption band that pointed to preservation of phenolic groups in these preparations, confirming the importance of FTIR as a tool to check the incorporation of active principles by the polymeric matrix. The results of this study demonstrate the potential of these analytical techniques in the evaluation of factors related to quality control in the stages of pre-formulation, formulation and product storage to maintain quality, efficacy and safety.
References
Carvalho JEU, Müller HC, Alves MR, Nazaré RFR. Cupuacuzeiro. Technical Note. Belém, PA, Brazil: Embrapa Amazônia Oriental; 2004;p. 1–3.
Pugliese AG, Tomas-Barberan FA, Truchado P, Genovese MI. Flavonoids, proanthocyanidins, vitamin C, and antioxidant activity of Theobroma grandiflorum (cupuassu) pulp and seeds. J Agric Food Chem. 2013;61(11):2720–8.
da Costa RS, Teixeira CB, Gabbay Alves TV, Ribeiro-Costa RM, Casazza AA, Aliakbarian B, et al. Optimization of spray drying conditions to microencapsulate cupuassu (Theobroma grandiflorum) seed by-product extract. Nat Prod Res. 2019. https://doi.org/10.1080/14786419.2018.1462178.
Silva da Costa R, de Farias Silva N, Gabbay Alves TV, Fernandes da Silva M, do Socorro Barros Brasil D, Ribeiro-Costa RM, et al. Antioxidant activity of an industrial cupuassu seed by-product: molecular modeling of phenolic compounds. Chem Eng Technol. 2019;42(2):397–406.
Aizpurua-olaizola O, Navarro P, Vallejo A, Olivares M, Etxebarria N. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food Chem. 2016;190:614–21.
Gabbay Alves TV, Silva da Costa R, Aliakbarian B, Casazza AA, Perego P, Carréra Silva Júnior JO, et al. Microencapsulation of Theobroma cacao L. waste extract: optimization using response surface methodology. J Microencapsul. 2017;34(2):111–20.
Saénz C, Tapia S, Chávez J, Robert P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009;114(2):616–22.
Jahurul MHA, Zaidul ISM, Norulaini NAN, Sahena F, Jinap S, Azmir J, et al. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J Food Eng. 2013;117(4):467–76.
da Costa RS, dos Santos OV, da Lannes SCS, Casazza AA, Aliakbarian B, Perego P, et al. Bioactive compounds and value-added applications of cupuassu (Theobroma grandiflorum Schum.) agroindustrial by-product. Food Sci Technol. 2020. https://doi.org/10.1590/fst.01119.
Gabbay Alves TV, da Costa RS, Gomes ATA, da Costa CEF, Perego P, Silva Júnior JOC, Converti A, Ribeiro-Costa RM. Quality control of Amazonian cocoa (Theobroma cacao L.) by-products and microencapsulated extract by thermal analysis. J Therm Anal Calorim. 2018;1:8–10.
Pena Muniz M, Ferreira dos Santos M, da Costa CE, Morais L, Lamarão MN, Ribeiro-Costa R, et al. Physicochemical characterization, fatty acid composition, and thermal analysis of Bertholletia excelsa HBK oil. Pharmacogn Mag. 2015;11(41):147–51.
Pereira Lima R, Souza da Luz PT, Braga M, dos Santos Batista PR, Ferreira da Costa CE, Zamian JR, et al. Murumuru (Astrocaryum murumuru Mart.) butter and oils of buriti (Mauritia flexuosa Mart.) and pracaxi (Pentaclethra macroloba (Willd.) Kuntze) can be used for biodiesel production: physico-chemical properties and thermal and kinetic studies. Ind Crops Prod. 2017;97:536–44.
Brazil. Farmacopeia Brasileira, volume 2/Agência Nacional de Vigilância Sanitária. Brasilia: Anvisa; 1959.
Aboulkas A, Hammani H, El Achaby M, Bilal E, Barakat A, El harfi K. Valorization of algal waste via pyrolysis in a fixed-bed reactor: production and characterization of bio-oil and bio-char. Bioresour Technol. 2017;243:400–8.
Ge Y, Sun M, Salomé-Abarca LF, Wang M, Choi YH. Investigation of species and environmental effects on rhubarb roots metabolome using 1H NMR combined with high performance thin layer chromatography. Metabolomics. 2018;14(10):1–11.
Salomé-Abarca LF, Pas JVD, Kim HK, Uffelen GAV, Klinkhamer PGL, Choi YH. Metabolic discrimination of pine resins using multiple analytical platforms. Phytochemistry. 2018;155(July):37–44.
Sampaio RCA, Da Costa RS, De Souza CRF, Duarte Júnior AP, Ribeiro-Costa RM, Da Costa CEF, De Oliveira WP, Converti A, Silva Júnior JOC. Thermal characterization of Arrabidaea chica (Humb. & Bonpl.) B. Verl. dry extracts obtained by spray dryer. J Therm Anal Calorim. 2016;123(3):2469–75.
de Oliveira TB, Genovese MI. Chemical composition of cupuassu (Theobroma grandiflorum) and cocoa (Theobroma cacao) liquors and their effects on streptozotocin-induced diabetic rats. Food Res Int. 2013;51:929–35.
Gabbay Alves TV, Silva da Costa R, Aliakbarian B, Casazza AA, Perego P, Pinheiro Arruda MS, et al. Bioactive compounds and antioxidant potential for polyphenol-rich cocoa extract obtained by agroindustrial residue. Nat Prod Res. 2019. https://doi.org/10.1080/14786419.2017.1399381.
da Silva Leite R, de Souza VG, de Souza Salvador I, de Oliveira AH, de Lima Neto A, Basílio IJLD, et al. Evaluation of compatibility between dried extracts of Myracrodruon urundeuva Allemão and pharmaceutical excipients by TG and DTA. J Therm Anal Calorim. 2018;133(1):633–9.
da Costa EM, Filho JMB, do Nascimento TG, Macêdo RO. Thermal characterization of the quercetin and rutin flavonoids. Thermochim Acta. 2002;392:79–84.
Saavedra-Leos Z, Leyva-Porras C, Araujo-Díaz SB, Toxqui-Terán A, Borrás-Enríquez AJ. Technological application of maltodextrins according to the degree of polymerization. Molecules. 2015;20(12):21067–81.
Lupo B, Maestro A, Gutiérrez JM, González C. Characterization of alginate beads with encapsulated cocoa extract toprepare functional food: comparison of two gelation mechanisms. Food Hydrocoll. 2015;49:25–34.
de Freitas EFM, de Lopes LL, de Alves SMF, de Campos AJ. Efeito da maltodextrina no sumo da polpa de abacaxi Pérola atomizado. Rev Ciências Agrárias. 2019;42(1):271–80.
Silverstein RM, Webster FX. Spectrometric identification of organic compounds. 7th ed. Rio de Janeiro: LTC; 2007.
Batista NN, de Andrade DP, Ramos CL, Dias DR, Schwan RF. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Res Int. 2016;90:313–9.
Circioban D, Ledeti A, Vlase G, Vlase T, Dehelean C, Ledeti I. Thermal stability and kinetic degradation study for dihydroartemisinin. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09902-6.
Acknowledgements
The authors would like to thank the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) (Grant No. 001) for the financial support of this work and for the sandwich Ph.D. fellowship of R.S.C, Process No 99999.003074/2015-03.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RSdaC and WBdeSP. The first draft of the manuscript was written by RSdaC and WBdeSP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva da Costa, R., Pinheiro, W.B.S., Arruda, M.S.P. et al. Thermoanalytical and phytochemical study of the cupuassu (Theobroma grandiflorum Schum.) seed by-product in different processing stages. J Therm Anal Calorim 147, 275–284 (2022). https://doi.org/10.1007/s10973-020-10347-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10347-0