Abstract
Rock phosphate is the fundamental component for the manufacture of phosphoric acid and phosphate fertilizers. The aim of this study is to predict how sample preparation of Kef Essennoun phosphate responds to heat treatment and to estimate the purity of the raw and calcined phosphates. The influence of temperature (600–900 °C) was evaluated. The evolution of thermal treatment and the characterization of the Kef Essennoun ore (Djebel Onk mine, Algeria) were carried out by various analytical techniques including X-ray diffraction (XRD), wavelength-dispersive X-ray fluorescence, Fourier-transform infrared spectroscopy, thermogravimetric analysis/differential thermal analysis, and scanning electron microscopy (SEM). The results showed that the raw phosphate is a mixture of six phases, of which carbonate hydroxyapatite [Ca10(PO4)3(CO3)3(OH)2] is the dominant phase. With heat treatment, at 700 °C, a fluorapatite [Ca5(PO4)3F] phase appeared, as confirmed by XRD analysis, with good crystallization indicated by SEM. The P2O5 content was increased from 28.389% in raw phosphate to 31.085% in the calcined product. The dissolution of the calcined phosphate at 900 °C was completed by HNO3 acid attack, and occurred rapidly at ambient temperature. The results show that the production of phosphoric acid by Ca5(PO4)3F was more easily achieved with optimized consumption of the acid attack.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Djebel Onk deposit in Algeria is a significant phosphate reserve. Djebel Onk is located approximately 100 km southwest of Tébessa, near the Tunisian border, with a capacity of about 2 billion tons of 53–61% tri-calcium phosphate (TPL) [1]. The current annual production is about 2.3–2.5 million tons of high-grade ore (25–26% P2O5). Organic matter is usually present in the phosphates in very small quantities, from 0.25 to 1%. Despite this low content, it hinders the purification of phosphoric acid. The main constituents of the organic matter in phosphate sediments include aliphatic and polynuclear aromatic hydrocarbon, lipids, fatty acids and humic compounds [2, 3]. The presence of organic matter in the phosphates is a major drawback because of the formation of foam that causes difficulties in separating phosphoric acid and calcium sulfate [4, 5], and it hinders the recovery of uranium. Among the processes proposed in the literature, the calcination process has very interesting advantages in terms of eliminating organic matter and also decomposing the gangue minerals (carbonate) to form CaO and MgO, which can be separated after calcination by extinction and washing [6].
In the present work, we consider calcining the natural phosphate in order to achieve both elimination of organic matter and changes by heat treatment, as considered by many authors [7,8,9]. The calcination of Kef Essennoun phosphate is studied in the temperature range of 600–900 °C. The evaluation of the calcination process is followed by characterization of the raw and calcined phosphate by various analysis techniques including X-ray diffraction (XRD), wavelength-dispersive X-ray fluorescence (WXRF), Fourier-transform infrared spectroscopy (FTIR), thermogravimetric analysis/differential thermal analysis (TG/DTA), and scanning electron microscopy (SEM). This process is necessary to obtain a suitable phosphate material for the production of light-green phosphoric acid, which in turn is used for the production of ultrapure phosphoric acid.
Experimental
Sample preparation
Phosphates used in this study came from the Djebel Onk deposit (Fig. 1), part of a succession of sedimentary layers formed at the end of the Cretaceous–Eocene. Djebel Onk is a limestone mountain range, about 20 km long. Phosphates are milled and sieved at a diameter of 80 μm.
Thermal treatment and characterization
Calcination was used to pretreat Djebel Onk phosphates. Traditionally, the heat treatment of phosphate ore involves heating the ore to a certain temperature in order to obtain a product with specific properties. The calcination method in this study was carried out by introducing samples of phosphate into a muffle furnace at fixed temperatures of 600 °C, 700 °C, 800 °C and 900 °C. The sample was then analyzed by XRD, WXRF, FTIR, and SEM, and %CaCO3 was calculated by determination of total limestone (Bernard calcimeter).
Simultaneous thermogravimetric (TG) and differential thermal analysis (DTA) of crude phosphate sample was carreid out on a Setaram Labsys TM TG-DTA16 instrument. The sample is introduced into a furnace in an inert nitrogen environment at a heating rate of 10 °C min−1. The temperature range employed was from ambient to 900 °C. The phase identification and the related properties of the calcined phosphates were investigated by XRD using a PANalytical X’Pert (Philips) instrument operated at 45 kV and 40 mA. All the data were processed by X'Pert HighScore Plus software with commercial databases [full width at half maximum (FWHM) deduction and peak identification]. The infrared spectra were recorded in the range of 400–4000 cm−1 with a Nicolet 380 FT-IR spectrometer.
Results and discussion
Composition of raw phosphate
The composition of the Djebel Onk phosphate is presented in Table 1. Chemical analysis as determined by WXRF shows that the content of P2O5 is 28.389% and CaO is 41.346%. The CaO/P2O5 mass ratio is a common measure of rock quality. The mass ratio for the raw phosphate was 1.456, which was increased to 1.784 for phosphate calcined at 900 °C. It is important that this value does not exceed the percentage required on the phosphates market [10]. The effect of temperature shows that the grade (P2O5 at.%) of phosphate ore increased for calcination at 900 °C, with MgO < 1%. Iron (Fe2O3) and aluminum (Al2O3) form phosphate sludge as the acid is concentrated to commercial concentrations, contributing to P2O5 losses and maintenance requirements (Fig. 2). As P2O5 concentrations increase, high iron content contributes to increased acid viscosity. Aluminum enhances the crystal properties; high aluminum content is associated with good filtration rates. Aluminum also reacts with fluoride ions to form aluminum fluoride complexes. Above a certain concentration, magnesium is linked to increased product acid viscosity and to the formation of struvite (MgNH4PO4·6H2O) when making ammonium phosphates (human kidney stones can also comprise struvite). In the industry, two ratios are used to describe these impurities, the iron–aluminum (I&A) ratio and the minor element ratio (MER): the lower the ratio, the purer the rock. I&A and MER are defined in the following equations:
The results in Table 2 show that the two values of I&A and MER calculated for the raw and calcined phosphates at 900 °C are very low, in the range of 0.036–0.038 and 0.070–0.078, respectively.
Thermal analysis
Many studies on natural phosphates have shown that when these materials are subjected to heat treatment, different mass loss as a function of temperature occurs [11]. A Labsys™ TG-DTA16 was used to sample 10–20 mg nacelle compound while also utilizing a high-precision microbalance. The nacelle is introduced into a furnace in an inert nitrogen environment with a heating rate of 10 °C min−1. The temperature range employed is from ambient to 900 °C. The results (Fig. 3) revealed three main successive mass losses. The first mass loss of 1.4% is observed between room temperature and 150 °C and is attributed to the loss of water (moisture). The second mass loss is observed between 220 and 550 °C, which can be considered as roasting [12]. It is attributed to the simultaneous loss of structural water and the decomposition of organic matter. [13]. It should be noted that it is 2.116% for the studied phosphates. The third mass loss, the most important, is recorded between 600 and 900 °C. It corresponds to the decomposition of calcite releasing CO2 and H2O. The importance of this loss is related to the abundance of dolomite cement (carbonated), estimated to be 6.123%. In the DTA curve, it was possible to observe three endothermic peaks at 97, 360 and 762 °C. They are respectively related to evaporation, melting and decomposition of organic matter, and the decarbonation reactions of endogangue (Fig. 3).
Structural characterization
XRD mineralogical analysis
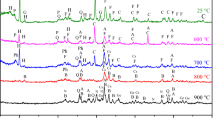
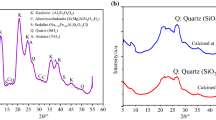
Figure 4 shows the XRD recorded at room temperature for the raw phosphate powder and for the powder calcined at different temperatures. The identification of the mineral phases of the samples showed the presence of the apatite mineral class, including hydroxyapatite [Ca9.74(PO4)6(OH)2.08], carbonate-hydroxyapatite [Ca10(PO4)3(CO3)3(OH)2] and carbonate-fluorapatite [Ca10(PO4)5CO3F1.5(OH)0.5]. Apatite mineral class phases are known by their non-neutrality due to the presence of carbonates in their structure and those of the matrix such as dolomite [CaMg(CO3)2], quartz (SiO2) and calcite (CaCO3) for raw phosphates [14, 15]. Carbonate-hydroxyapatite [Ca10(PO4)3(CO3)3(OH)2] is the dominant phase in raw phosphate. The ionic crystal belongs to the space group P63/m, and diffraction patterns are indexed according to JCPDS card 19-0272 [16, 17]. The spectra are similar and have the same hexagonal structure regardless of the temperature. We also note that the intensity is proportional to the temperature increase. This is due to the crystallization of phosphate calcined in the temperature range of 600–900 °C and the appearance of a very stable phase that is fluorapatite [Ca5(PO4)3F], as shown in Fig. 5. The results confirm the structural rearrangement and the decomposition of the carbonates included in the phases. However, with the presence of carbonate, the 211 and 112 reflections are superimposed in the raw phosphate and the phosphate calcined at 600 °C. The diffractograms of the phosphates show fine lines. In order to obtain more detailed structural information, the crystallite sizes (D) of fluorapatite at different temperatures were calculated from the full width at half maximum (FWHM) of all the diffraction peaks in the XRD patterns using the Scherrer formula [18, 19] as given below:
Here K is the Scherrer constant, which is its good approximation value is 0.9, λ (= 1.54059 Å) is the wavelength of CuKα radiation, \(\beta_{\text{sample}} = \sqrt {\beta_{\exp }^{2} - \beta_{\text{ins}}^{2} }\), βexp is the FWHM in all (hkl) reflections, βins is the correction factor for instrument broadening and θ is the diffraction angle.
The crystallographic parameters of the phosphate rock and of phosphate calcined at different temperatures are presented in Tables 3 and 4. The crystallite size increases with the increase in temperature from 48.6 nm at 600 °C to 74.4 nm at 800 °C and then remains constant. It was also found that the strain decreased with the increase in temperature (Fig. 6). The results confirm the rearrangement among the Ca9.74(PO4)6(OH)2.08, Ca10(PO4)3(CO3)3(OH)2 and Ca10(PO4)5CO3F1.5(OH)0.5 to give the fluorapatite Ca5(PO4)3F. For the latter component, several applications in different areas are known [20].
FTIR methods
FTIR spectroscopy analysis reveals several bands (Fig. 7), in particular those due to phosphates, carbonates and hydroxyapatite ions [21, 22]. The bands due to PO43− ions are observed at 1045.7 cm−1. The symmetric and antisymmetric adsorption bands of PO −34 groups lie between 1100 and 950 cm−1, and those of strain are between 571 and 607 cm−1 [23]. The peak obtained at 1428.5 cm−1 is the same observed for the fluorapatite phosphate carbonates, as indicated by Bonel [24]. The peak at 868.3 cm−1 corresponds to elongation of P–OH (HPO −24 ), and that at 3409 cm−1 is attributed to the vibration of hydroxyl ions OH−.
After heat treatment of phosphate at different temperatures from 600 to 900 °C, the characteristic bands of hydroxyapatite disappeared (Fig. 5), which indicates the change in its internal structure of the apatite phases. In addition, the band intensity for carbonate at 1400–1500 cm−1 becomes wider, and the carbonate peak at 873 cm−1 decreases dramatically, which indicates that a small amount of carbonate is retained in the sample.
Figure 8 shows the FTIR spectra of the phosphate samples heated at different temperatures of 600, 700, 800 and 900 °C for 4 h.
SEM analysis
SEM was utilized to investigate the morphology of the phosphate. At a scale of 10 µm, the crude beige phosphate is in the form of grains surrounded by a matrix (Fig. 9). The thermal effect on the calcined phosphates gave a well-defined morphology by way of example, and at 900 °C, phosphates become aggregates of rods. Thus, monitoring the organic matter contained in the phosphates before and after calcination at temperatures of 600 and 900 °C clearly shows the results found by energy dispersive X-ray analysis (EDAX), confirming the thermal effect on the removal of organic and inorganic material. This confirms the results of the thermogravimetric analysis on the one hand, and on the other hand the appearance of the elements which form the phases of the phosphate. The elemental mapping of phosphates calcined at 900 °C (Fig. 10) shows the distribution of elements including P, Ca, Si and O, confirming the results of XRD.
Bernard calcimeter
During the production of phosphoric acid by the wet process, carbonates react with sulfuric acid according to the following reaction:
During the reaction, the carbon dioxide produced is responsible for the foam and the production of small gypsum crystals, which can cause binding of the filter downstream, and consequently the production of phosphoric acid of low quality [25]. The volume of gas in the slurry must be controlled in order to control the reaction, which is achieved with antifoaming agents. In turn, these agents can cause problems downstream in solvent extraction units. Thus, the prior removal of this compound is an important step. The dissociation of the carbonates is an endothermic reaction, which requires high heat consumption. The proposed cleavage reaction [26] is as follows (5):
The dosage of calcite in raw phosphate and phosphate calcined at different temperatures for 4 h was assessed using a Bernard calcimeter according to the French standard NF P 94-048 [27]. The results obtained are shown in Fig. 11. We note that the percentage of CaO gradually decreases with increasing temperature. We obtain the best results for the removal of CaCO3 at a temperature of 900 °C. This result supports the fabrication of phosphoric acid by the wet process, as it enables reduced consumption of sulfuric acid to produce phosphoric acid of high quality. Phosphoric acid is considered the second mineral acid produced after sulfuric acid. The bulk of phosphoric acid production (90%) is dedicated to chemical fertilizers [28]. According to the literature, carbonates degrade differently during heat treatment. Degradation temperatures of some carbonates are shown in Table 5.
Tests of dissolution of calcined phosphates
The acid attack of phosphates has been the subject of several studies [30, 31]. In this study, the dissolution of 0.5 g of calcined phosphates at 900 °C with nitric acid attack carried out at ambient temperature and under stirring showed total dissolution. These results have important implications for the production of phosphoric acid by eliminating the problems caused by organic matter and carbonates, and also for optimization of phosphoric acid production conditions.
Conclusions
This study of the calcination of Kef Essennoun phosphate allowed several conclusions to be drawn:
-
Kef Essennoun raw phosphate is a mixture of six phases, of which the predominant phase is carbonate-hydroxyapatite. XRD analysis of the calcined phosphates showed the effect of temperature on the phase structures of the phosphates.
-
The formation of fluorapatite Ca5 (PO4)3F was very stable initially at 600 °C. The disappearance of the secondary phase improved the crystallinity of the Ca5(PO4)3F, and the increase in the crystallite size of Ca5(PO4)3F with temperature.
-
TG/DTA analysis confirmed the results obtained by EDAX and by the Bernard calcimeter. The elimination of organic matter and calcite in response to future challenges in the phosphate industry will greatly assist the economical production of high-quality phosphoric acid with low consumption of acid. The process consists of optimizing the acid attack of phosphates using the Ca5(PO4)3F formed.
References
Slansky M. Géologie des phosphates sédimentaires. Mém BRGM. 1980;114:92.
Biswas DR, Narayanasamy G. Rock phosphate enriched compost: an approach to improve low-grade Indian rock phosphate. Biores Technol. 2006;97:2243–51.
Sugiyama S. Approach using apatite to studies on energy and environment. Phosphorus Res Bull. 2007;21:1–8.
Blazy P, Jdid EA. Décarbonisation des phosphates sédimentaires par calcination dynamique. C R Acad Sci Paris. 1995;321:287–94.
Bounemia L, Mellah A. Effects of initial concentration and irradiation dose to degradation of di butyl phthalate from phosphoric acid (30 % P2O5). Radiochim Acta. 2018. https://doi.org/10.1515/ract-2018-2984.
Khoshjavan S, Rezai B. Beneficiation of refractory rock phosphate by calcination and flotation. Miner Metall Explor. 2011;28(4):187–92.
Abouzeid AZM, El Jallad IS, Orphy MK. Calcareous phosphates and their calcined product. Miner Sci Eng. 1980;12:3–83.
Becker P. Phosphate and phosphoric acid. In: Chapter 2, Process review. Marcel Dekker Inc; 1989. pp. 35–140.
Blazy P, Samama J-C. Évolution duCO2 lors de la calcination d’un phosphate apatitique suivie d’une carbonatation par CO2 gazeux. Earth Planet Sci. 2001;333:271–6.
Sis H, Chander S. Reagents used in the flotation of phosphate ores: a critical review. Miner Eng. 2003;16(7):577–85.
Malek N. Influence de la composition chimique et minéralogique du phosphate noirdu gisement de Djebel Onk sur le procédé de traitement. Ph. D. thesis, Université AMira de Bejaïa, Algeria; 2007.
Özer AK. The characteristics of phosphate rock for upgrading in a fluidized bed. Adv Powder Technol. 2003;14:33–42.
Merabet D, Benabdeslam N, Bezzi N, Ikhlef T, Arkoub H. Influence de la composition chimique et minéralogique du phosphate noir du gisement de Djebel Onk(Tébessa) sur le procédé de traitement. Ann Chim Sci Mater. 2004;29(5):69–85.
Abu-Eishah SI, El-Jallad IS, MuthakerM Touqan M, Sadeddin W. Beneficiation of calcareous phosphate rocks using dilute acetic acid solutions: optimisation of operating conditions for Ruseifa (Jordan) phosphate. Int J Miner Process. 1991;31:115–26.
Straaten PV. Rocks for crops: agrominerals of sub-Saharan Africa. Guelph: ICRAF; 2002.
Legeros RZ. Apatite crystallites: effects of carbonate on morphology. Science. 1967;155:1409.
Sakae T. Variations in dental enamel crystallites and micro-structure. J Oral Biosci. 2006;48(2):85–93.
Authier A. Early days of X-ray crystallography, OXFORD, ISBN 978-0-19-965984-5; (2013).
Hassanzadeh-Tabrizi SA. Low temperature synthesis and luminescence properties of YAG: Eunano powders prepared by modified sol-gel method. Trans Nonferrous Met Soc China. 2011;21:2443–7.
Leroy N, Bres E. Structure and substitutions in fluorapatite. Eur Cells Mater. 2001;2:36–48.
Silverstein M, Basler C, Morrill G. Identification spectrométrique des composés organiques. 2nd ed. Bruxelles: Deboock; 2007.
Rouessac F, Rouessac A. Analyse chimique. Méthodes et techniques modernes. 6th ed. Paris: Dunod; 2004.
Bezzi N, Aïfa T, Hamoudi S, Merabet D. Trace elements of Kef Es Sennoun natural phosphate (Djebel Onk, Algeria) and how they affect the various mineralurgic modes of treatment. Procedia Eng. 2012;42:1915–27.
Bonel G. Contribution à l’étude de la carbonatation des apatites. Ann Chim. 1972;7:127–44.
Heydarpour T, Rezai B, Gharabaghi MA. Kinetics study of the leaching of a calcareous phosphate rock by lactic acid. Chem Eng Res. 2011;89:2153–8.
Kunii D, Levenspiel O. Fluidization engineering. New York: Wiley; 1969. p. 1–63.
Afnor NF. Sols: reconnaissance et essais–Détermination de la teneur en carbonate–Méthode du calcimètre. 1996;11:94–048.
Belboom S, Szöcs C, Léonard A. Environmental impacts of phosphoric acid production using di-hemihydrate process: a Belgian case study. J Clean Prod. 2015;108:978–86.
Bisutti I, Hilke I, Schumacher J, Raessler M. A novel single dual temperature combustion method for the determination of organic, inorganic and total carbon in soil samples. Talanta. 2007;71:521–8.
Aouadi-Selmi H, Antar K, Khattech I. Thermochemical and kinetic study of the attack of fluorapatite by sulfuric acid solution at different temperatures. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-09044-4.
Soussi-Baatout A, Ibrahim K, Khattech I, Jemal M. Attack of Tunisian phosphate ore by phosphoric acid: kinetic study by means of differential reaction calorimetry. J Therm Anal Calorim. 2016;124:1671–8.
Acknowledgements
The authors are grateful for the financial support of this project by the Nuclear Research Center of Algiers (CRNA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bounemia, L., Mellah, A. Characterization of crude and calcined phosphates of Kef Essennoun (Djebel Onk, Algeria). J Therm Anal Calorim 146, 2049–2057 (2021). https://doi.org/10.1007/s10973-020-10167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10167-2