Abstract
Severe fire and explosions are frequent phenomena during handling of organic peroxides that are promoted supremely by conditions such as chemical impurities and thermal instability. As an initiator in the polymerization process, cumene hydroperoxide (CHP) has wide usage in the chemical process industry. This violently reactive chemical is studied here experimentally using differential scanning calorimeter (DSC), an isothermal mode of operation that can access the thermal hazards in the decomposition of CHP alone and later mixed with products following an autocatalytic reaction scheme. Importantly, DSC-evaluated thermokinetic parameters such as reaction enthalpy (ΔHd), time to maximum rate (TMRiso), and maximum heat flow (Qmax) were estimated to ascertain the degree of thermal hazard under various transportation and storage temperatures. The Heat-Wait-Search mode of accelerating rate calorimeter has been used to investigate decomposition kinetics parameters data under an adiabatic condition. Data such as initial exothermic temperature (T0), self-heating rate (dT/dt), pressure rise rate (dP/dt) and pressure–temperature profiles help to gauge the runaway reaction hazard of CHP alone and then mixed with its products to support the autocatalytic model of exothermic decomposition. The curve fitting data indicated that activation energy had reduced from 245.4 to 236.7 and 242.3 kJ mol−1, when CHP was mixed with acetone or dicumyl peroxide, respectively. The decrease in activation energy for autocatalytic material thermal decomposition reaction is depicted here with various experimental findings and mathematical analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive application and property of CHP
Cumene hydroperoxide (CHP) is an important intermediate product in the process of petrochemical production. It is normally produced via the cumene oxidation process and widely used in many industrial fields, as an initiator for polymerization that is used to yield the acrylonitrile–butadiene–styrene (ABS) resin and as the curing agent in radical polymerization process with unsaturated monomers. The participation is evident in a large number of reactions involving the formation of free radicals and subsequent production of market chemicals, such as phenol and acetone, which can be produced via the acid-catalyzed decomposition of CHP [1,2,3,4,5].
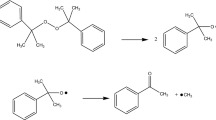
Phenol, an industrially important basic chemical, is widely produced by acidic cumene process acetone. Despite considerable efforts to develop and commercialize some new, one-step routes via partial benzene oxidation with O2, O3, H2O2, and N2O, the cumene process is still regarded as the most profitable. In sharp contrast with a two-step route via toluene, which renders about 5% of phenol, the cumene process provides currently more than 90% of phenol world production: in other words, 7 million tons per year. As a byproduct, moreover, acetone is produced abundantly in this process [3]. The base-catalyzed decomposition of CHP results in the formation of acetophenone, α-methylstyrene, dicumyl peroxide (DCPO), and α-cumylalcohol; furthermore, sodium hydroxide is often added to neutralize excess acid for the entire purification operation in the treating process of waste; the detailed process of CHP is shown in Fig. 1 [4,5,6]. In terms of the decomposition type, the thermal decomposition of CHP was a typical autocatalytic reaction, which was catalyzed by the product of the reactant. Fundamentally, CHP is also an organic peroxide that may cause runaway reaction; even severe fire and explosion incidents have happened due to its thermal instability and incompatibilities during transport, storage, and manufacturing processes [4, 7,8,9].
Multiple accidents analysis and relevant research summary of CHP
In the past few decades, there have been several accidents which resulted to be costly in terms of loss of human life and property in industrial premises. This has been due to negligence and stringent control measures over the production process parameters of CHP and complete lack of security management data. On the morning of September 9, 2002, an explosion and resulting fire broke out in the INEOS phenol plant in Mobile Alabama, USA. The main reason for the disaster was a failure in the emergency shutdown system, which caused the CHP feeding valve to remain open. Subsequently, an excessive amount of CHP had mixed with acid, resulting in the release of a lot of gaseous products and a subsequent runaway accident. On September 26, 2003, at the Changbin manufacturing district in Changhua, Taiwan, an explosion and fire occurred during the process of cumene oxidation to produce CHP in a batch reaction process, where sodium hydroxide was added as a catalyst. This process is an exothermic reaction with plentiful raw material and catalysts to react in a closed reactor tank. The reaction produces organic gas, and gradual heat accumulation was the primary cause of the explosion [10, 11]. Therefore, to avoid such industrial accidents triggered by fire or explosion, the thermal hazard parameter evaluation and reaction kinetics study of CHP had become a central issue to address. It has now been established as an useful integrated database in the process of petrochemical industry and academic fields.
Organic peroxides (OPs) are compounds that contain carbon and have at least two oxygen atoms linked together in the molecule. Oxygen is the primary source of the –O–O– linkages in OPs, whether they are prepared by direct air oxidation or by reactions of organic compounds with peroxide materials derived from oxygen, such as hydrogen peroxide, alkali metal peroxides, or ozone [12, 13]. Eventually, the thermal decomposition of CHP is an exothermic reaction involving the splitting of the peroxide bond with a huge heat release and gaseous product. Once the heat release rate in a reactive system exceeds the heat removed rate, a large accumulation of gaseous product would cause pressure increase sharply, which is the main reason for many accidents. In the past few decades, plentiful scholars had studied thermal hazards of CHP in various aspects and proposed that the thermal decomposition of CHP was caused by the cleavage of the peroxide bond and can result in fire and explosion under uncontrolled conditions [14, 15]. Levin et al. verified the influence of hydrogen ions on the thermal stability of CHP and concluded that the risk increased significantly with the increase in ion concentration [16]. Hou et al. analyzed the effects of several hydroxyl compounds used in the process on the stability of CHP and concluded that the potential thermal hazard increased when CHP was mixed with these hydroxyl compounds [17]. Talouba et al. studied the thermal stability of CHP by the calorimetric method [18]. The mechanism of the chain reaction of CHP was proposed under the isothermal condition, as represented in Fig. 2. Cao et al. evaluated the thermal hazard effects of CHP mixed with phenol and reported that the time to maximum rate is low while the maximum peak power is higher than pure CHP [19]. The simulated experimental curves also corroborated that CHP mixed with phenol has a significant potential hazard. All these studies were not comprehensive enough to investigate these accidents due to a scanty report of analysis on the runaway reaction of CHP mixed with products under the autocatalytic model [9, 19,20,21].
Research objectives and methods
Although previous studies have explored many aspects of CHP, there were still some gray areas which demand intervention. In particular, the effect of CHP mixed with its products under an acidic and alkaline environment on their autocatalytic reaction characteristics and thermal stability is not studied. Summarizing the experimentally obtained decomposition characteristics to safety recommendations for factual situations is generally a challenging procedure. In order to identify potential thermal hazards more accurately, this work intentionally investigated the effect of CHP alone and then mixed with products following the autocatalytic reaction model under both isothermal and non-isothermal conditions. Differential scanning calorimeter (DSC) and accelerating rate calorimeter (ARC) are used to obtain experimental data of thermal decomposition reaction. Afterward, the chemical kinetic parameters (reaction rate constant, frequency factor, and activation energy) are estimated through the Arrhenius equation and autocatalytic model. These parameters have a favorable significance in terms of calculating the thermal hazard data of CHP decomposition such as the time to maximum rate (TMR), no return temperature (TNR), and self-accelerating decomposition temperature (SADT) [22,23,24].
Experimental
Samples
Cumene hydroperoxide (CHP, 85.0 mass%, a light liquid) was purchased from Alsar (Massachusetts, USA), and acetone (99.5 mass%, a light liquid) and dicumyl peroxide (DCPO, 98.0 mass%, a white crystalline solid) were procured from Qiangsheng company (Jiangsu province, China). All samples were stored in a refrigerator at 4.0 °C to prevent the decomposition that might affect the experiments.
Differential scanning calorimetry (DSC)
DSC, a heat flux calorimeter, was manufactured by Mettler Toledo Company in Switzerland. As a widely used thermo-analytical technique, DSC is used to study phase transition, provides reaction kinetics, and supplies thermal stability information. Moreover, the STARe software was connected with DSC for data treating, and experiments were conducted in isothermal mode, in which this method had a long period to reflect commendably on the thermal stability of chemical substances. The experimental sample was kept on a gold-plated crucible for a period of time to observe the thermal decomposition reaction. The mass of CHP is 2.5 ± 0.1 mg, which was heated to 120.0, 125.0, 130.0, and 135.0 °C, and the product acetone or DCPO mixed with CHP as the reactant was 0.25 ± 0.01 mg under an isothermal temperature of 120.0 °C. They were held for 12.0 h to assure the sample decomposition completely for isothermal tests. The detailed experimental information on CHP alone and information on the other mixed with derivatives from DSC tests are the experimental results listed in Table 1.
Accelerating rate calorimeter (ARC)
ARC, a widely used calorimeter from the Thermal Hazard Technology Company of UK for estimating the explosive characteristics, has been used here. In this study, the Heat-Wait-Search (H-W-S) mode was selected. The temperature step of 5 °C min−1 and waiting time of 15 min were selected, and the detection sensitivity was set at 0.02 °C min−1. Essentially, ARC is an excellent insulation calorimeter which can analyze data to obtain decomposition kinetics parameters under an adiabatic condition, such as initial exothermic temperature (T0), self-heating rate (dT/dt), pressure rise rate (dP/dt), and pressure–temperature profiles.
Results and discussion
Analysis of DSC experimental
Dynamic experiment and thermal analysis of CHP
To conveniently obtain the decomposition temperature range of CHP, DSC under a scanning rate (β = 4 °C min−1) was applied in a dynamic condition. The thermal decomposition curve of CHP is shown in Fig. 3, and it demonstrated an obvious induction period in the initial stage. The entire exothermic curve was similar to a bell-shaped curve. This is an evident sign of autocatalytic reaction. From the curve, the onset temperature (Tonset) of CHP reads 148.7 °C and the peak temperature (Tpeak) is shown at 167.4 °C.
Thermal analysis of isothermal experiments and runaway reaction of CHP alone and then mixed with its products
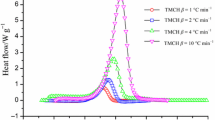
The thermal hazards of handling CHP alone and then mixed with its products are evaluated under isothermal conditions. Figure 4 shows that the decomposition curve of CHP has an obvious induction period of approximately 40 min when the isothermal temperature is at 120 °C. After the thermal induction, the reactants begin to undergo thermal decomposition. The products formed will catalyze the CHP as the reactant, continuing the reaction until it gets out of control. It is speculated that the change in enthalpy of the reaction is not an obvious tendency; the reason could be a low amount of sample and any other instrumental error. The large heat of accumulation during induction is usually ignored in actual industrial processes. This makes substances with autocatalytic reactions relatively more dangerous. On the other hand, as the isothermal temperature increased from 120.0 to 125.0, 130.0, and finally to 135.0 °C, the reaction time interval was brought forward from 39.93 to 19.03, 8.15, and 7.73 min, respectively. It is apparent from Fig. 4 that the reaction induction period decreased significantly due to isothermal temperature increase, and subsequently, the curves became steep and sharp. The reaction rate constant and peak power increased with the rise of temperature after a period of time. On the contrary, when the temperature was low, the wave crest appeared late and is relatively flat. This kind of reaction is a typical autocatalytic reaction, which was consistent with the analysis results in DSC dynamic experiments. Though high temperature was not attained during normal operation, it is important to pay attention to the risk of explosion and fire caused by the uncontrolled reaction of high temperature to materials of organic peroxides such as CHP in scenarios such as storage, transportation, and treatment.
For the accurate and safe study, Fig. 5 shows the effect of the products on CHP disintegration; the temperature of the incompatible experiments, in which CHP was mixed with acetone or DCPO in proportion, was set at 120.0 °C. The peak power of CHP alone and mixed with acetone or DCPO increased from 0.06136 to 0.06552 and 0.06485 W g−1, respectively. Meanwhile, Fig. 5a, b shows a phenomenon that the period time of CHP mixed with acetone or DPCO is shorter than that of CHP alone. These are all indicative of the thermal hazard of autocatalytic material that increases after products are added as a catalyst.
Kinetic analysis of isothermal experiments
The caution while handling a chemical manufacturing process can be studied more precisely by analyzing the reaction kinetics. By simulating the possible faulty operation under normal circumstances, the process risk can be quantified and the loss remedied can be taken as an important reference frame. The isothermal experiment can make the data more accurate and facilitate the simulation of a more realistic industrial production environment and data collection by reducing the impact of temperature function. The analysis of the isothermal experimental data and the theoretical model related to the reaction can be used to obtain the kinetic parameters such as the reaction rate constant (k), apparent activation energy (Ea), and frequency factor (A). The isothermal decomposition kinetics of CHP can be deduced from the relationship between temperature and peak power via TAM III [25]. The chemical kinetics equation under the autocatalytic model can be described by simple chemical equations as in Eqs. (1) and (2).
In the first step, the organic peroxide (A) decomposed into a free radical (B). Then, the radical (B) and the organic peroxide molecule interacted to generate more free radicals (B) in the second step. Alternatively, this study assumed that the initial decomposition reaction occurred at the same time, but its k1 value was very small, and its effect was to initiate the autocatalytic reaction.
In general, the Arrhenius equation was expressed as follows:
where k, Ea, R, and A represent reaction rate constant, apparent activation energy, molar gas constant (8.314 J mol−1 K−1), and the pre-exponential factor, respectively.
Taking the natural logarithm of the reaction rate at different temperatures produced the expression.
Assuming the reaction rate, k, to be proportional to the Qpeak (peak power) of the reaction process at this reaction temperature, Eq. (4) can be transformed into Eq. (5).
The values of ln Q versus 1/T are indicated in Fig. 6, using the equation described above under the isothermal conditions at 120.0, 125.0, 130.0, and 135.0 °C. The slope of its regression curve was –Ea/R. Hence, The Ea of CHP calculated was 76.0 kJ mol−1, and ln A was 20.47. Equation (6) can be solved and applied to predict the time to maximum rate under isothermal (TMRiso) condition at any other temperatures as appended as follows [16, 26].
The isothermal temperatures of CHP were substituted into Eq. (6) to obtain TMRiso as shown in Table 1. Obviously, the time to maximum under isothermal condition decreased with the rise of temperature, which indicated that TMRiso was negatively correlated with temperature. The results also indicated that the thermal hazard has increased with lower TMRiso and higher heat flow that could make the reaction dangerous.
Experimental analysis with ARC
The most possible risk of a thermal explosion is when the autocatalytic substances are placed in an adiabatic state such as a closed storage tank or reaction tank. The adiabatic thermal method is undoubtedly one of the best methods to study the thermal runaway reaction in the closed environment, and adiabatic calorimeter such as ARC can allow the reaction to safely proceed at thermal runaway stage that is usually characterized by high temperature and pressure, large heating value, and pressurization rates [26]. Onset temperature (Tonset), self-heating rate (rT), and pressure data obtained by ARC are the key parameters to evaluate the runaway hazard of exothermic decomposition reaction of CHP alone and then mixed with its products. Figure 7a reveals that Tonset of CHP alone and mixed with acetone or DCPO went from 120.5 to 115.3 °C and 110.5 °C, respectively. On the other hand, the peak pressure also rose from 10.3 to 13.5 and 14.7 bar, as shown in Fig. 7b. Figure 8a indicates that a sharp rise in the self-heating rate of CHP was observed throughout the exothermic interval and the process attained a maximum heat release rate of 661.2 °C min−1, but even more remarkable was the maximum heat release rate of CHP mixed with moderate DCPO that rises to 3726.8 °C min−1. The exothermic event was inevitably accompanied by a pressure rise. Figure 8b indicates that the pressure rise rate of CHP was compared with the experimental results of CHP alone and mixed with acetone or DCPO that went from 119.0 to 225.4 and 365.8 bar min−1, respectively. A high rate of pressure boost means an increase in the probability of an explosion in all prospects. A small range of temperature rise, which if ignored by field operators or custodians, can also be a risk factor for runaway events. It may be a worthwhile cause of fire or explosion accidents caused by human factors. Therefore, in the control of process safety and prevention, it is necessary to pay attention to the thermal runaway events caused by the self-decomposition reaction of autocatalytic materials, resulting in fire and explosion, even causing significant casualty and property losses (Table 2).
Calculation of thermal hazard parameters of ARC tests
In the ARC experiment, part of the heat released by the sample was inevitably used to heat the reactor. Therefore, the data obtained in the ARC experiment cannot be directly used as the final data for reference and calculation, and the test data need to be corrected with the thermal correction coefficient. The formula for thermal inertia (Φ) is as follows [26].
where Ms is mass of the sample, Mb is mass of bomb, Cvs is the average heat capacity of the sample, and Cvb is the average heat capacity of the bomb.
The rate constant increased exponentially with temperature, following the classic Arrhenius equation. Taking the logarithm of both sides of Eq. (3), Eq. (8) can be obtained as follows.
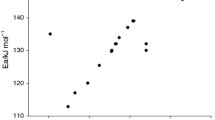
First-order (n = 1) model kinetics is assumed for decomposition of CHP and mixed with products. According to Eqs. (9) and (10), lnk versus inverse of temperature (T−1) is plotted, as shown in Fig. 9 [27].
The straight line confirmed the assumption made for the decomposition of CHP reaction as first-order kinetics. The slope of the plot was equal to −Ea/R. The activation energy and pre-exponential factor were calculated, and the value was 245.4 kJ mol−1 and 2.3 × 1029 s−1, respectively. Thus, the Arrhenius rate law for thermal decomposition of CHP can be given as:
The dynamic parameters of CHP mixed with acetone or DCPO obtained by simulation calculation based on Eqs. (8) and (10) are shown in Table 3. When the reaction absolute onset temperature of CHP was substituted into Eq. (11), the reaction rate constant k = 1.131 can be obtained. Similarly, the reaction rate constant of CHP mixed with acetone or DCPO was 1.132 and 1.148. Obviously, the reaction rate constant of CHP decomposition reaction and the potential risk of uncontrolled reaction increased after the addition of products. At the same time, the activation energy was found to be reduced from 245.4 to 236.7 and 242.3 kJ mol−1, respectively. The thermal decomposition reaction of autocatalytic materials is more likely to occur due to the decrease in activation energy. It is also indicative of the addition of the products of CHP decomposition such as acetone or DCPO that has pronounced effects on thermal hazard and is a necessary concern in the actual production process.
Conclusions
Thermal decomposition parameters such as Tonset, self-heating rate, and pressure data obtained by ARC were used as an index to measure hazardous nature of an autocatalytic reaction. It indicates the rapidity of the advent of a runaway situation when mixed with the reaction products by autocatalytic substances. DSC-evaluated parameters such as ΔHd, TMRiso, and Qmax were estimated for the degree of thermal hazard which can find usefulness in transportation and storage under 120.0, 125.0, 130.0, and 135.0 °C. The peak power of the reaction process is a quick and significant safety parameter to precisely evaluate the grade of thermal hazard under isothermal conditions. Ea and A were evaluated under various isothermal conditions through the Arrhenius equation with reaction rate and temperature. Thermal curves and experimental data provided evidence to show that the thermal hazard significantly increased with a decrease in activation energy when the CHP was mixed with acetone or DCPO.
Abbreviations
- A :
-
Pre-exponential factor (s−1)
- c vb :
-
Average heat capacity of the bomb (J g−1 K−1)
- c vs :
-
Average heat capacity of the mass (J g−1 K−1)
- dP/dt :
-
Pressure rise rate (bar min−1)
- dT/dt :
-
Self-heating rate (°C min−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- K :
-
Reaction rate constant
- M b :
-
Mass of bomb (mg)
- M s :
-
Mass of sample (mg)
- N :
-
Reaction order
- Q peak :
-
Peak power of the reaction (W g−1)
- R:
-
Molar gas constant (8.314 J mol−1 K−1)
- r T :
-
Self-heating rate (°C min−1)
- T :
-
Reaction temperature (°C)
- TMRiso :
-
Time to maximum rate under isothermal (min)
- T f,exo :
-
Final temperature of the exothermic reaction (°C)
- ∆Tad :
-
Adiabatic temperature rise (°C)
- ∆Tab :
-
Absolute temperature rise (°C)
- β :
-
Heating rates (°C min−1)
- Φ :
-
Thermal inertia (dimensionless)
References
Hou HY, Shu CM, Tsai TL. Reactions of cumene hydroperoxide mixed with sodium hydroxide. J Hazard Mater. 2008;152:1214–9.
Huang D, Han M, Wang J, Jin Y. Catalytic decomposition process of cumene hydroperoxide using sulfonic resins as catalyst. ChemEng J. 2002;88:215–23.
Lu KT, Luo KM, Lin SH, Su SH, Hu KH. The acid-catalyzed phenol-formaldehyde reaction critical runaway conditions and stability criterion. Process Saf Environ Protect. 2004;82:37–47.
Schmidt RJ. Industrial catalytic processes-phenol production. Appl Catal A Gen. 2005;208:89–108.
Sato H, Shimizu T. Marked effects of alcohols and imidazoles on the cumyl hydroperoxide reaction with the wild-type cytochrome P450 1A21. Arch Biochem Biophys. 1995;322:277–83.
Shen SJ, Wu SH, Chi CC, Horng JJ, Shu CM. Simulation of solid thermal explosion and liquid thermal explosion of dicumyl peroxide using calorimetric technique. Simul Model Pract Theory. 2011;19:1251–7.
Hou HY, Liao TS, Duh YS, Shu CM. Reactive incompatibility of cumene hydroperoxide mixed with alkaline solutions. J Therm Anal Calorim. 2006;85(1):145–50.
Huang D, Han M, Wang J. Catalytic decomposition process of cumene hydroperoxide using sulfonic resins as catalyst. ChemEng J. 2002;88:215–23.
Luttrell BW. Toxic tips: Phenol. Chem Health Saf. 2003;3:1074–9098.
Hsu JM, Su MS, Huang CY, Duh YS. Calorimetric studies and lessons on fires and explosions of a chemical plant producing CHP and DCPO. J Hazard Mater. 2012;17–18:19–28.
Iwata Y, Momota M, Koseki H. Thermal risk evaluation of organic peroxide by automatic pressure tracking adiabatic calorimeter. J Therm Anal Calorim. 2006;85:617–22.
Luo KM, Chang JG, Lin SH, Chang CT, Yeh TF, Hu KH, Kao CS. The criterion of critical runaway and stable temperatures in cumene hydroperoxide reaction. J Loss Prev Process Ind. 2001;14:229–39.
Zhu QC, Shen BX, Ling H, Gu R. Cumene hydrogenation over Pd/C catalysts. J Hazard Mater. 2010;175:646–50.
Ho TC, Duh YS, Chen JR. Case studies of incidents in runaway reactions and emergency relief. Process Saf Progress. 2004;17(4):259–62.
Kletz TA. Fires and explosions of hydrocarbon oxidation plants. Plant/Oper Progress. 1988;7:226–30.
Levin ME, Gonzales NO, Zimmerman LW, Yang J. Kinetics of acid-catalyzed the cleavage of cumene hydroperoxide. J Hazard Mater. 2006;130:88–106.
Hou HY, Su CH, Shu CM. Thermal risk analysis of cumene hydroperoxide in the presence of alkaline catalysts. J Loss Prev Process Ind. 2012;25:176–80.
Talouba IB, Balland L, Mouhab N, Abdelghani-Idrissi MA. Kinetics parameter estimation for decomposition of organic peroxides by means of DSC measurements. J Loss Prev Process Ind. 2011;24:391–6.
Cao CR, Liu SH, Das M, Shu CM. Evaluation for the thermokinetic of the autocatalytic reaction of cumene hydroperoxide mixed with phenol through isothermal approaches and simulations. Process Saf Environ Protect. 2018;117:426–38.
Chen KY, Wu SH, Wang YW, Shu CM. Runaway reaction and thermal hazards simulation of cumene hydroperoxide by DSC. J Loss Prev Process Ind. 2008;21:101–9.
Koltunov KY, Sobolev VI. Efficient cleavage of cumene hydroperoxide over HUSY zeolites: the role of Brønsted acidity. Appl Catal A Gen. 2008;336:29–34.
Duh YS, Kao CS, Hwang HH, Lee WL. Thermal decomposition kinetics of cumene hydroperoxide. Process Saf Environ Protect. 1998;76:271–6.
Stoessel F. What is your thermal risk. ChemEng Progress. 1993;10:68–75.
Wang YW, Shu CM, Duh YS, Kao CS. Thermal runaway hazards of cumene hydroperoxide with contaminants. IndEngChem Res. 2001;40:1125–32.
Liu SH, Shu CM, Hou HY. Application of thermal hazard analyses on process safety assessments. J Loss Prev Process Ind. 2015;33:59–69.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimetry. Thermochim Acta. 1980;37:1–30.
Andreozzi R, Marotta R, Sanchirico R. Thermal decomposition of acetic anhydride-nitric acid mixtures. J Hazard Mater. 2002;90(2):111–21.
Acknowledgements
The authors would like to express their sincere thanks to the Anhui University of Science and Technology in China under Contract Number QN201613 as well as to the Anhui Province Education Department, Natural Sciences Key Fund, China (Grant No. KJ2017A078).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, SH., Yu, CF. & Das, M. Thermal hazardous evaluation of autocatalytic reaction of cumene hydroperoxide alone and mixed with products under isothermal and non-isothermal conditions. J Therm Anal Calorim 140, 2325–2336 (2020). https://doi.org/10.1007/s10973-019-09017-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09017-7