Abstract
The biggest challenge in dispersion of nanoparticles in phase change materials (PCMs) is the physical stability of these particles in PCM. Numerous studies have evaluated the effect of different parameters on the stability of nanoparticles in PCM, but the effect of PCM polarity has rarely been investigated. In this study, the stability of functionalized and pristine multi-walled carbon nanotubes (MWCNTs) in three different PCMs with various polarity levels was investigated. The utilized PCMs were paraffin wax (nonpolar), stearic acid (partially polar), and polyethylene glycol (polar). Two different methods of functionalization of MWCNTs, with stearic acid and hexadecyl amine, were used to compare their stability in PCMs. The FTIR analysis and FESEM images reveal that the surface modification reactions were done thoroughly and MWCNTs are well dispersed in PCM. The results showed that pristine MWCNT is more stable in nonpolar PCMs (paraffin and stearic acid), while the samples containing functionalized MWCNT or surfactant had higher stability in polyethylene glycol. The three most stable samples were used to measure their thermal conductivity and heat release/storage capability. The addition of nanoparticles to all kinds of PCMs led to higher rate of heat storage and release. Moreover, the thermal conductivity of all PCMs improved by the introduction of nanoparticles. In both liquid and solid phases, nanoenhanced PCM had higher thermal conductivity compared to pure PCM with stearic acid containing 1 mass% of pristine MWCNT having the highest rate of enhancement at 16.82% for solid phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The limited fossil fuels resources and the resulted emissions from their consumption have made renewable energy sources like sun and wind a reliable source of energy. The intermittent nature of these sources makes it necessary to consider a storage system. One method of energy storage is storing thermal energy which is carried out in three different ways: sensible, latent, and thermochemical. Latent heat storage materials due to their unique characteristics have been widely used for various applications like buildings, automobiles, and solar water heaters [1,2,3,4,5]. Different advantages including easy controlling, low volume variation, and appropriate size have led to the wider usage of solid–liquid phase change than other kinds of phase changes. The biggest issue of these materials is their low thermal conductivity. In recent years, various methods have been utilized to enhance thermal conductivity and heat transfer in phase change materials. These methods include using fins, putting PCM in a porous environment, and dispersing high conductive materials into the PCM [6,7,8].
Several researchers have focused their studies on the dispersion of high conductive particles, especially nanoparticles, in PCM. The successful experience of using nanoparticles in fluids to make nanofluids was the main reason for incorporation of this idea for PCMs. Several studies have been conducted to evaluate the stability of nanoparticles in fluids and their effect of improving the thermal properties of the fluid. Cacua et al. [9] investigated the stability and thermal conductivity of TiO2/water nanofluid. They studied the effect of amplitude and duration of ultrasonication on the stability of nanofluids. They reported that the thermal conductivity of nanofluids increased as the temperature increased. Shamaeil et al. [10] found out that the thermal conductivity of nanofluids increases significantly as the concentration of nanoparticles in it increases. Mousavi et al. [11] prepared five types of water-based ternary hybrid nanofluids (THNFs) containing CuO/MgO/TiO2 nanoparticles and studied the effect of particles concentration and temperature on the thermophysical properties of the hybrid nanofluid. They realized that as the particles content and temperature increased, the thermal conductivity and viscosity of the THNF enhanced. Zadkhast et al. [12] also prepared a hybrid water-based nanofluid containing MWCNT and CuO nanoparticles and found out that the thermal conductivity of the nanofluid has a direct relationship with mass concentration of nanoparticles and temperature. Hassan et al. [13] found out that hybrid nanofluids have higher thermal conductivity and convective heat transfer rate compared to base fluids and single nanofluids. Khan et al. [14] compared the heat transfer enhancement in a channel by using different shapes of nanoparticles, meaning spherical, disk, and cylindrical. Raei et al. [15] evaluated the effect of using \(\gamma\)-Al2O3/water nanofluid in a double-tube heat exchanger on the heat transfer rate. They reported that nanofluids had higher Nusselt number compared to pure water.
Different nanoparticles including metallic nanoparticles and their oxides and carbon nanoparticles have been widely used to enhance thermal characteristics of PCM. Liu et al. [16] reviewed the studies that metallic oxides and carbon nanoparticles have been used by various researchers. Shah et al. [17] published a review article on the usage of nanoparticles for enhancing the properties of PCMs. Jegadheesvaran et al. [18] presented a comprehensive review of the properties of NePCM.
Metallic nanoparticles and their oxides due to their high thermal conductivity have had many applications in this context. Al Ghossein et al. [19] observed that by increasing the mass fraction of nanosilver in icosane, the thermal conductivity of icosane would be enhanced, while its latent heat and phase change temperature will decrease. Nanowires and nanotubes of MnO2 were dispersed in four different PCMs (paraffin wax, myristic acid, palmitic acid, and stearic acid) by Liang et al. [20] to improve their thermal properties. They observed that after 100 cycles, the latent heat of composites falls only by 2.8%. Mishra et al. [21] reported the thermal conductivity enhancement of phenol–water mixture by dispersing α-Al2O3, SiO2, hydrophobic SiO2, and TiO2 nanoparticles in it. Jeyaseelan et al. [22] used NaNO3/KNO3 (solar salt) as PCM and investigated the effect of adding Al2O3 and TiO2 nanoparticles on its thermal properties. They observed that by adding 3 mass% of Al2O3 and TiO2 nanoparticles, thermal conductivity of NaNO3/KNO3 was improved by 8.3 and 8.1%, respectively. Selvaraj et al. [23] reported that the thermal conductivity, thermal diffusivity, and latent heat capacity of polyethylene glycol could be enhanced by 15, 30, and 23% for using 2 vol% of BeO nanoparticles. The energy efficiency of a solar water heater in case of using no PCM, with PCM, and with NePCM was measured 58.74%, 69.62%, and 74.79%, respectively, by Kumar and Mylsamy [24]. Harikrishnan et al. [25] showed that both melting and solidification times of myristic acid could be reduced by introducing SiO2 nanoparticles in it. Suresh Kumar and Kalaiselvam [26] found out that adding CuO nanoparticles to palmitic acid decreases both melting and solidification times. Wei et al. [27] prepared microencapsulated PCM with Al2O3 nanoparticles as shell and observed that by using 8 mass% of Al2O3, the thermal conductivity of PCM increased by 287.4%. He et al. [28]
Carbon nanoparticles for their high thermal conductivity and low density are suitable for enhancing thermal properties of phase change materials. Various carbon nanoparticles like graphene, graphite, Gnp, xGnp, carbon nanofiber, and carbon nanotubes have been used in many studies [18,19,20,21,22,23,24]. Prabakaran et al. [29] studied the melting of a fatty acid PCM containing functionalized graphene nanoplateletes (GNPs). The thermal conductivity of PCM was enhanced by 102% when 0.5 vol% of nanoparticles were added. The viscosity of NePCM increased at higher volume fractions of nanoparticles. The thermal conductivity of paraffin was observed to increase linearly by Temel et al. [30] as the mass fraction of GNP dispersed in it increased. The latent heat of PCM decreased by 2.2, 8.6, and 15.6% when 3, 5, and 7% of GNP were added. They also reported that after 50 melting/solidification cycles, the composites remained stable. Hou et al. [31] investigated the effect of the addition of expanded graphite with three different volume expansion ratios at various mass fractions to sodium acetate trihydrate (SAT). They found out that by adding 5 mass% of expanded graphite, the thermal conductivity of SAT became three times higher. Although using expanded graphite with higher expansion ratio leads to higher heat transfer efficiency, it causes serious supercooling. Xia et al. [32] prepared composite PCM with n-octadecane and expanded graphite with different mass ratios. They found out that after 50 thermal cooling cycles, phase change temperatures and phase change enthalpy almost remain the same. Cheng et al. [33] used the binary eutectic mixture of tetradecanol–palmitic acid as PCM, expanded perlite as supporting material, and utilized carbon fiber for enhancing thermal properties of the mixture. The thermal conductivity of the PCM increased from 0.48 to 1.081 W m−1 K−1 in the presence of carbon fibers. Zhou et al. [34] also used expanded graphite to improve the thermal performance of adipic acid (AA) as PCM. They showed that the addition of expanded graphite resulted in significant enhancement of the thermal conductivity of AA, but as the content of expanded graphite increased, the latent heat and thermal conductivity of composites decreased. Gu et al. [35] reported that while the thermal conductivity of sodium acetate trihydrate as PCM containing expanded graphite nanoparticles could be enhanced to 1.589 W m−1 K−1, the latent heat of the composite is not much different of pure PCM. Yu et al. [36] investigated the impact of using expanded graphite for improving the thermal properties of stearic acid as PCM. They reported that after 500 and 1000 melting/solidification cycles, phase change temperature and latent heat of PCM just slightly changed which indicates a good cycle thermal stability.
Carbon nanotubes have unique mechanical, electrical, and thermal properties which makes them suitable for different applications. In recent years, nanotubes have been used in different studies to enhance the thermal conductivity of phase change materials. Multi-walled carbon nanotubes and carbon nanotubes with different mass fractions were dispersed in n-octadecane by Motahar et al. [37], and its thermal properties were investigated. They observed that the thermal conductivity of composite containing 0.5, 1, 2, and 5 mass% of nanotubes increased by 10, 18, 22, and 33% in solid phase and 15, 30, 38, and 45% in the liquid phase. Harish et al. [38] compared the variation of thermal conductivity of lauric acid in both solid and liquid phases by dispersing 1 vol% of graphite nanoplatelets, multi-walled carbon nanotubes, and single-walled carbon nanotubes and found that due to their higher aspect ratio and boundary heat conduction, graphite nanoplatelets have better performance in improving the properties of lauric acid. Salyan and Suresh [39] enhanced the thermal conductivity of mannitol (with a melting point of 168.38 °C) by 7.1% and 32.7% by adding 0.1 and 0.5 mass% of multi-walled carbon nanotube, respectively. Also, both complete charge time and discharge time reduce by 10.42% and 25.73%, respectively. The thermal conductivity of carbon foam saturated with pure PCM and PCM containing MWCNT in different mass fractions was compared by Nada and Al-Shaer [40]. They found out that the thermal conductivity of the composites enhances with the increase in MWCNT percentage. Temel et al. [41] studied the impact of the mass fraction and size of MWCNT on thermophysical properties of paraffin wax. They reported that using longer MWCNT with higher diameter has a better performance in enhancing the thermal conductivity of paraffin. Nanoencapsulated PCM was prepared using lauric acid and CNT by Wu et al. [42], and they realized that the thermal conductivity of lauric acid confined in CNTs is 20 times higher than that of pure lauric acid.
Dispersing nanoparticles in PCM uniformly and preventing them from agglomeration are a challenging task which has prevented their widespread usage in researches and industries. There are different methods for improving the stability of nanoparticles in PCM like using surfactants, functionalization of nanoparticles, ultrasonication, and homogenization.
Functionalization of nanoparticles is absorbing different functional groups such as hydroxyl, carboxyl, amine, and vinyl on the surface of nanoparticles. These functional groups which all of them contain oxygen could be absorbed on the surface of nanoparticles using mechanical, chemical, and electrochemical methods. The type and amount of functional group depend on the surface modification method. Meng et al. [43] have reviewed the methods used for functionalization of carbon nanotubes. Chen et al. [44] investigated modification of carbon nanotubes with stearic acid, and they observed that the dispersion of modified nanotubes was improved. Hasanabadi et al. [45] used an amine functionalization method for improving the stability of MWCNT in concrete. Sun et al. [46] studied the stability and thermal properties of polyethylene glycol (PEG) and carbon microspheres as a composite PCM. Carbon microspheres have rich oxygen-based functional groups which helps its uniform dispersion in PEG. The results showed that the thermal conductivity of PEG was enhanced by 65.07% and after 500 thermal cycles, no significant change in phase change temperature and latent heat was observed. Genc and Karagoz Genc utilized TiO2 nanoparticles and myristic acid for preparing form-stable microencapsulated PCM. They found out that the TiO2 shell can well prevent the leakage of myristic acid and the microencapsulated PCM has good thermal reliability. Feng et al. [47] prepared composites using polyethylene glycol as PCM and pure multi-walled carbon nanotube and functionalized multi-walled carbon nanotubes to improve its thermal characteristics. They observed that phase change enthalpy and temperature of composites containing functionalized nanotubes were lower than those of containing pristine nanotube. Shen et al. [48] used three different methods of acidification, mechanochemical process, and ball milling to modify the multi-walled carbon nanotubes and resulted that acidification is the best way of surface modification of nanotubes. Composites containing acidic nanotubes showed better cyclic stability and had a better performance in inhibition of subcooling in erythritol. Li et al. [49] used the acidification method to functionalize carbon nanotubes and found out that functionalized nanotubes are shorter than pristine ones and have better dispersibility and composites containing them have higher thermal conductivity. Putra et al. [50] used beeswax as PCM and different types of MWCNT to improve its characteristics. They used pristine, ball-milled, and acid-treated MWCNT. They observed that thermal conductivity and heat storage capability of composites improved compared to pure PCM.
By far, the effect of different parameters including nanoparticles type, volume concentration of nanoparticles, the shape of nanoparticles, preparation methods of NePCM, dimensions of nanoparticles, and using surfactant on stability and thermal properties of NePCM has been widely studied. However, there is lack of enough knowledge about the impact of the polarity of chosen PCM on the stability of nanoparticles in it. In this study, the stability of three different PCMs with different levels of polarity, paraffin wax (nonpolar), stearic acid (partially polar), polyethylene glycol (polar), containing pristine and functionalized MWCNT, which was investigated. Due to the widespread utilization of MWCNTs, the study has only been focused on this type of nanoparticles. Stearic acid and hexadecyl amine were used to prepare functionalized MWCNT. The stability of 15 different samples after consecutive melting and solidification cycles was analyzed. After identification of the most stable samples, thermal storage and conductivity of these samples were analyzed.
Mathematical
Mathematical description of PCM melting
Generally, the melting process of PCM without considering the shape of the container and boundary conditions is shown in Fig. 1. A container with an arbitrary shape contains the PCM with melting temperature of Tm, and the PCM at first was at solid state with the initial temperature of Tini. Since the phase change from solid to liquid is always followed by volume enhancement, a part of the container is always occupied with a gas. Depending on the physical conditions of the problem, the melting heat of PCM is supplied through the contact of the walls with a constant temperature surface or fluid (Th and Tc for hot and cold walls, respectively) or a constant heat flux (\(q^{{\prime \prime }}\)). All the other walls should be insulated. The melting front is at the temperature of Tm, and H length is a measure of phase transition from solid to liquid. As the molten zone grows bigger, natural convection increases and its direction depends on the gravitational force. Transition to natural convection is determined by Rayleigh number which is the ratio of buoyancy forces to heat penetration. Rayleigh number could be calculated using the following equation:
where \(\Delta T\) for the constant temperature difference boundary condition is \(T_{\text{H}} - T_{\text{C}}\) and for constant heat flux is \(\frac{{q^{{\prime \prime }} H}}{k}\).
Melting process of phase change materials without considering the boundary conditions and the shape of container [51]
Due to working with solid and liquid zones, modeling of solidification and melting processes is conducted with different methods. The heat transfer problem in melting and solidification problems is named boundary problem as the boundary between liquid and solid moves. Since the boundary of the two phases depends on the speed of latent heat absorbing or releasing, its location is not only a parameter and it is a part of the problem solution. The following equation which is known as Stephan problem describes this process:
In this equation, L denotes the melting latent heat, \(\rho\) the density, \(s\left( t \right)\) the location of the surface, k the thermal conductivity, t the times and T the temperature. In this situation, the place and velocity of the boundaries are unknown. Additionally, the difference in the physical properties of the two phases causes non-physical discontinuities in the numerical model which should be specified. Generally, there are two fundamental viewpoints for phase change modeling which are based on fixed grid and adaptive grid. These methods are described in detail in more relevant studies [52, 53].
Thermophysical properties of NePCM
The addition of nanoparticles to PCM affects its different thermophysical properties. The density specific heat, latent heat, and thermal expansion coefficient of NePCM could be calculated with the following equations based on the model of mixing [54,55,56,57]:
The model developed by Vajjha et al. [58] is used for the calculation of the dynamic viscosity and thermal conductivity of NePCM:
In this equation, K is the thermal conductivity, B is the Boltzmann constant equaling 1.381 × 10−23 J K−1, \(\zeta\) is the correction factor due to Brownian motion, and \(f\left( {T,\varphi_{\text{n}} } \right)\) is the following function:
Materials and characteristics
Materials
Stearic acid (melting point 55–62 °C), paraffin wax (melting point 55–62 °C), and polyethylene glycol (melting point 58–63 °C) all supplied by Merck Company were purchased to conduct comparative studies. Three kinds of carbon nanoparticles including pure multi-walled carbon nanotube (\(\varphi = 10 - 20 \,{\text{nm}}\)) and two kinds of functionalized carbon nanotubes with –COOH (\(\varphi < 7\,{\text{nm}}\)) and –OH (\(\varphi = 10 - 20\,{\text{nm}}\)) functional groups were also purchased to improve thermal properties of PCMs. All of these nanoparticles were supplied by the US Nano, with the density of 2.1 g cm−3 and length of 10–30 µm
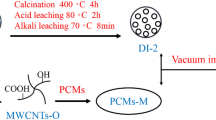
Functionalization of MWCNT surface with stearic acid
Figure 2 shows the process of functionalization of MWCNT with stearic acid. During esterification reaction between carboxyl functional group in stearic acid and the hydroxyl group in MWCNT, along with the production of water, the stearic acid will be absorbed to the walls of carbon nanotubes.
First, oxidized carbon nanotubes containing 1.76 mass% of the hydroxyl group are stirred by a magnetic stirrer (AMSTAT Basic, Indonesia) in deionized water in ambient temperature for 30 min. The mixture was ultrasonicated for 30 min (Elma S 30 H, Germany). In the following step, 10% of the mass of the mixture and five times of the mass of carbon nanotubes, sulfuric acid, and stearic acid were added to the mixture, respectively. After heating the mixture to 100 °C, the mixture is stirred by a magnetic stirrer for 4 h. To provide an inert environment and prevent oxidation of mixture vapors, nitrogen was used. The mixture vapors were also condensed in a two-shell heat exchanger and then entered the experiment cycle again. Figure 3 shows the schematic of the experiment.
The final mixture was washed with chloroform and hexane to remove residuals of stearic acid. In the final step, functionalized nanotubes were separated from the mixture using a filter paper and were put into an oven with 80 °C temperature for 8 h to completely dry.
Functionalization of MWCNT surface with hexadecyl amine
Figure 4 shows the process of functionalization of MWCNT with hexadecyl amine. During the acid and base reaction between the basic group in hexadecyl amine and acidic group (carboxyl) in MWCNT, in addition to producing water, the hexadecyl amine is absorbed to MWCNT’s walls.
In the first step, carbon nanotubes containing 1.23 mass% of carboxyl group were put in 1-Methyl- 2-pyrrolidone as solvents and then were stirred for 30 min by a magnetic stirrer. The solution was ultrasonicated for 30 min. Hexadecyl amine in the amount of five times of MWCNT’s mass was added to the mixture and then was put in a 110 °C constant temperature bath and was stirred with a magnetic stirrer for 48 h. Nitrogen was used to provide an inert environment and to avoid oxidization of the mixture vapors. A two-shell heat exchanger was also used to prevent the exiting of the mixture vapors from the experiment environment. In order to remove the remaining hexadecyl amine, the final mixture was washed with ethanol and chloroform. In the final step, the functionalized nanotubes were separated from the mixture with filter paper and were put in an 80 °C oven for 8 h to completely dry.
Preparation of NePCM
NePCM was prepared by the conventional two-step method. In order to better dispersion of carbon nanoparticles in high viscose organic PCM, chloroform with lower viscosity was used as the medium fluid. First, for the samples containing surfactant, sodium dodecyl benzene sulfonate (SDBS) was solved in chloroform as a surfactant and nanoparticles were dispersed in the solution by a magnetic stirrer and then were ultrasonicated. The PCM was melted entirely, and the mixture of chloroform, nanoparticles, and SDBS (in case of use) was added. A magnetic stirrer first homogenized the mixture in the melting point of the PCM, and then, the mixture was ultrasonicated. During this process, chloroform with a boiling point of 61 °C is evaporated entirely and removed from the mixture. Table 1 shows prepared samples with different compositions of PCM and nanoparticles.
PCMs were chosen based on their difference in polarity. That is, paraffin is nonpolar, stearic acid is relatively polar, and polyethylene glycol is polar. Among the nanostructures, carbon black and MWCNT are nonpolar and do not make ionic bonds with surfactants.
FTIR analysis
Fourier-transform infrared (FTIR) analysis was conducted in the frequency range of 400–4000 (1 cm−1), using Thermo AVATAR. Figure 5 shows the spectrums for HDA, MWCNT-COOH, and surface modified MWCNT using hexadecyl amine. In high frequencies, the 3250 and 3170 peaks in the spectrum of HDA are associated with asymmetric and symmetric tensile vibrations of the N–H graft in NH2 functional group, respectively. The 3956 peak represents the asymmetric tensile vibrations of the C–H graft in the CH3 group. The associated asymmetric and symmetric tensile vibrations of the C–H link in the CH2 group are indicated in strong peaks of 2919 and 2850. In low frequencies, the appeared peaks in the wavelength of 1467 and frequency range of 880–950 are related to the N–H link in NH2 group. The 1057 and 723 peaks are associated with tensile and rotational movements of C–N and C–H links, respectively. The wide peak in the spectrum of MWCNT-COOH which is located in the wavelength of 3432 is due to the tensile vibrations of the O–H link. As mentioned in the spectrum of HDA, the 2918 and 2855 peaks refer to tensile vibrations of the C–H link. The relatively sharp peak at 1626 is associated with tensile vibrations of C=C link in MWCNT and its COOH functional group.
Figure 6 shows the FTIR spectrum of stearic acid (SA), MWCNT-OH, and surface modified MWCNT using stearic acid (MWCNT-SA). The location of the peaks associated with C=H bond in the CH2 and CH3 functional groups of stearic acid was precisely similar to that of the spectrum of hexadecyl amine. The 1705 and 1464 peaks were attributed to the tensile vibrations of C=O link and transformation vibrations of CH2 and CH3 functional groups, respectively. The 1302 and 937 peaks were due to the vibrations of in-plane and off-plane links of OH functional group in stearic acid, respectively. The swinging vibrations of the OH functional group also appeared in the peaks 725 and 683.
By comparing the FTIR spectra of MWCNT before and after the experiments, it is clear that the associated peaks of OH link in carboxyl and hydroxyl groups (in the location of 3432 1 cm−1) are disappeared in both experiments which indicates that the reaction was done thoroughly and successfully.
FESEM
Field emission scanning electron microscopy (FESEM) images of MWCNT before and after surface modification by stearic acid with a magnification of 140,000 times using TESCAN, MIRA3, Czech Republic, are shown in Fig. 7. According to these figures, the volumetric density of carbon nanotubes has decreased after surface modification.
Experimental setup
Figure 8 shows the schematic of the temperature control circuit for conducting the melting and solidification experiments of samples. Melting process of the samples was carried out in a cylindrical constant temperature bath with a 28 cm diameter and 12 cm3 capacity. The water level in this bath was 20 cm. For keeping the temperature of hot water at a constant level, a Hanyoung-AX4-2A thermostat with a PID controller was used. The applied thermostat was 8-mm-diameter PT100 thermocouple with 0.1 °C accuracy, which relayed to a Hanyoung-HSR-2D202Z solid-state relay. This system was capable of providing a constant temperature water bath for an extended period. A mechanical stirrer guarantees the temperature uniformity of the bath. The results showed that the temperature difference in different sections of bath above the fan was always less than 0.2 °C.
Results and discussion
Sedimentation analysis
Figure 9 shows the PW01–PW06 samples after ten consecutive melting and solidification cycles. In each cycle, the samples were first put in the temperature of 60 °C for 10 min to be assured of their complete melting. Then, the samples were put in an environment with the temperature of 27 °C for 20 min to solidify completely.
Samples PW02–PW05 were prepared using nonpolar paraffin and polar functionalized MWCNT, while PW01 sample contains nonpolar nanoparticles (pristine MWCNT). More sediment and less physical stability of PW02–PW05 samples, as it is clear in Fig. 9, could be due to their polar–nonpolar composition. The nonpolar–nonpolar composition in samples PW01 has fewer sediment and has better physical stability. It is noteworthy that polarity of nanoparticles and base fluid were the dominant factors in the stability of these samples and other factors like using surfactant and surface modification of MWCNT compared to them do not have a considerable effect. Saydem et al. [59] also investigated the stability of MWCNT in paraffin wax. They reported that regardless concentration or dispersion method, after a number of heating/cooling cycles, nanoparticles deposited. Using surfactants, stirrer, and sonication extended the stability of composites for only a few more cycles. Zeng et al. [60] also observed that in a bottom-heated vertical cylindrical cavity, CNTs settled significantly after the third thermal cycle. These two papers show that the results of this study are in compliance with other studies.
Figure 10 demonstrates SA06–SA10 samples after ten consecutive solidification and melting cycles. In these cycles, the samples were kept in 65 °C to make sure of their melting. Then, the samples were kept in 27 °C to solidify completely.
According to Fig. 10, the stability of different nanoparticles in stearic acid was similar to paraffin. Polar nanoparticles (samples SA07–SA10) showed lower stability than that of nonpolar nanoparticles (samples SA06). This was due to the molecular structure of stearic acid. The molecule of stearic acid has a polar head (–COOH) with a molar mass of 45 g mol−1 and a nonpolar head (CH3[CH2]16) with a molar mass of 239 g mol−1 which makes the nonpolar part of stearic acid dominant and leads to a behavior like paraffin. The comparison of the stability of nonpolar nanoparticles in paraffin (sample PW01) and stearic acid (sample SA06) showed that these nanoparticles have lower stability in stearic acid because stearic acid is more polar than paraffin.
The notable point in these experiments was the difference between the mechanism of instability in paraffin and stearic acid. The dominant mechanism of instability of MWCNT in paraffin was sedimentation, and due to the density difference, paraffin and MWCNT were completely separated after a while. Instability in stearic acid is mostly in the form of agglomeration, and MWCNTs tend to form bigger particles. This phenomenon as it is evident in Fig. 10 makes the mixture heterogeneous. Agglomeration of particles together and increasing the size of particles led to sedimentation of them in the base fluid. Figure 10 shows that the nonpolar sample (SA06) experienced more sedimentation, whereas polar samples (SA07–SA10) have become more heterogeneous without being completely precipitated. This observation may be due to formation of ionic and hydrogen bond between polar nanoparticles and the carboxyl group in stearic acid. At first, when the particles were well dispersed in the base fluid, and their size was small, the associated force of these bonds overcomes the mass force of nanoparticles. However, with agglomeration of nanoparticles, the weak ionic and hydrogen force was not capable of overcoming the mass force, and sedimentation occurred.
Figure 11 demonstrates PEG11–PEG15 samples after ten consecutive solidification and melting cycles. Similar to other groups of samples, in every cycle, samples were put in 60 °C for 10 min to melt completely, and then, the samples were left for 60 min in − 5 °C to become solid completely.
Utilization of a completely polar base fluid like polyethylene glycol with a chemical formula of HO[C2H4O]nH was a better choice to show the effect of using surfactant and surface modification of nanoparticles on the physical stability of nanofluid rather than a nonpolar base fluid. In addition to the density difference between nanoparticles and the base fluid, another reason for the instability of PEG11 sample was the polar–nonpolar composition of the mixture of PEG and MWCNT. Utilization of functionalized MWCNT containing carboxyl group in sample PEG12 has led to a polarization of nanoparticles and improving their stability compared to sample PEG11. The formation of van der Waals bonds between polyethylene glycol and the carboxyl group of MWCNT makes the composite more stable. Adding SDBS as a surfactant in sample PEG13 has improved the stability of the mixture. SDBS is an ionic polymer and uses both electrostatic forces and spatial stability mechanisms to create repulsive forces between nanoparticles [1025]. As it is shown in Fig. 12, SDBS has a polar head and a long nonpolar tail. Formation of van der Waals bonds between the polar head of SDBS and the carboxyl group of MWCNT in addition to enhancing the electrical charge on the surface of the particles which leads to the creation of electrostatic repulsion between them creates spatial repulsion between them. This is due to the connection of long hydrocarbon tails.
The effect of surface modification of MWCNTs on their physical stability is well observed in PEG14 and PEG15 samples. The covalent bonds that are formed in surface modification reactions are immensely stronger than van der Waals bonds in PEG13 and show higher stability especially during consecutive solidification and melting cycles.
In the next stage, the three most stable samples, meaning PW01, SA06, and PEG15, were selected to compare their heat storage and release as well as their thermal conductivity with pure PCMs.
Thermal storage and release curves
The effect of adding 1 mass% of the MWCNT nanoparticles in paraffin, stearic acid, and polyethylene glycol (PW01, SA06, and PEG15, respectively) was estimated using thermal storage and release curves of pure PCM and nanoenhanced PCM.
A constant temperature bath (Isotech Jupiter Basic RS422, England) with the temperature range of − 50 to 150 °C was used. In each case, the sample which initially was at the ambient temperature was immersed in the 80 °C bath and its temperature variation over time was drawn. For recording the temperatures, a K-type thermocouple and a PICO-TC08 data logger were used. When the samples reached the temperature of 72 °C, the paraffin and stearic acid samples were cooled down to 40 °C. The polyethylene glycol samples were put in the temperature of − 25 °C. In this case, the tests continued until the samples reached the temperature of − 20 °C.
According to Fig. 13, the temperature of all samples increased slowly with the time. Pure paraffin and stearic acid reached their melting point after 273, and 164 s, while that time was 240 and 86 s for PW01, and SA06 samples, respectively. Although PEG15 and pure polyethylene glycol samples reached equilibrium temperature faster than all the other samples, they did not show any specific phase change temperature which was a drawback for them.
It is shown that the addition of MWCNT to paraffin and stearic acid led to the enhancement of heat transfer in them at the beginning of the phase change process. To reach 57 °C, pure paraffin and paraffin containing MWCNT needed 334 and 295 s, respectively. Therefore, the slope of temperature enhancement has increased by 11.7% due to the addition of MWCNT. For stearic acid, this slope enhancement is 37.6% as pure stearic acid and stearic acid containing MWCNT needed 234 and 143 s to reach 57 °C, respectively. In case of pure PEG and PEG containing functionalized MWCNT, the time for reaching 57.8 °C was 216 and 153 s, and the slope of temperature increase enhanced by 29.2%.
Based on the results of thermal release process (Fig. 14), pure paraffin wax and stearic acid needed 1120 and 1467 s to reach their solidifying temperature. Samples containing nanoparticle needed less time to reach the solidifying point so that it took 924, 1256, and 126 s for PW01 and SA06 samples, respectively. Polyethylene glycol samples were fastest to release heat, but they did not show a specific phase change temperature.
Figure 14 shows that adding nanoparticles does not have a significant effect on the changing of the slope of temperature reduction after finishing of solidification process, but solidification time in paraffin and stearic acid is reduced by 253 and 243 s, respectively.
In general, the thermal storage/release rates of all PCMs are improved by using nanoparticles.
Thermal conductivity
The thermal conductivity of the nanocomposites was measured by the hot wire method. KD2-Pro thermal property analyzer with the standard uncertainty of ± 3%, manufactured by Decagon, USA, was used to obtain the thermal conductivity of the samples at variant temperatures. The rate of energy storage and release was highly dependent on the thermal conductivity of the PCMs at both liquid and solid states. Figure 15 shows the variation of thermal conductivity via the temperature for pure PCMs and nano-PCMs. Paraffin, stearic acid, and polyethylene glycol as PCMs and their composites with 1 mass% of MWCNT (PW01, SA06, and PEG14, respectively) were applied. The thermal conductivities of paraffin and stearic acid and their composites changed slightly with the temperature at both liquid and solid states. A sudden increase occurred in thermal conductivity near the phase change temperature (about 60 °C) in paraffin and stearic acid and their composites. This higher thermal conductivity near the phase change temperature could be mentioned a desirable factor for TES applications of PCMs. This property may be related to the instability of solid crystalline structure and acceleration of the molecular vibrations in the matrix of the regular solid structure [61]. As can be seen, composites containing polyethylene glycol as PCM behaved differently. According to “Thermal storage and release curves” section, these composites have no specified phase change temperature; therefore, no sharp increase in their thermal conductivity curves was observed.
It is shown that the thermal conductivity curves of the nano-PCMs were higher than the curves of pure PCMs. The average thermal conductivity enhancement in both solid (30–50 °C) and liquid (70 °C) phases for various nanocomposites is also shown in Fig. 16. The maximum enhancement of thermal conductivity in solid and liquid phases occurs in SA06 and PEG15 with 16.83 and 16.58%, respectively.
Conclusions
In this study, two different methods for functionalization of CNTs have been used to enhance their stability in organic PCMs. Stearic acid and hexadecyl amine were reacted with MWCNTs to prepare functionalized MWCNT. The effect of using (SDBS) as surfactant was investigated. Three PCMs were chosen based on their polarity: paraffin wax (completely nonpolar), stearic acid (partially polar), and polyethylene glycol (completely polar).
Samples containing paraffin wax and functionalized MWCNTs or surfactant showed lower stability than that of containing paraffin wax and pristine MWCNT, due to their polar–nonpolar composition. The samples containing stearic acid as PCM also showed the same pattern. In these samples, the one that had pristine MWCNT showed better stability because of its nonpolar–nonpolar structure. In contrast, since polyethylene glycol was a complete polar substance, the samples containing functionalized MWCNTs or SDBS as surfactant showed better stability than that of containing pristine MWCNT.
In the next stage, the three most stable samples (PWo1, SA06, and PG15) were selected to measure their thermal storage and release properties as well as their thermal conductivity. Based on the thermal storage and release curves, the addition of nanoparticles to PCMs enhanced both storage and release rate of all composites.
The thermal conductivity of all samples was improved both in the solid phase and liquid phase. The highest enhancement in the solid state was recorded for stearic acid containing 1 mass% of MWCNT (sample SA06) with 16.83%, while the highest increase in liquid phase belonged to the composite containing polyethylene glycol and 1 mass% of MWCNT (sample PG15) with 16.57%.
Abbreviations
- k :
-
Thermal conductivity (W m−1 K−1)
- T :
-
Temperature (K)
- t :
-
Time (s)
- C p :
-
Specific heat (J kg−1 K−1)
- d :
-
Diameter (nm)
- g :
-
Acceleration gravity (m s−2)
- H :
-
Vertical length (m)
- Ra:
-
Rayleigh number
- Gr:
-
Grashof number
- Pr:
-
Prandtl number
- \(\beta\) :
-
Thermal expansion coefficient (K−1)
- \(\varphi\) :
-
Volume fraction of nanoparticles
- \(\rho\) :
-
Density (kg m−3)
- \(\zeta\) :
-
Correction factor
- \(\mu\) :
-
Dynamic viscosity (kg m−1 s−1)
- \(\nu\) :
-
Kinematic viscosity (m2 s−1)
- \(\alpha\) :
-
Thermal diffusivity (m2 s−1)
- s:
-
Solid
- l:
-
Liquid
- np:
-
Nanoparticle
- pcm:
-
Phase change material
- NePCM:
-
Nanoenhanced phase change material
- FESEM:
-
Field emission scanning electron microscope
- FTIR:
-
Fourier-transform infrared spectroscopy
- PEG:
-
Polyethylene glycol
- SA:
-
Stearic acid
- PW:
-
Paraffin wax
References
Alva G, Liu L, Huang X, Fang G. Thermal energy storage materials and systems for solar energy applications. Renew Sustain Energy Rev. 2017;68:693–706.
Jaguemont J, Omar N, Van den Bossche P, Van Mierlo J. Phase-change materials (PCM) for automotive applications: a review. Appl Therm Eng. 2017;132:308–20.
Ladekar C, Choudhary S, Khandare S. Experimental investigation for the optimization of heat pipe performance in latent heat thermal storage. J Mech Sci Technol. 2017;31(6):2627–34.
Manikandan S, Selvam C, Praful PPS, Lamba R, Kaushik S, Zhao D, et al. A novel technique to enhance thermal performance of a thermoelectric cooler using phase-change materials. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08348-9.
Swami VM, Autee AT, Anil T. Experimental analysis of solar fish dryer using phase change material. J Energy Storage. 2018;20:310–5.
Ibrahim NI, Al-Sulaiman FA, Rahman S, Yilbas BS, Sahin AZ. Heat transfer enhancement of phase change materials for thermal energy storage applications: a critical review. Renew Sustain Energy Rev. 2017;74:26–50.
Qureshi ZA, Ali HM, Khushnood S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: a review. Int J Heat Mass Transf. 2018;127:838–56.
Tao Y, He Y-L. A review of phase change material and performance enhancement method for latent heat storage system. Renew Sustain Energy Rev. 2018;93:245–59.
Cacua K, Murshed SMS, Pabón E, et al. Dispersion and thermal conductivity of TiO2/water nanofluid. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08817-1.
Shamaeil M, Firouzi M, Fakhar A. The effects of temperature and volume fraction on the thermal conductivity of functionalized DWCNTs/ethylene glycol nanofluid. J Therm Anal Calorim. 2016;126(3):1455–62.
Mousavi S, Esmaeilzadeh F, Wang X. Effects of temperature and particles volume concentration on the thermophysical properties and the rheological behavior of CuO/MgO/TiO2 aqueous ternary hybrid nanofluid. J Therm Anal Calorim. 2019;137(3):879–901.
Zadkhast M, Toghraie D, Karimipour A. Developing a new correlation to estimate the thermal conductivity of MWCNT-CuO/water hybrid nanofluid via an experimental investigation. J Therm Anal Calorim. 2017;129(2):859–67.
Hassan M, Marin M, Ellahi R, Alamri SZ. Exploration of convective heat transfer and flow characteristics synthesis by Cu–Ag/water hybrid-nanofluids. Heat Transf Res. 2018;49(18):1837–48.
Khan LA, Raza M, Mir NA, Ellahi R. Effects of different shapes of nanoparticles on peristaltic flow of MHD nanofluids filled in an asymmetric channel. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08348-9.
Raei B, Shahraki F, Jamialahmadi M, Peyghambarzadeh S. Experimental study on the heat transfer and flow properties of γ-Al2O3/water nanofluid in a double-tube heat exchanger. J Therm Anal Calorim. 2017;127(3):2561–75.
Liu L, Su D, Tang Y, Fang G. Thermal conductivity enhancement of phase change materials for thermal energy storage: a review. Renew Sustain Energy Rev. 2016;62:305–17.
Shah KW. A review on enhancement of phase change materials-A nanomaterials perspective. Energy Build. 2018;175:57–68.
Jegadheeswaran S, Sundaramahalingam A, Pohekar SD. High-conductivity nanomaterials for enhancing thermal performance of latent heat thermal energy storage systems. J Therm Anal Calorim. 2019;138:1137–66. https://doi.org/10.1007/s10973-019-08297-3.
Al Ghossein RM, Hossain MS, Khodadadi J. Experimental determination of temperature-dependent thermal conductivity of solid eicosane-based silver nanostructure-enhanced phase change materials for thermal energy storage. Int J Heat Mass Transf. 2017;107:697–711.
Liang W, Wang L, Zhu H, Pan Y, Zhu Z, Sun H, et al. Enhanced thermal conductivity of phase change material nanocomposites based on MnO2 nanowires and nanotubes for energy storage. Sol Energy Mater Sol Cells. 2018;180:158–67.
Mishra AK, Lahiri B, Philip J. Thermal conductivity enhancement in organic phase change material (phenol-water system) upon addition of Al2O3, SiO2 and TiO2 nano-inclusions. J Mol Liq. 2018;269:47–63.
Jeyaseelan TR, Azhagesan N, Pethurajan V. Thermal characterization of NaNO3/KNO3 with different concentrations of Al2O3 and TiO2 nanoparticles. J Therm Anal Calorim. 2019;136(1):235–42.
Selvaraj V, Morri B, Nair LM, Krishnan H. Experimental investigation on the thermophysical properties of beryllium oxide-based nanofluid and nano-enhanced phase change material. J Therm Anal Calorim. 2019;137:1527–36. https://doi.org/10.1007/s10973-019-08042-w.
Kumar PM, Mylsamy K. Experimental investigation of solar water heater integrated with a nanocomposite phase change material. J Therm Anal Calorim. 2019;136(1):121–32.
Harikrishnan S, Hussain SI, Devaraju A, Sivasamy P, Kalaiselvam S. Improved performance of a newly prepared nano-enhanced phase change material for solar energy storage. J Mech Sci Technol. 2017;31(10):4903–10.
Kumar KS, Kalaiselvam S. Experimental investigations on the thermophysical properties of CuO-palmitic acid phase change material for heating applications. J Therm Anal Calorim. 2017;129(3):1647–57.
Wei S, Duan Z, Xia Y, et al. Preparation and thermal performances of microencapsulated phase change materials with a nano-Al2O3-doped shell. J Therm Anal Calorim. 2019;138:233–41. https://doi.org/10.1007/s10973-019-08097-9.
He Y, Zhang N, Yuan Y, Cao X, Sun L, Song Y. Improvement of supercooling and thermal conductivity of the sodium acetate trihydrate for thermal energy storage with α-Fe2O3 as addictive. J Therm Anal Calorim. 2018;133(2):859–67.
Prabakaran R, Kumar JPN, Lal DM, Selvam C, Harish S. Constrained melting of graphene-based phase change nanocomposites inside a sphere. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08458-4.
Temel UN, Somek K, Parlak M, Yapici K. Transient thermal response of phase change material embedded with graphene nanoplatelets in an energy storage unit. J Therm Anal Calorim. 2018;133(2):907–18.
Hou P, Mao J, Liu R, Chen F, Li Y, Xu C. Improvement in thermodynamic characteristics of sodium acetate trihydrate composite phase change material with expanded graphite. J Therm Anal Calorim. 2019;137(4):1295–306.
Xia Y, Cui W, Zhang H, Zou Y, Xiang C, Chu H, et al. Preparation and thermal performance of n-octadecane/expanded graphite composite phase-change materials for thermal management. J Therm Anal Calorim. 2018;131(1):81–8.
Cheng F, Huang Y, Wen R, Zhang X, Huang Z, Fang M, et al. Preparation and characterization of form-stable tetradecanol–palmitic acid expanded perlite composites containing carbon fiber for thermal energy storage. J Therm Anal Calorim. 2019;136(3):1217–25.
Zhou W, Li K, Zhu J, Li R, Cheng X, Liu F. Preparation and thermal cycling of expanded graphite/adipic acid composite phase change materials. J Therm Anal Calorim. 2017;129(3):1639–45.
Gu X, Qin S, Wu X, Li Y, Liu Y. Preparation and thermal characterization of sodium acetate trihydrate/expanded graphite composite phase change material. J Therm Anal Calorim. 2016;125(2):831–8.
Yu H, Gao J, Chen Y, Zhao Y. Preparation and properties of stearic acid/expanded graphite composite phase change material for low-temperature solar thermal application. J Therm Anal Calorim. 2016;124(1):87–92.
Motahar S, Alemrajabi AA, Khodabandeh R. Enhanced thermal conductivity of n-octadecane containing carbon-based nanomaterials. Heat Mass Transf. 2016;52(8):1621–31.
Harish S, Orejon D, Takata Y, Kohno M. Enhanced thermal conductivity of phase change nanocomposite in solid and liquid state with various carbon nano inclusions. Appl Therm Eng. 2017;114:1240–6.
Salyan S, Suresh S. Multi-walled carbon nanotube laden with D-Mannitol as phase change material: characterization and experimental investigation. Adv Powder Technol. 2018;29:3183–91.
Nada S, Alshaer W. Experimental investigation of thermal conductivity enhancement of carbon foam saturated with PCM and PCM/MWCNTs composite for energy storage systems. Heat Mass Transf. 2019;55:2667–77. https://doi.org/10.1007/s00231-019-02610-4.
Temel UN, Kurtulus S, Parlak M, Yapici K. Size-dependent thermal properties of multi-walled carbon nanotubes embedded in phase change materials. J Therm Anal Calorim. 2018;132(1):631–41.
Wu S, Ma X, Peng D, Bi Y. The phase change property of lauric acid confined in carbon nanotubes as nano-encapsulated phase change materials. J Therm Anal Calorim. 2019;136(6):2353–61.
Meng L, Fu C, Lu Q. Advanced technology for functionalization of carbon nanotubes. Prog Nat Sci. 2009;19(7):801–10.
Chen C, Chen X, Xu L, Yang Z, Li W. Modification of multi-walled carbon nanotubes with fatty acid and their tribological properties as lubricant additive. Carbon. 2005;43(8):1660–6.
Hasanabadi S, Sadrameli SM, Soheili H, Moharrami H, Heyhat MM. A cost-effective form-stable PCM composite with modified paraffin and expanded perlite for thermal energy storage in concrete. J Therm Anal Calorim. 2019;136(3):1201–16.
Sun Q, Yuan Y, Zhang H, Cao X, Sun L. Thermal properties of polyethylene glycol/carbon microsphere composite as a novel phase change material. J Therm Anal Calorim. 2017;130(3):1741–9.
Feng L, Wang C, Song P, Wang H, Zhang X. The form-stable phase change materials based on polyethylene glycol and functionalized carbon nanotubes for heat storage. Appl Therm Eng. 2015;90:952–6.
Shen S, Tan S, Wu S, Guo C, Liang J, Yang Q, et al. The effects of modified carbon nanotubes on the thermal properties of erythritol as phase change materials. Energy Convers Manag. 2018;157:41–8.
Li M, Chen M, Wu Z, Liu J. Carbon nanotube grafted with polyalcohol and its influence on the thermal conductivity of phase change material. Energy Convers Manag. 2014;83:325–9.
Putra N, Rawi S, Amin M, Kusrini E, Kosasih EA, Mahlia TMI. Preparation of beeswax/multi-walled carbon nanotubes as novel shape-stable nanocomposite phase-change material for thermal energy storage. J Energy Storage. 2019;21:32–9.
Dhaidan NS, Khodadadi J. Melting and convection of phase change materials in different shape containers: a review. Renew Sustain Energy Rev. 2015;43:449–77.
Ziskind G. Modelling of heat transfer in phase change materials (PCMs) for thermal energy storage systems. In: Cabeza LF, editor. Advances in thermal energy storage systems. Elsevier: Amsterdam; 2015. p. 307–24.
Liu S, Li Y, Zhang Y. Mathematical solutions and numerical models employed for the investigations of PCMs׳ phase transformations. Renew Sustain Energy Rev. 2014;33:659–74.
Arıcı M, Tütüncü E, Kan M, Karabay H. Melting of nanoparticle-enhanced paraffin wax in a rectangular enclosure with partially active walls. Int J Heat Mass Transf. 2017;104:7–17.
Mahdi JM, Nsofor EC. Solidification enhancement of PCM in a triplex-tube thermal energy storage system with nanoparticles and fins. Appl Energy. 2018;211:975–86.
Parsazadeh M, Duan X. Numerical and statistical study on melting of nanoparticle enhanced phase change material in a shell-and-tube thermal energy storage system. Appl Therm Eng. 2017;111:950–60.
Said M, Hassan H. Effect of using nanoparticles on the performance of thermal energy storage of phase change material coupled with air-conditioning unit. Energy Convers Manag. 2018;171:903–16.
Vajjha RS, Das DK, Namburu PK. Numerical study of fluid dynamic and heat transfer performance of Al2O3 and CuO nanofluids in the flat tubes of a radiator. Int J Heat Fluid Flow. 2010;31(4):613–21.
Saydam V, Duan X. Dispersing different nanoparticles in paraffin wax as enhanced phase change materials. J Therm Anal Calorim. 2019;135(2):1135–44.
Zeng Y, Fan L-W, Xiao Y-Q, Yu Z-T, Cen K-F. An experimental investigation of melting of nanoparticle-enhanced phase change materials (NePCMs) in a bottom-heated vertical cylindrical cavity. Int J Heat Mass Transf. 2013;66:111–7.
Wang J, Xie H, Xin Z. Thermal properties of paraffin based composites containing multi-walled carbon nanotubes. Thermochim Acta. 2009;488(1–2):39–42.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjbar, S., Masoumi, H., Haghighi Khoshkhoo, R. et al. Experimental investigation of stability and thermal conductivity of phase change materials containing pristine and functionalized multi-walled carbon nanotubes. J Therm Anal Calorim 140, 2505–2518 (2020). https://doi.org/10.1007/s10973-019-09005-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09005-x