Abstract
Nowadays, various types of engine oils are widely used in lubricating and cooling internal combustion engines. In this study, the behavior of MWCNTs–SiO2 (30–70)/10W40 hybrid nanofluid as part of a new generation of engine oil is investigated experimentally. A mixture of SiO2, with 20–30 nm particle diameter, and MWCNT, with 3–5 nm inner and 5–15 nm outer nanoparticle diameter was dispersed into a base fluid of 10W40 engine oil. Then, the viscosity of the product was measured at nanofluid concentrations and temperatures, respectively, ranging from 0.05 to 1% and 5 to 55 °C, for different values of shear rate. Also, a sensitivity analysis to the solid volume fraction was performed at different temperatures. The results show that the behavior of the samples is well fitted with the pseudo-plastic Ostwald de Waele non-Newtonian model. The viscosity of the produced hybrid nano-lubricant is found to be 35% greater than that of pure engine oil. Because of the significant deviation between the measured viscosity and the values predicted by existing classical viscosity models, a new regression model is obtained. The R2 and adj. R2 for the model are computed as 0.988 and 0.977, respectively, signifying strong predictability with ± 3% margin of deviation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term nanofluid refers to a base fluid in which nanoparticles under 100 nm are suspended. The study of nanofluids has attracted considerable attention because of its wide applications, including cooling systems, energy harvesting, cooling electronic chips, heat exchangers, microelectronics, microfluidics, automotive industry, biomechanics, etc. [1,2,3,4,5]. According to the literature, researchers have attempted to better understand the underlying physics of thermophysical properties that distinguish nanofluids from other traditional fluids.
The dispersion of nano-additives within a base fluid is why nanofluids have higher thermal conductivity compared to traditional fluids and have become a popular subject of research [6,7,8,9,10,11]. On the other hand, this leads to viscosity enhancement of the mixture, which is an unfavorable effect. Rheological properties are as important as thermophysical characteristics. Viscosity is an important property of a nanofluid, indicating the resistance force against the flow. It plays an important role in convection heat transfer and the pumping power of systems. Clearly, the efficiency of devices is directly linked to fluid viscosity. Hence, there is compensation between the unfavorable increase in the viscosity and favorable thermal conductivity enhancement. Of course, a cooling device will be economically competitive in the energy market if it has low pumping power. Although an increase in viscosity is inevitable, fortunately, the rectifying effects of thermal conductivity, convection heat transfer coefficient, and the critical boiling heat flux are much larger. Seemingly, the production of such fluids has enormous potential for use in the next generation of cooling systems [3, 4, 12]. There are fewer published studies on nanofluids dealing with examining dynamic viscosity than thermal conductivity simultaneously [11]. Many classical correlations have been proposed to predict the viscosity of nanofluids; however, they are neither general nor usable for a wide range of nanofluids [13,14,15]. Almost all of them were obtained based on curve fitting over experimental data. Thus, contradictions among the models have been reported in the literature [15,16,17,18,19]. These might occur because of different nanoparticle sizes and morphologies, purity, uncertainties, the range of shear rate, agglomeration, suspensions, and/or preparation methods [11].
Rheological behavior of nanofluids
The rheology of nanofluids is an important issue in engineering applications since it defines the flow pattern and affects the pressure drop. Therefore, there is a need for accurate measurement of the rheological behavior of nanofluids. This can be done by examining the shear stress–shear rate dependency and studying the apparent viscosity of nanofluids.
Rheological studies show that multiphase flows can be classified into two classes of Newtonian and non-Newtonian fluids [1, 14, 16,17,18,19,20,21,22]. Additionally, a nanofluid might reveal Newtonian behavior at a low solid volume fraction, while it might be non-Newtonian at a high solid volume fraction. Some of the characteristics determining this behavior include the nanoparticle volume fraction, temperature, fluidity of base fluid, and size of nanoparticles [16, 23, 24]. In addition, the effect upon rheology of the use or non-use of surfactant in nanofluids must not be disregarded. A study was conducted on the viscosity of silicone oil suspended with TiO2 and SiO2 by Murshed et al. [23]. They showed that the shear stress of the investigated silicone oils varies linearly with the shear rate. In fact, the apparent viscosity was found to be shear rate independent, namely the investigated nanofluid shows Newtonian behavior. Similar results were reported earlier by Chen and Xie [24] for silicon oil-based MWCNT nanofluid. Another study was conducted on ethylene glycol-based titania nanofluids by Chen et al. [16]. They found that the viscosity varies strongly with temperature and solid volume fraction. The mixture displays non-Newtonian behavior in shear rates lower than 100 (s−1), beyond which Newtonian behavior becomes apparent [25]. The variations of viscosity with respect to solid volume fraction and temperature were experimentally studied by a number of researchers [26,27,28,29,30,31]. All of them have noted nonlinear relations for viscosity with nanofluid concentration and temperature. This is because of the interaction between the suspended nanoparticles.
Classic and empirical models
In spite of the presence of a large number of studies on the viscosity prediction, establishing theoretical correlations has been rarely investigated in the open literature due to their complexity [17, 32, 33]. It can be stated that the lack of such models to accurately calculate viscosity is very sensible. Therefore, researchers use those obtained by themselves via curve fitting to their experimental data, for calculating viscosity.
Einstein’s model is the oldest and the most well-known model among the theoretical models, which is the basic correlation for viscosity prediction [34]. The model was expressed based on a linear relationship between the viscosity and the volume fraction of suspended spherical nano-additives in diluted mixtures. Modified Einstein models were proposed by Frankel and Acrivos [35] and Nielsen [36], which are valid for low-concentration nanofluids. Mooney [37] made another modification for extremely diluted mixtures containing spherical particles. The Graham dynamic viscosity model extended the model of Frankel and Acrivos by considering the nanoparticle diameter, the distance between two nanoparticles, the van der Waals forces effect, and the Brownian motion of spherical nanoparticles [38]. The model already shows an excellent agreement with the Einstein model at low concentrations. Nowadays, concentrated suspensions are more used in engineering applications than diluted systems. De Bruijn [39] modified the Einstein model to provide a better agreement at higher concentrations. The Einstein model was also modified by Brinkman [40] for mixtures with a solid volume fraction larger than 4%. Taking the Brownian motion of spherical nanoparticles into account, Bachelor [41] proposed a model able to accurately predict the viscosity of high-concentration mixtures, even up to 10% solid volume fraction.
Considering the probability of forming agglomerates and appearing new structures within dispersed systems, Krieger et al. [42] established their power-law-based semi-empirical effective viscosity model as follows.
where μ (= 2.5) is the intrinsic viscosity of hard spherical particles, and the maximum concentration, \(\varphi_{\text{m}}\), is equal to 0.605 at high shear rates and takes values in the range of 0.495–0.54 in the quiescent condition. The above relation is usable for any volume fraction of monodisperse nanoparticles suspended in a base fluid.
Hemmat Esfe et al. [19] obtained a regression model for the viscosity prediction of nano-lubricant containing zinc oxide nanoparticles (ZnO) in terms of concentration (Tables 1, 2).
where the subscripts nf and bf denote nanofluid and base fluid, respectively, and \(\varphi\) denotes the nano-lubricant concentration. The constants \(a_{i}\) are calculated at distinct temperature intervals ranging from 5 to 55 °C in order to offer a more accurate model [19].
Nanofluids containing CNT
Nanofluids suspended with CNTs are the most popular among other types of nanofluids because of the high thermal conductivity of CNTs as compared to other nanoparticles. However, few studies have been conducted on viscosity measurement [16,17,18].
Estelle et al. [50] studied the effect of shear rate on the nanofluid flow behavior of water-based MWCNT. They reported that such a nanofluid shows viscoelastic and pseudo-plastic behavior at low and high shear rates, respectively. In another study, Halelfadl et al. [51] considered the impact of temperatures ranging from 0 to 40 °C in various MWCNT concentrations. A summary of rheological properties and important outcomes is gathered in Table 3. Some of the studies show a decreasing trend of viscosity with shear rate [52]; in contrast, an inverse trend has been reported by many studies [53,54,55]. Reference [56] reported that viscosity depends on the duration of sonication, so that the viscosity increases as sonication is increased, reaches a peak point, then decreases after about 7-min sonication.
Nanofluids containing silicon dioxide
Regardless of the properties of the base fluid, nanofluids containing SiO2 reveal Newtonian behavior at a vast range of shear rates. Richmond et al. [57] found that adding TiO2 nanoparticles to water-based SiO2 nanofluids change the shear stress–shear rate relation from linear to nonlinear. As a matter of fact, the suspension of SiO2 into any base fluid causes the mixture to behave as Newtonian [58, 59]. Rheological properties of various types of nanofluids containing SiO2 are listed in Table 4. Since the current study has mainly emphasized viscosity measurement, examining the viscosity of both the base fluid and the mixture shows how viscosity is affected by other factors.
The dispersion of CNT, SWCNT, and MWCNT in pure liquids has been the subject of numerous studies, due to their attractive thermophysical and rheological properties [60,61,62,63,64]. However, nanofluid merely suspended by CNT is not widespread or commonplace for industrial purposes because of its high production costs. One of the current challenges in front of scientists is the production of nanofluids for various applications. The simultaneous use of more than one type of nanoparticle might reduce production costs and provide better heat transfer efficiency and lubricating performance. This is why, in the last decade, studies have tended to focus on oxide nanoparticles, e.g., SiO2 and CNT as hybrid nanofluids.
Hybrid nanofluids
Given the importance of the rheological properties of water-based drilling fluids, Baghbanzadeh et al. [66] carried out a comparative examination of four different water suspensions containing SiO2, MWCNT, MWCNTs–SiO2 (30–70), and MWCNTs–SiO2 (50–50) nanoparticles. According to the results, the viscosity and density of nanofluids increase with concentration while decrease with temperature. It was shown that the viscosity of MWCNTs–SiO2 (50–50)/water nanofluid increases (at least up to 8.2%) at high nanofluid concentration (1 mass%). The results also indicated that the density of base distilled water of MWCNTs–SiO2 (80–20) nanoparticles was almost independent of solid volume fraction; the figure for MWCNTs–SiO2 (50–50) nanoparticles did not exceed of 0.9% even at high concentrations. The rheological behavior of Al2O3–MWCNT (65–35%)/5W50 hybrid nano-lubricant was studied by Hemmat Esfe et al. [67] to facilitate its use in the automotive industry. Aluminum oxide nanoparticles with an average diameter of 50 nm combined with MWCNTs with the internal diameter of 3–5 and an outer diameter of 15–15 nm. The dynamic viscosity of the hybrid nano-lubricant samples was measured for solid volume fraction, temperature, and shear rate, respectively, in the ranges of 0–1%, 5–55 °C, and 10.664–666.5 s−1. As a result, it turned out that the investigated hybrid nano-lubricant behaves as a non-Newtonian pseudo-plastic (shear thinning) nanofluid. Engine oil is used to reduce friction between moving elements. The rheological behavior of the TiO2–MWCNT (45–55%)/10W40 composite nano-lubricant was investigated by Hemmat Esfe et al. [22]. The base fluid (10W40 engine oil), as well as the nano-lubricant, behaves non-Newtonian at temperatures ranging from 5 to 55 °C and at a high shear rate of 12,000 s−1.
It is favorable that the viscosity of a nano-lubricant whose base fluid is an oil engine increases with temperature. Accordingly, viscosity is an important property in engine oil. Researchers have increased the viscosity of ZrO2–MWCNT/10W40 hybrid nano-lubricant by dispersing nanoparticles in engine oil at high temperatures [21]. Viscosity determination and optimization of thermophysical properties of nano-oils have attracted the attention of many researchers; however, many questions still remain unanswered [68, 69].
Although extensive research has been conducted on rheological behavior of nanofluids, to the best of our knowledge, very few studies have been carried out experimentally for the case of oil-based suspensions containing MWCNT and SiO2 nanoparticles. The main purpose of the current paper is to study the rheological behavior of MWCNT–SiO2/10W40 nano-lubricant with the relative fraction of 30:70 (30% corresponds to the MWCNT and 70% corresponds to the SiO2 nanoparticles). All of the experiments were performed in nanofluid concentrations and temperatures, respectively, ranging from 0.05 to 1% and 5 to 55 °C, for different values of shear rate. The results were compared against pure 10W40 engine oil. The nonlinear feature of viscosity with temperature has been tackled through the development of a third-order polynomial regression correlation with temperature-dependent coefficients. So that six set of coefficients associated with 5, 15, 25, 35, 45, and 55 °C have been obtained. Then, the sensitivity of viscosity to solid volume fraction and temperature was discussed.
Experiments
Samples were prepared by dispersing SiO2 nanoparticles with diameters ranging from 20 to 30 nm and MWCNT nanoparticles with inner and outer diameters ranging from 3 to 5 nm and 5 to 15 nm, respectively, into 10W40 engine oil. They were mixed together at the fraction of \({\text{MWCNT}}/{\text{SiO}}_{2} = 3/7\). Equation (3) was used to calculate the nanofluid concentration.
where \(w\), \(\rho\), and \(\varphi\) denote the mass, density, and volume fraction of nanoparticles, respectively (Tables 5, 6).
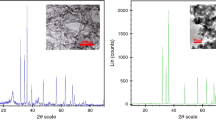
Figure 1 shows the morphology identification and structural specifications of SiO2 and MWCNT. The phase, shape, and size of a crystal could be determined via X-ray diffraction (XRD) along with other structural characteristics including the distance between the crystal layers, the orientation, and position of the crystal, the crystallinity measurement of the sample, and the composition of the crystal atoms and its structure. It works based on X-ray radiation to sample at various angles and analyze the pattern of diffraction or reflection. Figure 1 also shows a magnified image provided by transmission electron microscope (TEM). The basis of TEM is similar to that of optical microscopes, except that electron beam is used instead of light. The use of light is limited to transparent structures while using electrons remove this restriction. As a matter of fact, TEM is a nano-sized slide show projector in which the beam of electrons passes through the image. The electrons that pass through the object collide with the phosphorescent curtain, causing the image of the object to appear on the curtain. Darker parts indicate that fewer electrons have crossed this part of the body (this part of the sample has a higher density), and the lighter areas are places wherein the electrons pass through (low-density parts).
In the first step, using digital mass scales, MWCNT and SiO2 nanoparticles corresponding to their mixing ratio (3:7) and based on 1% maximum concentration were weighted with the maximum error of 0.0001 g. They were well mixed together prior to being suspended into 10W40 engine oil. Using a magnetic blender, the suspensions containing the hybrid nanoparticles dispersed into the engine oil were perfectly blended for 75 min. Because of the high probability of agglomeration and clustering even in nanoscale, an ultrasonic processor (Kimia Nano Danesh (KND), I. R. Iran), with a power of 800–1200 W and frequency of 20 kHz, was utilized to crush them. The stable and homogeneous samples, with volume fractions of 0.05%, 0.1%, 0.25%, 0.5%, 0.75%, and 1%, are depicted in Fig. 2.
Viscosity measurement
The dynamic viscosity of the samples was measured by changing the shear rate from 666.50 to 11997 s−1 at temperatures of 5 °C, 15 °C, 25 °C, 35 °C, 45 °C, and 55 °C. A Brookfield viscometer, made by Brookfield engineering laboratories (USA), was utilized to measure the viscosity of samples. The instrument measured the viscosity with a maximum uncertainty and reliability of 0.2%.
Accuracy of measurements
Apparatus calibration was performed prior to sample testing by examining the viscosity of glycerin at room temperature, 300 K. After doing three tests, it was measured as 9.26 poise, while the figure was documented as 9.5 poise. Therefore, it can be said that the viscometer works with 2.5% uncertainty. The tests were repeated at all of the nanoparticle volume fractions and temperatures, and then, mean values were reported to reduce the noise of the setup.
Results and discussion
Rheological behavior investigation
The rheological behavior of fluids, particularly nanofluids, is an important factor affecting thermal conductivity and pressure drop. In other words, the convection heat transfer coefficient and pumping power exhibit significant sensitivity to viscosity. Many studies have reported that the increase in heat transfer due to adding nanoparticles is associated with an increase in viscosity. Needless to say, the former is favorable and the latter is unfavorable although the heat transfer enhancement is more than the increase in viscosity [7, 8, 70, 71].
Bearing in mind that the lubrication efficiency of fluids is a function of their rheological behavior, it is clear that the prediction of rheological behavior is key to designing efficacious cooling devices. The rheological behavior of fluids can be studied by exploring the responses of the apparent viscosity and the shear stress with respect to changes in the imposed shear rate. The apparent viscosity and shear stress of pure oil 10W40 and MWCNTs–SiO2 (30–70)/10W40 were examined at various temperatures and solid volume fractions, as depicted in Fig. 3. Nonlinear variations of the apparent viscosity and shear stress with respect to the shear rate are seen in Fig. 3. From this, it can be inferred that the nano-lubricant is sorted as a non-Newtonian fluid. Since various types of non-Newtonian fluids exist and are widely utilized in industry, it is important to find out which of the non-Newtonian models best describes the behavior of the present nano-lubricant. We must compare the experimental data against well-known models and establish the best correlation, if necessary.
Using the Herschel–Bulkley model [72] and the Ostwald de Waele relationship, the function followed by the nano-lubricant can be explored.
where \(\dot{\gamma }\) is shear rate and m and n are consistency and power-law indexes, respectively. Equation (4) is used when the mixture has no minimum yield stress, in other words, when the variation of the shear stress with respect to the shear rate starts from the origin. According to Eq. (4), the fluid is Newtonian if n is equal to 1, since the shear stress would be in linear relation to shear rate. n > 1 and n < 1 correspond to pseudo-plastic (shear thinning) and dilatant (shear thickening) fluids, respectively. Also, \(t\) and \(K\) are structure-dependent parameters. Equation (5) is used for the so-called Bingham fluids, in detail, when a fluid behaves as a solid until a minimum yield stress, \(\tau_{y}\), is exceeded. A summary of the models and their corresponding correlations are tabulated in Table 7.
Passing on all the curves of Table 7 on the experimental results illustrated in Fig. 3, the behavior of the nano-lubricant would be cleared. The R2 values for the four models are listed in Tables 8–11 in order to analyze the accuracy and the deviation of the models.
Tables 10 and 11 show that the Ostwald de Waele and Herschel–Bulkley models are well fitted to the experimental data in comparison with the Newtonian and Bingham plastic models. The difference R2 data are gathered in Table 12 to provide a better insight into how the Ostwald de Waele and Herschel–Bulkley models work, and which one is better. According to Table 12, the positive values are more than the negative values, which means that Ostwald de Waele model is more capable of capturing the true behavior as compared to the Herschel–Bulkley model.
Disregarding the uncertainties, the rheological behavior of MWCNTs–SiO2 (30–70)/10W40 nano-lubricant can be estimated by the power-law model with a high desirability. The rheological behavior of MWCNTs–SiO2 (30–70)/10W40 nano-lubricant follows the pseudo-plastic model as shown in Tables 13 and 14.
The constants m and n were calculated by the following equation. The slope and y-intercept are denoted by n and m, respectively.
Figure 4 graphically shows the logarithmic variation of shear stress with respect to the logarithmic variation of shear rate. Similar behavior for zinc oxide nano-lubricant has been reported by Hemmat et al. [19].
Figure 5 shows how the consistency index, m, varies with the solid volume fraction and temperature. The consistency index has a decreasing trend, reaching a trough near 45 °C before increasing. As is seen from Fig. 5a, the sensitivity of m to temperature becomes less as the temperature is increased. It can be concluded that increasing the temperature hampers the severe decreasing trend of the viscosity by the temperature. Therefore, the interaction of temperature with itself is negative. Although m is highly affected by temperature, it is a weak function of the solid volume fraction, which is more evident at higher values of φ.
The variation of the power-law index, n, with respect to the temperature in various solid volume fractions is illustrated in Fig. 6. As previously depicted in Table 13, the investigated nano-lubricant behaves like a pseudo-plastic fluid. The inference is better supported by Fig. 6. As shown, a higher temperature is associated with a larger deviation from Newtonian behavior. The departure becomes higher with a maximum of 25% at 55 °C. Also, 55 °C is the temperature at which n experiences a significant variation, and the sensitivity of n to the temperature became highest at high temperature and low volume fraction.
The impacts of concentration and temperature on dynamic viscosity
Nano-lubricant viscosity at various solid volume fractions and temperatures and at a special shear rate is depicted in Fig. 7. Generally speaking, the viscosity of the nano-lubricant is higher than that of the base fluid, the engine oil. The dynamic viscosity is in a direct and in an inverse relationship with the temperature and solid volume fraction, respectively. The monotonically increasing impact of the solid volume fraction on dynamic viscosity is evident. The interaction forces between the SiO2 and MWCNT molecules are responsible for the changes in viscosity.
Nanoparticles adhering together leads to cluster formation. The existence of clusters in the suspension is followed by increasing the friction between the solid surfaces and oil, so that the more the number of clusters, the rougher the flow. Consequently, concentration plays an important role in viscosity enhancement. On the other hand, the effect of temperature on viscosity is inevitable. As expected and mentioned before, the viscosity has an inverse relation with the temperature. The reason is that temperature increases Brownian motion, which is followed by the reduction in adhesion forces between nanoparticles and fluid molecules, resulting in weaker friction.
Figure 8 shows the difference in dynamic viscosity at the highest and the lowest solid volume fractions of MWCNTs–SiO2 (30–70)/10W40 nanofluid. The increasing trend of viscosity with respect to solid volume fraction is more significant at lower temperatures, as shown in Figs. 7b and 8. In fact, the interaction between these two variables is negative, and the viscosity becomes less sensitive to the solid volume fraction at higher temperatures. Focusing on Fig. 7a, it can be seen that the highest and the lowest values of the viscosity of hybrid nano-lubricant MWCNT–SiO2/10W40 are 1.4 times and 1.1 times greater than that in the figure for pure engine oil. It is worth noting that the maximum and minimum values occurred at 5 °C with 1% solid volume fraction and 55 °C with 0.05% solid volume fraction, respectively.
Proposed correlation
A comparison between the current experimental data against some of the accurate theoretical and empirical models has been carried out and illustrated in Fig. 9. The figure reveals that the models are not even able to provide an acceptable estimation for the relative viscosity. Meanwhile, the results are predicted precisely by the obtained regression correlation. Also, it is obvious that the power-law model cannot predict the relative viscosity precisely at different temperatures. One of the main reasons for the weakness of the four prescribed models might be neglect of Brownian motion, which is implicitly taken into in the coefficients of Eq. (7). To overcome this issue, a typical regression model in the form of a single-variable polynomial function is considered while the variable is the solid volume fraction and the coefficients are temperature-dependent.
where \(\mu_{\text{nf}}\), \(\mu_{\text{bf}}\), and \(\mu_{\text{nf}} /\mu_{\text{bf}}\) are the nanofluid viscosity, base fluid viscosity, and relative viscosity, respectively. The constants, along with the values of R2 and adj. R2, are tabulated in Table 15. It is obvious that the constants are temperature-dependent.
The margins of deviation (MOD) and residuals are illustrated in Fig. 10. The MOD were calculated in order to evaluate the accuracy of the established correlation. Figure 10b shows a scatter plot around a 45° line, indicating the goodness of fit: The closer to the line the dots are placed, the more capable the model is of predicting the design points [73]. Equation (7) predicts viscosity within a temperature range of 5–55 °C and a solid volume fraction range of 0.05–1%, with a maximum error of 3%.
Figure 11 shows the dynamic viscosity enhancement (DVE) versus the temperature and the nanoparticle volume fraction. Adding nanoparticles to the oil leads to DVE, as depicted in Fig. 11a. This result is supported by the fact that it leads to a friction increase between the nano-lubricant flow layers, due to a large number of nanoparticles. Needless to say, the friction increases the viscosity of the nano-lubricant, and consequently the DVE. As shown in Fig. 11b, the maximum of DVE with respect to base lubricant is about 35%, which corresponds to the highest values of both solid volume fraction and temperature. Such a significant enhancement meets the demands of various industries for the lubrication of machinery with high-viscosity oils.
Conclusions
In the current study, the rheological behavior of MWCNT–SiO2 (30–70%)/10W40 hybrid nano-lubricant has been investigated experimentally. Since oils are widely used to cool engines and as lubricant for moving mechanical parts, this paper has explored ways to improve their efficiency. The average, inner, and outer diameters of oxide nanoparticles were considered in the range of 20–30 nm, 3–5 nm, and 5–15 nm, respectively. In order to prepare stable and homogeneous suspensions, the two-step method was used. The viscosity of the samples was measured at the solid volume fractions of 0.05%, 0.1%, 0.25%, 0.5%, 0.75%, and 1%, and in the temperature range of 5–55 °C. The main outcomes of this study are summarized as follows:
-
The rheological behavior of the investigated nano-lubricant obeys a non-Newtonian power law (or Ostwald de Waele) model for all values of solid volume fraction and temperature. Also, measurements show that the non-Newtonian behavior of the nano-lubricant flows similar to a pseudo-plastic fluid.
-
The temperature and the solid volume fraction have a decreasing and an increasing impact on the nanofluid viscosity and the cooling efficiency of the mixture, respectively.
-
The viscosity of the produced nano-lubricant is higher than that of the pure engine oil, as the pure base oil, for all values of solid volume fraction and temperature. For instance, the viscosity of MWCNTs–SiO2 (30–70)/10W40 hybrid nanofluid is 1.4 times greater than that in the figure for pure 10W40 engine oil at T = 5 °C and φ = 1%. In addition, the viscosity of MWCNTs–SiO2 (30–70)/10W40 is 1.1 times that of pure oil at the lowest and highest values of solid volume fraction (0.05%) and temperature (55 °C), respectively.
-
The impact of adding nanoparticles to the engine oil is much more significant at lower temperatures. In fact, the changes in viscosity are not remarkable at high temperatures.
-
DVE rises as the solid volume fraction is increased. The maximum difference between the viscosity of the produced nano-lubricant and engine oil is about 35%, which occurs at the maximum values of temperature and nanofluid concentration.
-
Since the measured viscosity could not be predicted with high desirability by the existing models, a new regression model with R2 = 0.988, adj. R2 = 0.977, and MODmax = ±3% is established and proposed for future studies.
Abbreviations
- a :
-
The distance between any two nanoparticles (m)
- h :
-
Nanoparticle diameter (m)
- K :
-
Structure-dependent parameters
- m :
-
Consistency index
- n :
-
Power-law index
- t :
-
Structure-dependent parameter
- T :
-
Temperature (°C)
- w :
-
Mass (g)
- \(\dot{\gamma }\) :
-
Shear rate (s−1)
- µ :
-
Dynamic viscosity (poise)
- ρ :
-
Density (kg m−3)
- τ :
-
Shear stress (dyne cm−2)
- φ :
-
Nanoparticles volume fraction
- bf:
-
Base fluid
- nf:
-
Nanofluid
- HB:
-
Herschel–Bulkley
- OW:
-
Ostwald de Waele
- Y:
-
Yield stress
References
Murshed SMS, Leong KC, Yang C. Thermophysical and electrokinetic properties of nanofluids—a critical review. Appl Therm Eng. 2008;28:2109–25. https://doi.org/10.1016/j.applthermaleng.2008.01.005.
Boungiorno J, Hu L, Kim S, Hannink R, Truong B, Forrest E. Nanofluids for enhanced economics and safety of nuclear reactors: an evaluation of the potential features issues, and research gaps. Nucl Technol. 2008;162:80–91.
Saidur R, Leong KY, Mohammad HA. A review on applications and challenges of nanofluids. Renew Sustain Energy Rev. 2011;15:1646–68. https://doi.org/10.1016/j.rser.2010.11.035.
Murshed SMS, de Castro CAN. Nanofluids as advanced coolants. In: Mohammad A, editor. Green solvents I prop. appl. chem. Dordrecht: Springer; 2012. p. 397–415. https://doi.org/10.1007/978-94-007-1712-1_14.
Sidik NAC, Mohammed HA, Alawi OA, Samion S. A review on preparation methods and challenges of nanofluids. Int Commun Heat Mass Transf. 2014;54:115–25. https://doi.org/10.1016/j.icheatmasstransfer.2014.03.002.
Yu WH, France DM, Routbort JL, Choi SUS. Review and comparison of nanofluid thermal conductivity and heat transfer enhancements. Heat Transf Eng. 2008;29:432–60. https://doi.org/10.1080/01457630701850851.
Sarkar J. A critical review on convective heat transfer correlations of nanofluids. Renew Sustain Energy Rev. 2011;15:3271–7. https://doi.org/10.1016/j.rser.2011.04.025.
Godson L, Raja B, Mohan Lal D, Wongwises S. Enhancement of heat transfer using nanofluids-an overview. Renew Sustain Energy Rev. 2010;14:629–41. https://doi.org/10.1016/j.rser.2009.10.004.
Kleinstreuer C, Feng Y. Experimental and theoretical studies of nanofluid thermal conductivity enhancement: a review. Nanoscale Res Lett. 2011;6:229. https://doi.org/10.1186/1556-276X-6-229.
Murshed SMS, Nieto de Castro CA. Superior thermal features of carbon nanotubes-based nanofluids—a review. Renew Sustain Energy Rev. 2014;37:155–67. https://doi.org/10.1016/j.rser.2014.05.017.
Murshed SMS, Estellé P. A state of the art review on viscosity of nanofluids. Renew Sustain Energy Rev. 2017;76:1134–52. https://doi.org/10.1016/j.rser.2017.03.113.
Prasher R, Song D, Wang J, Phelan P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl Phys Lett. 2006;89:67–70. https://doi.org/10.1063/1.2356113.
Vajjha RS, Das DK. A review and analysis on influence of temperature and concentration of nanofluids on thermophysical properties, heat transfer and pumping power. Int J Heat Mass Transf. 2012;55:4063–78. https://doi.org/10.1016/j.ijheatmasstransfer.2012.03.048.
Sundar LS, Sharma KVV, Naik MTT, Singh MK. Empirical and theoretical correlations on viscosity of nanofluids: a review. Renew Sustain Energy Rev. 2013;25:670–86. https://doi.org/10.1016/j.rser.2013.04.003.
Meyer JP, Adio SA, Sharifpur M, Nwosu PN. The viscosity of nanofluids: a review of the theoretical, empirical, and numerical models. Heat Transf Eng. 2016;37:387–421. https://doi.org/10.1080/01457632.2015.1057447.
Chen H, Ding Y, Tan C. Rheological behaviour of nanofluids. New J Phys. 2007;9:367–367. https://doi.org/10.1088/1367-2630/9/10/367.
Mahbubul IMM, Saidur R, Amalina MAA. Latest developments on the viscosity of nanofluids. Int J Heat Mass Transf. 2012;55:874–85. https://doi.org/10.1016/j.ijheatmasstransfer.2011.10.021.
Mishra PC, Mukherjee S, Nayak SK, Panda A. A brief review on viscosity of nanofluids. Int Nano Lett. 2014;4:109–20. https://doi.org/10.1007/s40089-014-0126-3.
Hemmat Esfe M, Saedodin S, Rejvani M, Shahram J. Experimental investigation, model development and sensitivity analysis of rheological behavior of ZnO/10W40 nano-lubricants for automotive applications. Phys E Low-Dimens Syst Nanostructures. 2017;90:194–203. https://doi.org/10.1016/j.physe.2017.02.015.
Mondragón R, Segarra C, Martínez-cuenca R, Juliá JE, Carlos J. Experimental characterization and modeling of thermophysical properties of nano fluids at high temperature conditions for heat transfer applications. Powder Technol. 2013;249:516–29. https://doi.org/10.1016/j.powtec.2013.08.035.
Hemmat Esfe M, Rostamian H, Rejvani M, Emami MRS. Rheological behavior characteristics of ZrO2–MWCNT/10w40 hybrid nano-lubricant affected by temperature, concentration, and shear rate: an experimental study and a neural network simulating. Phys E Low-Dimens Syst Nanostructures. 2018;102:160–70. https://doi.org/10.1016/j.physe.2017.12.023.
Hemmat Esfe M, Rostamian H, Reza Sarlak M, Rejvani M, Alirezaie A. Rheological behavior characteristics of TiO2–MWCNT/10w40 hybrid nano-oil affected by temperature, concentration and shear rate: an experimental study and a neural network simulating. Phys E Low-Dimens Syst Nanostructures. 2017;94:231–40. https://doi.org/10.1016/j.physe.2017.07.012.
Murshed SM, Santos FJ, de Castro CA. Investigations of viscosity of silicone oil-based semiconductor nanofluids. J Nanofluids. 2013;2:261–6.
Chen L, Xie H. Silicon oil based multiwalled carbon nanotubes nanofluid with optimized thermal conductivity enhancement. Colloids Surf A Physicochem Eng Asp. 2009;352:136–40. https://doi.org/10.1016/j.colsurfa.2009.10.015.
He Y, Jin Y, Chen H, Ding Y, Cang D, Lu H. Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int J Heat Mass Transf. 2007;50:2272–81. https://doi.org/10.1016/j.ijheatmasstransfer.2006.10.024.
Abdollahi-Moghaddam M, Rejvani M, Alamdari P. Determining optimal formulations and operating conditions for Al2O3/water nanofluid flowing through a microchannel heat sink for cooling system purposes using statistical and optimization tools. Therm Sci Eng Prog. 2018;8:517–24. https://doi.org/10.1016/j.tsep.2018.10.009.
Rejvani M, Moghaddam MA, Alamdari P. Using statistical and optimization tools for determining optimal formulations and operating conditions for Al2O3/(EG + Water) nanofluids for cooling system. Therm Sci Eng Prog. 2018;7:230–40. https://doi.org/10.1016/j.tsep.2018.07.003.
Esfe MH, Wongwises S, Rejvani M. Prediction of thermal conductivity of carbon nanotube-EG nanofluid using experimental data by ANN. Curr Nanosci. 2017;13:1–6. https://doi.org/10.2174/1573413713666161213114458.
Lee J-HH, Hwang KS, Jang SP, Lee BH, Kim JH, Choi SUSS, Choi CJ. Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int J Heat Mass Transf. 2008;51:2651–6. https://doi.org/10.1016/j.ijheatmasstransfer.2007.10.026.
Yang L, Du K, Bao S, Wu Y. Investigations of selection of nanofluid applied to the ammonia absorption refrigeration system. Int J Refrig. 2012;35:2248–60. https://doi.org/10.1016/j.ijrefrig.2012.08.003.
Hemmat Esfe M, Hassani Ahangar MR, Rejvani M, Toghraie D, Hajmohammad MH. Designing an artificial neural network to predict dynamic viscosity of aqueous nanofluid of TiO2 using experimental data. Int Commun Heat Mass Transf. 2016;75:192–6. https://doi.org/10.1016/j.icheatmasstransfer.2016.04.002.
Sharma AK, Tiwari AK, Dixit AR. Rheological behaviour of nanofluids: a review. Renew Sustain Energy Rev. 2016;53:779–91. https://doi.org/10.1016/j.rser.2015.09.033.
Thomas S, Sobhan CBP. A review of experimental investigations on thermal phenomena in nanofluids. Nanoscale Res Lett. 2011;6:377. https://doi.org/10.1186/1556-276x-6-377.
Einstein A. Eine neue bestimmung der moleküldimensionen. Ann Phys. 1906;324:289–306. https://doi.org/10.1002/andp.19063240204.
Frankel NA, Acrivos A. On the viscosity of a concentrated suspension of solid spheres. Chem Eng Sci. 1967;22:847–53.
Nielsen LE. Generalized equation for the elastic moduli of composite materials. J Appl Phys. 1970;41:4626. https://doi.org/10.1063/1.1658506.
Mooney M. The viscosity of a concentrated suspension of spherical particles. J Colloid Sci. 1951;6:162–70. https://doi.org/10.1016/0095-8522(51)90036-0.
Graham AL. On the viscosity of suspensions of solid spheres. Appl Sci Res. 1981;37:275–86.
De Bruijn H. The viscosity of suspensions of spherical particles. (The fundamental η-c and φ relations). Recl Des Trav Chim Des Pays-Bas. 1942;61:863–74.
Brinkman HC. The viscosity of concentrated suspensions and solutions. J Chem Phys. 1952;20:571.
Batchelor BGK. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J Fluid Mech. 1977;83:97–117.
Krieger IM, Dougherty TJ, Nielsen LE. A mechanism for non-newtonian flow in suspensions of rigid spheres. Trans Soc Rheol. 1959;3:137–52. https://doi.org/10.1122/1.548848.
Wang X, Xu X, Choi SUS. Thermal conductivity of nanoparticle—fluid mixture. J Thermophys Heat Transf. 1999;13:474–80. https://doi.org/10.2514/2.6486.
Hemmat Esfe M, Saedodin S, Mahian O, Wongwises S. Thermophysical properties, heat transfer and pressure drop of COOH-functionalized multi walled carbon nanotubes/water nanofluids. Int Commun Heat Mass Transf. 2014;58:176–83. https://doi.org/10.1016/j.icheatmasstransfer.2014.08.037.
Hemmat Esfe M, Saedodin S. An experimental investigation and new correlation of viscosity of ZnO–EG nanofluid at various temperatures and different solid volume fractions. Exp Therm Fluid Sci. 2014;55:1–5. https://doi.org/10.1016/j.expthermflusci.2014.02.011.
Hemmat Esfe M, Saedodin S, Mahian O, Wongwises S. Heat transfer characteristics and pressure drop of COOH-functionalized DWCNTs/water nanofluid in turbulent flow at low concentrations. Int J Heat Mass Transf. 2014;73:186–94. https://doi.org/10.1016/j.ijheatmasstransfer.2014.01.069.
Esfe MH, Saedodin S, Asadi A. An empirical investigation on the dynamic viscosity of Mg (OH) 2–ethylene glycol in different solid concentrations and proposing new correlation based on experimental data. Int J Nat Eng Sci. 2014;8:29–34.
Hemmat Esfe M, Saedodin S, Wongwises S, Toghraie D. An experimental study on the effect of diameter on thermal conductivity and dynamic viscosity of Fe/water nanofluids. J Therm Anal Calorim. 2015;119:1817–24. https://doi.org/10.1007/s10973-014-4328-8.
Hemmat Esfe M, Abbasian Arani AA, Rezaie M, Yan WM, Karimipour A. Experimental determination of thermal conductivity and dynamic viscosity of Ag–MgO/water hybrid nanofluid. Int Commun Heat Mass Transf. 2015;66:189–95. https://doi.org/10.1016/j.icheatmasstransfer.2015.06.003.
Estellé P, Halelfadl S, Doner N, Maré T. Shear history effect on the viscosity of carbon nanotubes water-based nanofluid. Curr Nanosci. 2013;9:225–30. https://doi.org/10.2174/1573413711309020010.
Halelfadl S, Estellé P, Aladag B, Doner N, Maré T. Viscosity of carbon nanotubes water-based nanofluids: influence of concentration and temperature. Int J Therm Sci. 2013;71:111–7. https://doi.org/10.1016/j.ijthermalsci.2013.04.013.
Phuoc TX, Massoudi M, Chen R-HH. Viscosity and thermal conductivity of nanofluids containing multi-walled carbon nanotubes stabilized by chitosan. Int J Therm Sci. 2011;50:12–8. https://doi.org/10.1016/j.ijthermalsci.2010.09.008.
Estellé P, Halelfadl S, Maré T. Lignin as dispersant for water-based carbon nanotubes nanofluids: impact on viscosity and thermal conductivity. Int Commun Heat Mass Transf. 2014;57:8–12. https://doi.org/10.1016/j.icheatmasstransfer.2014.07.012.
Hung Y, Chou W. Chitosan for suspension performance and viscosity of MWCNTs. Int J Chem Eng Appl. 2012;3:343–6. https://doi.org/10.7763/IJCEA.2012.V3.215.
Harish S, Ishikawa K, Einarsson E, Aikawa S, Inoue T. Temperature dependent thermal conductivity increase of aqueous nanofluid with single walled carbon nanotube inclusions. Mater Express. 2012;2:213–23.
Sadri R, Ahmadi G, Togun H, Dahari M, Kazi SN, Sadeghinezhad E, Zubir N. An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanoscale Res Lett. 2014;9:151–66. https://doi.org/10.1186/1556-276X-9-151.
Richmond WR, Jones RL, Fawell PD. The relationship between particle aggregation and rheology in mixed silica–titania suspensions. Chem Eng J. 1998;71:67–75. https://doi.org/10.1016/S1385-8947(98)00105-3.
Mondragon R, Enrique Julia J, Barba A, Jarque JC. Determination of the packing fraction of silica nanoparticles from the rheological and viscoelastic measurements of nanofluids. Chem Eng Sci. 2012;80:119–27. https://doi.org/10.1016/j.ces.2012.06.009.
Chevalier J, Tillement O, Ayela F. Structure and rheology of SiO2 nanoparticle suspensions under very high shear rates. Phys Rev E Stat Nonlinear Soft Matter Phys. 2009;80:1–7. https://doi.org/10.1103/physreve.80.051403.
Farbod M, Ahangarpour A. Improved thermal conductivity of Ag decorated carbon nanotubes water based nanofluids. Phys Lett A. 2016;380:4044–8. https://doi.org/10.1016/j.physleta.2016.10.014.
Esfe MH, Rejvani M, Karimpour R, Abbasian Arani AAAA. Estimation of thermal conductivity of ethylene glycol-based nanofluid with hybrid suspensions of SWCNT–Al2O3 nanoparticles by correlation and ANN methods using experimental data. J Therm Anal Calorim. 2017;128:1–13. https://doi.org/10.1007/s10973-016-6002-9.
Chen H, Ding Y. Heat transfer and rheological behaviour of nanofluids – a review. In: Wang L, editor. Advances in transport phenomena, vol 1. Berlin: Springer; 2009. p. 135–77.
Hemmat Esfe M, Esfandeh S, Rejvani M. Modeling of thermal conductivity of MWCNT–SiO2 (30:70%)/EG hybrid nanofluid, sensitivity analyzing and cost performance for industrial applications: an experimental based study. J Therm Anal Calorim. 2018;131:1437–47. https://doi.org/10.1007/s10973-017-6680-y.
Hemmat Esfe M, Abbasian Arani AA, Shafiei Badi R, Rejvani M. ANN modeling, cost performance and sensitivity analyzing of thermal conductivity of DWCNT–SiO2/EG hybrid nanofluid for higher heat transfer: an experimental study. J Therm Anal Calorim. 2018;131:2381–93. https://doi.org/10.1007/s10973-017-6744-z.
Anoop K, Sadr R, Al-Jubouri M, Amani M. Rheology of mineral oil-SiO2 nanofluids at high pressure and high temperatures. Int J Therm Sci. 2014;77:108–15. https://doi.org/10.1016/j.ijthermalsci.2013.10.016.
Baghbanzadeh M, Rashidi A, Soleimanisalim AH, Rashtchian D. Investigating the rheological properties of nanofluids of water/hybrid nanostructure of spherical silica/MWCNT. Thermochim Acta. 2014;578:53–8. https://doi.org/10.1016/j.tca.2014.01.004.
Hemmat Esfe M, Karimpour R, Abbasian Arani AA, Shahram J. Experimental investigation on non-Newtonian behavior of Al2O3-MWCNT/5W50 hybrid nano-lubricant affected by alterations of temperature, concentration and shear rate for engine applications. Int Commun Heat Mass Transf. 2017;82:97–102. https://doi.org/10.1016/j.icheatmasstransfer.2017.02.006.
Esfe MH, Esfandeh S, Afrand M, Rejvani M, Rostamian SH. Experimental evaluation, new correlation proposing and ANN modeling of thermal properties of EG based hybrid nanofluid containing ZnO–DWCNT nanoparticles for internal combustion engines applications. Appl Therm Eng. 2018;133:452–63. https://doi.org/10.1016/j.applthermaleng.2017.11.131.
Hemmat Esfe M, Alirezaie A, Rejvani M. An applicable study on the thermal conductivity of SWCNT–MgO hybrid nanofluid and price-performance analysis for energy management. Appl Therm Eng. 2017;111:1202–10. https://doi.org/10.1016/j.applthermaleng.2016.09.091.
Daungthongsuk W, Wongwises S. A critical review of convective heat transfer of nanofluids. Renew Sustain Energy Rev. 2007;11:797–817. https://doi.org/10.1016/j.rser.2005.06.005.
Chang TB, Syu SC, Yang YK. Effects of particle volume fraction on spray heat transfer performance of Al2O3-water nanofluid. Int J Heat Mass Transf. 2012;55:1014–21. https://doi.org/10.1016/j.ijheatmasstransfer.2011.10.009.
Herschel WH, Bulkley R. Konsistenzmessungen von Gummi-Benzoll-sungen. Kolloid-Zeitschrift. 1926;39:291–300. https://doi.org/10.1007/BF01432034.
Pordanjani AH, Vahedi SM, Rikhtegar F, Wongwises S. Optimization and sensitivity analysis of magneto-hydrodynamic natural convection nanofluid flow inside a square enclosure using response surface methodology. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7652-6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rejvani, M., Saedodin, S., Vahedi, S.M. et al. Experimental investigation of hybrid nano-lubricant for rheological and thermal engineering applications. J Therm Anal Calorim 138, 1823–1839 (2019). https://doi.org/10.1007/s10973-019-08225-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08225-5