Abstract
In this study, three different volume expansion ratios of expanded graphite (EG) are prepared and investigated to enhance the heat transfer efficiency of the sodium acetate trihydrate (SAT) composites. A series of SAT composite phase change materials (CPCMs) with EG were prepared. The influence of volume expansion ratio and mass fraction of EG on thermodynamic characteristics of SAT CPCMs was examined, including thermal conductivity, phase change temperature, enthalpy, latent heat storage and release time, and the degree of supercooling. Results showed that SAT CPCMs can be absorbed adequately by EG, and EG could enhance the heat transfer efficiency effectively. But it also brought some problems with the addition of all the three volume expansion ratios of EG, such as the poor enthalpy and serious supercooling. Particularly, the situation gets worse with the increase in mass and expansion ratio of EG. Therefore, it is better to choose the EG with proper expansion ratio or reduce the proportion of the EG which possesses higher expansion ratio. Besides, thermal cycling test and thermogravimetric analysis revealed that the SAT CPCMs with 3 mass% EG showed a good thermal stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
PCMs can store thermal energy and regulate temperature in the process of charge and discharge, which are becoming an attractive way to solve energy crisis and environmental pollution. Compared with sensible heat storage materials, PCMs have more strength of large thermal storage density, small volume, and basically constant temperature during phase transition. Therefore, PCMs have been extensively applied to building energy conservation, heat recovering, solar energy storage, and other fields [1,2,3,4].
SAT is a kind of inorganic PCM with large latent heat of fusion and good heat transfer performance, which has been used in a lot of areas such as solar energy storage [5,6,7]. But SAT is not perfect and has some shortcomings such as supercooling and phase segregation. Besides, there is still a large space to improve the heat transfer performance of the SAT.
A large number of researches have been presented to modify the SAT. Wada et al. compared the enthalpy of SAT with polyvinyl alcohol and SAT during thermal cycling [8]. Cabeza et al. [9] found SAT can be successfully thickened with starch, bentonite, and cellulose. Jin et al. [10] studied the cooling processes of SAT starting from three different states, which could affect the performance of SAT. Nanoparticles with super-specific alien surface and high surface activity have also been used as a nucleating agent, including silver [11], aluminum nitride [12], copper [13], and so on [14].
EG has the advantages of huge surface area, strong adsorption, and high thermal conductivity and has been particularly chosen as a PCM additive to enhance the heat transfer performance. Sarı et al. [15] determined the proper amount of paraffin absorbed into EG, and the thermal conductivity of paraffin containing 10% EG was measured as 0.82 W m−1 K−1. Liu et al. [16] prepared a kind of ternary eutectic mixture with EG as CPCM, which showed good thermal properties. Gu et al. [17] combined 95% SAT and 5% EG, and the CPCM not only has form-stable property, but also has high heat transfer ability. Shin et al. [18] investigated the influence of EG and CMC addition on enthalpy and stability of SAT. A large amount of form-stable composite PCMs were prepared by using EG as the supporting materials. The thermal properties of the EG-based form-stable composite PCMs showed a significant difference in different studies [19]. This could be due to the difference in the expansion ratio of the EG used by the researchers. Therefore, exploring the influence of EG with different volume expansion ratios on the phase transition characteristics of PCMs is of great significance for the selection of EG as a high thermal conductivity medium.
In this report, three volume expansion ratios of EG and a series of SAT CPCMs with varying content of EG were synthesized. The characteristics and effects of the different volume expansion ratios of EG addition on thermodynamic characteristics of the SAT CPCMs were evaluated by scanning electron microscope (SEM), thermal conductivity tester, differential scanning calorimetry (DSC) analysis and thermocouple data acquisition system. The thermal stability and reliability of SAT CPCMs with 3 mass% EG were analyzed by thermal gravimetric analyzer (TG) and thermal cycling test.
Experimental
Materials and equipment
The materials used in the research are shown in Table 1, and SAT and DSP should be kept sealed in the experiments to prevent the effect of water loss on material properties. The equipment used in the research is shown in Table 2.
Preparation of SAT CPCMs with EG
EG’ (50, 80, and 100 mesh) was heated and dried in a vacuum oven for 24 h at 65 °C; then, the EG’ was heated and expanded for 30 s at 700 W by microwave oven, which can make sure that EG has a large porosity, and SAT CPCMs could be absorbed into EG as much as possible.
SAT CPCMs contained in a test tube are heated and melted in a thermostatic water bath at 70 °C. CMC was selected as thickener and DSP was selected as nucleating agent [20], then added EG and stirred the mixture thoroughly for more than 20 min [21]. A series of SAT CPCMs samples containing 1–5 mass% EG (50, 80, and 100 mesh) were prepared and weighed 10 g.
Characterizations of SAT CPCMs with EG
Morphology of the EG and SAT CPCMs with EG
EG is obtained through microwave puffing from EG’ (50, 80, and 100 mesh), and the volume expansion ratio is 357, 234, and 180 mL g−1, respectively. As shown in Fig. 1, the EG exhibits a worm-like appearance with a layered and porous structure. Volume expansion ratio of the EG decreases with the increase in mesh. The appearance of EG with 50 mesh looks coarse and long, which possesses the highest porosity. But the EG with 100 mesh is relatively small and slender, and the appearance of 80 mesh is just between the two.
The SEM images of EG, SAT CPCMs (0 mass%), and SAT CPCMs with different volume expansion ratios of EG are shown in Fig. 2. As shown in Fig. 2a, EG has a porous structure. It can be seen from Fig. 2b that SAT CPCMs are composed of granular crystals. Figure 2c–e shows the SEM image of the SAT CPCMs with different volume expansion ratios of 3 mass% EG. It can be seen from Fig. 2c and d that the SAT CPCMs can be absorbed adequately by EG prepared from 50 to 80 mesh EG’, and there is no SAT CPCMs leakage. Some SAT CPCMs on the surface of EG were prepared from 100 mesh (Fig. 2e); this is because the expansion ratio and pore of EG prepared from 100 mesh EG’ are smaller than others and cannot absorb SAT CPCMs absolutely.
Thermal conductivity
Figure 3 shows the thermal conductivities of the SAT CPCMs with varying content of EG (50, 80, and 100 mesh). All the three volume expansion ratios of EG have an important impact on improving thermal conductivity, and the thermal conductivity of the SAT CPCMs increased as the content of EG increased. Particularly, when the content of EG (50, 80, and 100 mesh) was up to 5%, the thermal conductivities of the composites reached 1.67, 1.58, and 1.49 W m−1 K−1, respectively, almost three times compared with SAT CPCMs without EG (0.58 W m−1 K−1). It is mainly because that EG is a kind of porous thermal transfer enhance filler, which can absorb SAT CPCMs and forms a connected heat transfer path inside the materials. By comparing the effect of different meshes of EG on the thermal conductivities, it can be found that the EG with higher expansion ratio shows better performance in improving heat transfer efficiency. This could be because the composites with higher expansion ratio EG can form more connected heat transfer paths. In this way, it is possible to make heat transfer through EG possess high thermal conductivity instead of SAT PCMs.
Thermal analysis by DSC
DSC analysis was carried out to determine the effect of different volume expansions and mass fractions of EG on thermodynamic characteristics including phase change temperature and enthalpy of SAT CPCMs. Figure 4 shows the DSC curves, and all composites are similar, which means that the main part of the CPCMs in melting is SAT. EG only plays a role of physical adsorption and does not change the basic latent energy storage characteristics of SAT in the channel. However, the content and expansion ratio of EG have an obvious significant effect on the latent characteristics.
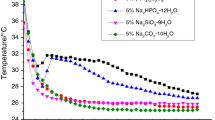
Figure 5 shows the phase change temperature (peak temperature) of SAT CPCMs with varying content of EG. The phase change temperature of all composites with different volume expansion ratios has a general downward trend with the increase in EG addition. Particularly, when the content of EG (50, 80, and 100 mesh) up to 5%, the phase change temperature of the composites decreased to 57.8, 58.2, and 57.7 °C, respectively. This is because as the mass fraction of EG increases, the percentage of SAT in materials decreased and the thermal conductivity of the materials increased and consequently accelerated the phase change speed. In addition, EG is the impurity to the SAT eutectic hydrate salt and may cause lattice defects and may disrupt the normal crystallization, resulting in crystals that may be destroyed and melted at lower temperature [17, 22].
Figure 6 shows latent heat of SAT CPCMs with varying content of EG. It is obvious that the latent heat of all composites with different volume expansion ratios has a general downward trend with the increase in EG addition. On the one hand, the mass fraction of SAT CPCMs reduced with the share of EG increased. On the other hand, the crystallization value of SAT decreased [17, 23]. By comparing the effects of three volume expansion ratios of EG addition on the enthalpy value of SAT CPCMs, it can be found that the greater the volume expansion ratio of EG, the lower the enthalpy of the composite PCMs. This could be because the 50 mesh EG with a higher volume expansion ratio can absorb more SAT CPCMs and limit its free movement with the same proportion, and it is becoming more hard for SAT crystals to aggregate and crystallize.
Cooling curve analysis
Figure 7 shows the cooling curves of SAT CPCMs with varying content of EG. The latent heat storage and release process (rectangular interval marked S and R) and the degree of supercooling were marked in the figure. From Fig. 7a, it can be seen that the heat charge and discharge process of the SAT CPCMs without EG lasted 35 min and 31 min with only 0.7 °C of subcooling. This means the SAT CPCMs have good phase change heat storage capacity and nucleation performance. The effect of the volume expansion ratio and mass fraction of EG on cooling curves of the SAT CPCMs is shown in Fig. 7b–p. From Fig. 7b–p, it can be seen that the supercooling of SAT CPCMs increased and the time of heat charge and discharge process reduced with the EG addition.
Detailed thermal characteristics of SAT CPCMs with EG are summarized in Fig. 8. Figure 8a, b shows the effect of the volume expansion ratio and mass fraction of EG on latent heat storage and release time of the SAT CPCMs. It can be seen that both the latent heat storage and release time of SAT CPCMs reduced with the increase in EG addition. The reduction in latent heat storage/release time may be due to an increase in thermal conductivity and a decrease in enthalpy. Besides, more EG could lead to more heterogeneous nucleation (for crystallization)/crystal defects (for melting), which in turn reduces release/storage time.
Figure 8c shows the effect of the volume expansion ratio and mass fraction of EG on the subcooling degree of SAT CPCMs. It is obvious that the addition of different expansion ratios of EG will lead to various increases in supercooling of the SAT CPCMs, and the degree of supercooling has a general upward trend as EG addition increased. It is supposed that the excessive EG will form a package and limit the free movement of SAT CPCMs, and it is becoming more hard for SAT crystals to aggregate and crystallize [17]. Additionally, the composites with the addition of 50 mesh EG possess greater degree of supercooling than others. It could be because 50 mesh EG with a larger expanded volume makes it easier to form a package on the SAT. Therefore, it is very important to ensure that the volume expansion ratio and mass fraction of EG are in a reasonable range.
Through the previous analysis in the research, all the three volume expansion ratios of EG could improve the heat transfer performance of SAT CPCMs effectively. However, it also has some negative effects on the thermodynamics characteristics, mainly including the poor enthalpy and serious supercooling. Therefore, it is necessary to choose the content of EG as much as possible in the condition of the enthalpy and subcooling of SAT CPCMs that are not deteriorated.
Thermal cycling test
The SAT CPCMs with three volume expansion ratios of 3 mass% EG showed satisfying thermodynamics characteristics, and the basic data before reciprocating heating and cooling test are shown in Table 3. In order to determine the thermal reliability of the SAT CPCMs with 3 mass% EG, a reciprocating heating and cooling test was performed. Cooling and DSC curve analysis of SAT CPCMs with 3 mass% EG were carried out before and after 100 thermal cycles. And the curves are shown in Figs. 9 and 10. It can be seen that all of the SAT CPCMs with different volume expansion ratios of EG showed good reliability. Table 3 gives the detail data of the composites before and after 100 thermal cycles. It is obvious that the properties of SAT CPCMs with 3 mass% EG have no significant change after 100 thermal cycles and showed good thermal reliability. The phase change temperature and enthalpy almost keep the original value after 100 thermal cycles. And the degree of supercooling has no serious deterioration. This means porous EG combined with the CMC can effectively prevent phase separation.
Thermogravimetric analysis
The thermal reliability of the SAT CPCMs with 3 mass% EG was measured by the thermogravimetric analyzer. Samples are measured under a constant argon flow of 40 mL min−1 at a heating rate of 20 °C min−1, and the temperature in the measurement is from 5 to 600 °C. Figure 11 shows the TG curves of SAT CPCMs with different volume expansion ratios of EG, and Table 4 shows the initial decomposition temperature (onset temperature) and the residual mass of the samples at 600 °C. It can be seen in Fig. 11 that the degradation process of the SAT CPCMs was divided into two parts. The first thermal degradation process of SAT CPCMs occurred between about 10 and 170 °C. This is mainly caused by the release of water of crystallization. The second thermal degradation process occurred between about 420 and 525 °C. This process corresponds to the decomposition of sodium acetate. It can be seen in Table 4 that the SAT CPCMs with the EG addition have higher initial decomposition temperature and residue amount than the SAT CPCMs without EG. It is supposed that EG could absorb PCM and form a physical protective barrier. Besides, EG with higher expansion ratio shows better performance in preventing SAT CPCMs to lose its water of crystallization. This is because the EG with higher expansion ratio possesses the higher porosity and could absorb PCM more adequately.
Conclusions
In this study, three volume expansion ratios of EG are prepared and investigated to enhance the heat transfer efficiency of the SAT CPCMs. SEM images showed that EG has a porous structure and can absorb SAT CPCMs adequately. Thermal conductivities were improved almost three times after the addition of 5 mass% EG, and EG with higher expansion ratio shows better performance in improving heat transfer efficiency. However, it also has some negative effects on the thermodynamics characteristics, mainly including the poor enthalpy and serious supercooling. Particularly, the situation is more serious with the EG addition with higher expansion ratio. Therefore, it is better to choose the EG with lower expansion ratio or reduce the proportion of the EG which possesses higher expansion ratio. Besides, thermal cycling test and thermogravimetric analysis revealed that the SAT CPCMs with 3 mass% EG showed a good thermal reliability.
References
Akeiber H, Nejat P, Majid MZA, et al. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew Sustain Energy Rev. 2016;60:1470–97.
Nomura T, Okinaka N, Akiyama T. Waste heat transportation system, using phase change material (PCM) from steelworks to chemical plant. Resour Conserv Recy. 2010;54(11):1000–6.
Pielichowska K, Pielichowski K. Phase change materials for thermal energy storage. Prog Mater Sci. 2014;65(10):67–123.
Yuan Y, Gao X, Wu H, et al. Coupled cooling method and application of latent heat thermal energy storage combined with pre-cooling of envelope: method and model development. Energy. 2017;119:817–33.
El-Bassuoni AMA, Tayeb AM, Helwa NH, et al. Modification of urea–sodium acetate trihydrate mixture for solar energy storage. Renew Energy. 2014;28(10):1629–43.
Cabeza LF, Ibáñez M, Solé C, et al. Experimentation with a water tank including a PCM module. Sol Energy Mater Sol Cells. 2006;90(9):1273–82.
Dannemand M, Schultz JM, Johansen JB, et al. Long term thermal energy storage with stable supercooled sodium acetate trihydrate. Appl Therm Eng. 2015;91:671–8.
Wada T, Yamamoto R, Matsuo Y. Heat storage capacity of sodium acetate trihydrate during thermal cycling. Sol Energy. 1984;33(3):373–5.
Cabeza LF, Svensson G, Hiebler S, et al. Thermal performance of sodium acetate trihydrate thickened with different materials as phase change energy storage material. Appl Therm Eng. 2003;23(13):1697–704.
Jin X, Zhang S, Medina MA, et al. Experimental study of the cooling process of partially-melted sodium acetate trihydrate. Energy Build. 2014;76(11):654–60.
Ramirez BMLG, Glorieux C, Martinez ESM, et al. Tuning of thermal properties of sodium acetate trihydrate by blending with polymer and silver nanoparticles. Appl Therm Eng. 2014;62(2):838–44.
Hu P, Lu DJ, Fan XY, et al. Phase change performance of sodium acetate trihydrate with AlN nanoparticles and CMC. Sol Energy Mater Sol Cells. 2011;95(9):2645–9.
Cui W, Yuan Y, Sun L, Cao X, Yang X. Experimental studies on the supercooling and melting/freezing characteristics of nanocopper/sodium acetate trihydrate composite phase change materials. Renew Energy. 2016;99(11):1029–37.
Lu DJ, Hu P, Zhao BB, Liu Y, Chen ZS. Study on the performance nanoparticles nucleating agents for sodium acetate trihydrate. J Eng Thermophys. 2012;33(8):1279–82.
Sarı A, Karaipekli A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Appl Therm Eng. 2007;27(8):1271–7.
Liu C, Yuan Y, Zhang N, et al. A novel PCM of lauric–myristic–stearic acid/expanded graphite composite for thermal energy storage. Mater Lett. 2014;120(4):43–6.
Gu X, Qin S, Wu X, et al. Preparation and thermal characterization of sodium acetate trihydrate/expanded graphite composite phase change material. J Therm Anal Calorim. 2016;125(2):831–8.
Shin HK, Park M, Kim HY, et al. Thermal property and latent heat energy storage behavior of sodium acetate trihydrate composites containing expanded graphite and carboxymethyl cellulose for phase change materials. Appl Therm Eng. 2015;75(1):978–83.
Zhang N, Yuan Y, Cao X, et al. Latent heat thermal energy storage systems with solid–liquid phase change materials: a review. Adv Eng Mater. 2018;20:1700753.
Mao J, Li J, Peng G, et al. A selection and optimization experimental study of additives to thermal energy storage material sodium acetate trihydrate. Int Conf Energy Environ Technol. 2009;1:14–7.
Mao J, Hou P, Liu R, et al. Preparation and thermal properties of SAT-CMC-DSP/EG composite as phase change material. Appl Therm Eng. 2017;119:585–92.
Yuan Y, Li T, Zhang N, et al. Investigation on thermal properties of capric–palmitic–stearic acid/activated carbon composite phase change materials for high-temperature cooling application. J Therm Anal Calorim. 2016;124(2):881–8.
Liu S, Yang H. Stearic acid hybridizing coal–series kaolin composite phase change material for thermal energy storage. Appl Clay Sci. 2014;101:277–81.
Acknowledgements
Authors would like to thank the project of the National Natural Science Foundation of China (No. 51708551).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hou, P., Mao, J., Liu, R. et al. Improvement in thermodynamic characteristics of sodium acetate trihydrate composite phase change material with expanded graphite. J Therm Anal Calorim 137, 1295–1306 (2019). https://doi.org/10.1007/s10973-019-08061-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08061-7