Abstract
Depletion of non-renewable energy sources are at elevated manner due to the rapid growth of industrialization and transportation sector in last few decades and leads to further energy demand. Biodiesels especially second-generation fuels from non-edible oil resources are alternate sources for replacement of diesel fuel in CI engines due to their considerable environmental benefits. In the present work, non-edible feedstock of Calophyllum inophyllum seed oil (tamanu oil) is used for biodiesel production. Transesterification method is used for preparation of biodiesel in the existence of methanol with NaOH as catalyst. The copper nanoparticles are synthesized by electrochemical method, and it is characterized by using X-ray diffraction analysis (XRD) and scanning electron microscopy (SEM). XRD and SEM results confirm the presence of copper nanoparticle and size of around 30 nm. This paper aims to investigate the effects of the copper additive nanoparticles with biodiesel blends on the engine performance, combustion and emission characteristics of single-cylinder direct-injection diesel engine and compared that with diesel fuel. The results showed that the addition of nano-additives enhances brake thermal efficiency and reduces specific fuel consumption compared to biodiesel blends but slightly lower than diesel. Combustion characteristics also are enhanced by improved oxidation reaction inside the combustion chamber which resulted in higher heat release rate. The emissions of HC, NOx and O2 are significantly reduced for nano-additive blends compared to diesel but increased CO2 emission was observed. It is noticed that higher CO2 emission and substantial reduction of unused O2 emissions from engine fueled with nano-additive are evident for enhanced oxidation and better combustion. Energy and exergy analysis of the diesel engine is carried out to estimate the effect of using nanoparticle additive with biodiesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy is one of the most significant essential things necessary for economic growth of the country, as well as for modernization of the nation, increasing industrialization, improved lifestyle of human and especially for transportation. Currently, demand for energy is going away from the limits and it is not promising to control it in the future because of increasing price of petroleum products. In order to relieve those things, we are in need of best alternative to petroleum products. Out of many alternate fuels, one best solution is using extracted oils from plants seed. Generally, petroleum-based crude oil resources are obtained from certain countries which are highly concentrated in certain regions of the world. Consequently, those countries that are scarce in these resources like India are facing a foreign exchange crisis, primarily due to the import of crude oil. Therefore, it is essential to look for substitute fuels, which can be produced within the country from available resources [1].
Biodiesel from non-edible oil feedstock
In the future, the demand for biodiesel may be predictable to increase due to fast exhaustion of fossil fuels and improvement of vehicle transportation. A lot of researches in the world are taken up to develop the biodiesel sources like edible and non-edible oils. But the edible oil source may not be a suitable and sustainable source for biodiesel production that would build food scarcity. In addition, most of the non-edible plants have better potential to be grown in wasteland which does not affect the food crop-cultivating regions. This proves the need to use non-edible oil seeds that can be the dependable, sustainable and potential resource for biodiesel production [2, 3]. Biodiesel can be prepared from both edible and non-edible oils. These edible oils produced are mostly from croplands. The production of edible oils involves usage of larger areas and is expensive which leads to the deletion of more usable resources. If it continues, surely it will lead to acute shortage of land and also food supply. But nowadays, it becomes a big issue that shortage of these resources competes with food storages. So the ultimate way to get rid off all this is non-edible oil. Due to such tremendous increase in edible oil, there should be such justification in order to use non-edible oil. There are many non-edible oil crops found globally, out of which nearly 150 crops are found to exist. Some of the crops include Jatropha curcas, Pongamia pinnata, mahua, Garcinia indica and Moringa oleifera [4]. The non-edible oils from seeds may vary in their properties according to the content of fatty acid, and it can be used successively in engine without any modification. The fuel properties are very much closure to diesel, and it is suitable for conventional engine. Hence, non-edible oils are considered as second-generation biodiesel feedstock [5]. The advantage of using non-edible oil is high resistance to pest and disease. It can eliminate food shortages and they restore degradable lands [6].
Nanoparticle as additive for biodiesel
Nano-metal particles suspended in the base fluids result in improvement of the thermophysical properties, which makes them an observable choice for use in number of commercial applications including agriculture technology, biotechnology, engineering, medical sciences, transportation, etc [7]. With the enhancement in nanotechnology during last few decades, researchers focus on improvising combustion behavior, stability aspects, performance parameters and emission characteristics of conventional diesel engine using nanoparticle as an additive in diesel and biodiesel fuel blends. Recently, a minimum number of experimental works on using nano-sized metallic, nonmetallic and organic particles in the diesel and biodiesel fuel for diesel engine have appeared. The results obtained were very hopeful due to enhancement in chemical and thermophysical properties of modified fuel such as elevated reactive medium for combustion, better heat and mass transport properties due to high thermal conductivity, more surface to volume ratio, improvement in fire point, flash point, pour point, [8].
Addition of cerium oxide nanoparticles to the biodiesel resulted in increased viscosity, insignificant variation in cold temperature properties, improved efficiency and reduction of HC and NOx emissions. It is understood that cerium oxide thermally promotes the oxidation of hydrocarbon and reduction of nitrogen oxide, thus acting as an effective catalyst, when added in the nanoparticle form [9]. Thermal efficiency of Calophyllum inophyllum biodiesel (B100) is lower compared with diesel. Nano-additives-blended biodiesel showed slight increase in thermal efficiency and reduction in specific fuel consumption compared to diesel and biodiesel. HC emission reduction takes place 80% at full load for cobalt oxide and 70% reduction at 75% load for titanium dioxide. NOx emission of cobalt oxide and titanium dioxide showed gradual increase at all loads as compared with pure biodiesel. CO emission of cobalt oxide-blended biodiesel showed 30% reduction at full load, and titanium dioxide-blended biodiesel showed 25% increase in CO emission at the full-load condition. It was found the mixing of nanoparticles with biodiesel has many advantages and it has a lot of promise for the future [10]. Addition of copper nanoparticles as additive in soya bean biodiesel enhanced the combustion process due to high surface reactivity. A maximum improvement of 1.03% in the brake thermal efficiency was obtained in B10 with 40 nm copper particle compared with pure diesel. In addition, it was observed that the decrease in the size of the nanoparticle increases the surface area of reaction which increases the efficiency. A maximum increase of 1.01% in the brake thermal efficiency was obtained when the particle size was decreased from 50 to 40 nm. Nitrogen oxide emissions for B10 with nano-copper were low compared with pure diesel at all the levels of loads. A maximum of 7.46% reduction in NOx emission was achieved by using B10 nano-copper compared with pure diesel at maximum loading condition. A maximum of 16.33% reduction in NOx was achieved when using B10 with 40 nm copper particle than B10 with 50 nm copper particle [11]. Additive like magnalium (Al-Mg) and cobalt oxide (Co3O4) resulted in significant improvement of efficiency compared to neat biodiesel operation without additive. Nearly 1% improvement in the thermal efficiency was seen for magnalium additive compared to B100 without additive. The reduction of HC emission observed for cobalt oxide-blended biodiesel was 75% at 75% load, whereas with magnalium additive, it was 70% at 50% load. By comparing the above two additives, cobalt oxide nano-fuel additive showed 47% reduction in NOx emission, whereas reduction in CO emission was observed in both cobalt oxide and magnalium nano-fuel additive [12]. Addition of rice husk nanoparticle in the size of 20 nm and 0.1% with Pongamia seed oil methyl ester showed increased performance, combustion and reducing emission characteristics. The results indicated that the additives improved brake thermal efficiency, higher combustion duration and heat release rate and reduced HC, CO and NOx emissions. It is observed that higher cylinder peak pressure was obtained for B20, because of the presence of oxygen in this biodiesel [13].
Nano-fuels which were prepared by sonicating nanoparticles of aluminum, iron and boron in base diesel resulted in shortened ignition delay, agglomerate ignition, improved combustion rate and longer flame sustenance. At maximum loading conditions, brake thermal efficiencies and exhaust gas temperatures of nano-fuels were elevated by 2 to 9% and 5 to 8%, respectively. Compared to diesel, a reduction of CO and HC was observed but a marginal increase in NOx emission was noted at elevated temperatures [14]. Metal-based additives like CNT, ZnO2, Cu2O, FeCl3, CeO2, Co3O4, Al and TiO2 nanoparticles showed an increase in peak pressure, heat release rate, cylinder gas temperature with prior start of combustion and shortened ignition delay. Existence of oxygen content, improved fuel mixing, better oxidation of hydrocarbons and enhanced combustion rate in both premixed and diffusion combustion phases are primarily attributed to this. Introducing metal-based additives offer additional oxygen to prompt the combustion of fuel [15]. Commonly, factors such as elevated temperatures, presence of air or existence of metals assist in oxidation. Oxidation studies of biodiesel have confirmed that copper showed the strongest catalytic effect on oxidation [16].
Finally, it is concluded that numerous researchers have identified that inclusion of nanoparticles in diesel and biodiesel fuels showed better properties over unmodified base fuel such as availability of enhanced reactive surface area (high surface to volume ratio) responsible for further complete combustion due to shortened ignition delays. The inherent characteristics of the additives such as nanolength scale and higher surface area to volume ratio makes more surface area of contact between fuel and oxygen for rapid oxidation, thus accomplished in releasing more energy compared to base fuels.
In this work, Calophyllum inophyllum oil is chosen for investigation because it is non-edible oil source and available in the coastal regions of South India and South Asia. It contains higher amount of unsaturated fatty acids (70.8%) than saturated fatty acids (29.2%). The oil can be converted into alkyl esters by transesterification process. The Cu nanoparticles are synthesized by electrochemical deposition with low cost and least time. After the completion of the above two processes, nanoparticles are blended with biodiesel by suitable method. Investigation has been performed to find out the effects of using nano-copper additives with biodiesel and their blends on the performance, combustion and emission characteristics of single-cylinder diesel engine with varying loads. The present study emphasized to compare the behavior of diesel engine fueled by diesel, biodiesel and their blends with copper nano-additives. Energy and exergy analysis of the diesel engine is carried out to estimate the effect of using nanoparticle additive with biodiesel.
Experimental
Production of Biodiesel from Calophyllum inophyllum
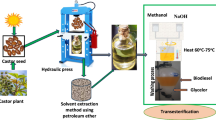
Adequate amount of tamanu oil seeds is to be collected and dried for some days in daylight to reduce the moisture. During the dehydration process, a kernel becomes loose and reduces its mass. Finally, the seed turns brownish in color and has high aromatic odor and enhances its oil content. In this work, seeds are to be positioned in the screw press for mechanical extraction. The oil extracted is dark green in color and is used as feedstock for the synthesis of biodiesel. Numbers of approaches are available for production of biodiesel from the feedstock oil to the purification of the esters (biodiesel) and the coproduct. In contrast to other methods, transesterification was extensively used in the earlier researches because of high yield with negligible side reactions and minimum reaction time. This is the most flourishing method for reducing the viscosity of oil and makes the properties to meet the ASTM D 6751 [17].
Transesterification
It is the chemical process of breaking of oils into esters and glycerol in the presence of alcohol and catalyst. Depending upon the quality of the oil, especially free fatty acid (FFA) content, the esterification process parameters may vary. If the FFA content is more than 4%, two-stage esterification (acid and alkaline esterification) is required. The oil extracted has FFA content of 18% (36 mg KOH/g). Therefore, two-stage esterification process is adopted [18]. The esterification arrangement is shown in Fig. 1.
Acid esterification
The unprocessed oil containing high FFA has triglyceride compounds which were converted into diglyceride through acid esterification. The oil is heated up to 60 °C in a flask and required quantity of methanol, and anhydrous H2SO4 is dissolved in it. The mixture is constantly stirred at constant speed and temperature constant at 60 °C for 2 h and is not allowed to go above 60 °C to avoid methanol loss. The mixture is transferred to separating funnel, and after settling, the top layer contains excess methanol and the bottom layer remains as diglyceride which is shown in Fig. 2.
Alkaline esterification
The bottom layer of diglyceride undergoes alkaline esterification, where it is mixed with sodium methoxide (methanol + sodium hydroxide) and heated to 60 °C. The mixture is continuously stirred for 1 h and allowed to settle naturally in separating funnel. The top layer formed is ester and bottom layer is glycerol. Then, the ester is allowed for water wash process till the water reaches the pH 7 which is shown in Fig. 2. The FFA of tamanu oil after the above process is around 1.2% (2.4 mg KOH/g).
Synthesis of nano-copper additive
The Cu nanoparticles are synthesized by electrochemical deposition from aqueous CuSO4 solution. Experiments were carried out in an electrochemical cell of 500 cm3 beaker which consists of aqueous CuSO4 solution (prepared by pouring the distilled water into the CuSO4 salt) acting as an electrolyte. Electrochemical cell contains two electrodes (surface cleaned) with cathode (cylindrical rod made of Cu) and anode (Cu plate) which are in contact with an electrolyte that brings out a chemical reaction when connected to an outside source of electricity, say, DC power supply unit (5 amperes). Electrolysis of this solution was obtained by passing constant current inside solution through anode and cathode as is shown in Fig. 3. After the electrolyzing process, Cu nanoparticles deposited on the cathode surface are removed carefully. The obtained Cu nanoparticles were washed several times with distilled water [19, 20].
The XRD study confirms that the resultant particles are copper nanopowder which is shown in Fig. 4. The experimental diffraction angle [2θ] and standard diffraction angle [2θ] of Cu specimen are shown in Table 1, which shows both the results are almost the same.
The SEM analysis of Cu nanoparticles is shown in Fig. 5. From the results, it was clear that hexagonal-like crystalline Cu nanoparticles were formed from the electrochemical process.
Preparation of nano-fluids
To prepare nano-fluids by suspending nanoparticles into base fluids, some special requirements are necessary such as even suspension, durable and stable suspension, low agglomeration of particles and no chemical change of fluid. In this study, surface active agents and/or dispersants were added to nanoparticles to disperse particles into fluid and allowed for ultrasonic vibration for few hours. The Cu nanoparticles are then suspended in biodiesel for many days, and this type of preparation of nano-fluids prevents the sedimentation and agglomeration of nanoparticles in the base fluids.
Results and discussions
The experiments are carried out at constant speed of 1500 rpm for different loads for the different blends, which is shown in Fig. 6. The fuel samples used are diesel, B10, B20, B100, B10 + 30 mg L−1 Cu, B20 + 30 mg L−1 Cu and B100 + 30 mg L−1 Cu at compression ratio of 17.5:1. The specifications of the engine are shown in Table 2. The exhaust emissions are recorded by an AVL 444 di gas analyzer; the gases measured are CO, HC, CO2, O2 and NOx. Smoke measurement can be done by AVL 437 smoke meter in terms of smoke opacity.
Performance characteristics
Brake thermal efficiency
For all the fuel blends tested in the engine, the brake thermal efficiency increased with respect to increase in engine load. The maximum brake thermal efficiency was 32.23% for diesel, 31.1% for B10, 30.71% for B20, 29.76% for B100, 31.5% for B10 + Cu, 31.1% for B20 + Cu and 30.9% for B100 + Cu at the maximum load. The brake thermal efficiency of biodiesel and their blends are lower than that of pure diesel. The decrease in brake thermal efficiency of biodiesel is mainly due to the lower heat of combustion. Addition of biofuels to commercial diesel fuel results in a linear decrease of the heat of combustion with the quantity of the biodiesel added to the diesel, because the organic chain found in vegetable oil is longer than that in diesel [21]. Brake thermal efficiency of biodiesel and their blends with additive is higher compared to biodiesel without additives but slightly lower than diesel which is shown in Fig. 7. An increase of 1.2% is achieved for B10 and B20 with additive and 3.8% for B100 with additive. The increase in brake thermal efficiency is due to the presence of additive which enhances better oxidation and high heat release rate.
Specific fuel consumption
Figure 8 shows the variation of brake specific fuel consumption (BSFC) with respect to load. BSFC of biodiesel blends and biodiesel with metal additive is slightly greater than pure diesel. At all loads, BSFC of all biodiesel blends is higher compared to biodiesel blends with additive at higher loads. The addition of additives shows a significant reduction of SFCs at all loads. A reduction of 3% to 6% was achieved for biodiesel blends with additives compared to normal biodiesel blends at maximum load. This may be due to the fact that increase in combustion temperature leads to increase in the conversion efficiency of heat energy into mechanical work and results in reduction of BSFC with respect to engine loads [21].
Emission characteristics
NOx emission
From Fig. 9, an important conclusion can be made. Generally, NOx emission phenomenon is strongly dependent on temperature. It is observed that the NOx emissions in the case of biodiesel fuel and its blends are lower compared to neat diesel at all loads. It is seen that the NOx emission of biodiesel blends is lower than pure diesel at 100% load. This reduction in NOx emissions of biodiesel may be owing to lesser temperatures of the gas in combustion chamber which is shown in Table 3. The same trend is also obtained in [22] using tamanu oil as a biodiesel. However, these observations disagree with some previous studies. The NOx emissions from biodiesel-fueled diesel engines were found to be higher than conventional diesel fuel. This is because of difference in fuel properties, ignition rate, combustion pressure and temperature, etc. The reduction in NOx is mainly due to reduced premixed phase (lower heat release rate), and it leads to lower cylinder temperature [23]. However, the NOx emission of biodiesel blends with their additives is less than the pure diesel at maximum load, but it is higher than biodiesel blends. This is expected because the presence of nano-copper additive in biodiesel enhances the oxidation reaction which leads to increase in combustion chamber pressure and temperature which results in oxidation of nitrogen to nitric oxide and hence promotes higher rate of NOx formation. It is evident from higher NOx for biodiesel with additives. The existence of nanoparticles leads to higher NOx emissions [13].
HC emission
Hydrocarbon emissions (HC) of biodiesel-blended fuels were reduced due to higher cetane number and higher oxygen quantity. The presence of higher amount of the oxygen in the biodiesel leads to complete combustion, and the higher cetane number reduces the ignition delay, as the reduced ignition delay decreases the unburned hydrocarbon emissions [24]. Figure 10 shows the hydrocarbon emissions for selected fuels. Compared to diesel, HC emission of biodiesel blends was lower than diesel at all loads, respectively. This is due to higher oxygen content, which improves the combustion further and reduces the fuel-rich region. The most important reasons for HC emissions are fuel-rich region misfiring, flame quenching and desorption of lubrication oil [12]. However, the addition of nano-copper additives with biodiesel resulted in further reduction in HC emission. The inclusion of nanoparticles acts as oxidizing catalyst which promotes complete combustion.
CO2 emission
Figure 11 shows the variation of carbon dioxide (CO2) level with respect to various loads for diesel and different blends of biodiesel and their additives. Generally, biodiesel blends have higher CO2 emissions compared to diesel fuel. The study exposed that with an increase in volume fraction of biodiesel in a diesel, higher CO2 emissions were resulted. This is due to longer and stable diffusion combustion phase, and oxygen availability in the biodiesel made the combustion complete. Further, it was found that with the addition of Cu nanoparticles, there has been a considerable drop in the CO2 emissions level. The CO2 emissions are slightly reduced with metal additives at different load conditions. This is because of the addition of nanoparticles, which enhanced the combustion due to the catalytic action, thereby increasing CO2 emissions compared to diesel. Oxidation reaction takes place with the copper nanoparticles in nano-additive blends. From Fig. 10, the variations are large at high loads because of rapid oxidation reaction and they do not happen at low loads.
CO emission
The variation of carbon monoxide (CO) emission for different fuels tested in the engine is shown in Fig. 12. The CO emission of biodiesel blends and biodiesel blends with their additives was lower than diesel. The reduction in CO emission is due to higher oxygen content and higher cetane number possessed by biodiesel. There is no significant difference in the CO concentrations between the biodiesel blends and blend with their additives. Finally, it is concluded that copper nanoparticles-blended biodiesel has no major influence on the CO emission compared to biodiesel without additive.
O2 emission
As seen in Fig. 13, biodiesel with nano-additives emits lower unused O2 emissions compared to all other blends. It is clear that oxygen consumption was higher or oxidation rate was higher in the case of biodiesel with additives. The catalytic oxidation of nanoparticle enhances complete combustion inside the combustion chamber. This is evident from lower unused O2 emission for biodiesel with additive. However, biodiesel also has lower O2 emissions compared to diesel fuel. Since oxygen content in the fuel is highly utilized during diffusion phase of combustion, it made the combustion better and leads to lower O2 emissions.
Smoke opacity
The smoke opacity is appreciably reduced for biodiesel blends and their additives when compared to conventional diesel fuel as shown in Fig. 14. The reduction in smoke opacity for biodiesel blends and their additives is mainly due to inherent oxygen content and presence of copper nano-additives which enhanced oxidation reaction as well as complete combustion of the fuel. The combustion is stable and complete because biodiesel contains less aromatics and additional oxygen content. Smoke opacity is decreased when the concentration of biodiesel was increased in the blend. Nonexistence of aromatics is more predominant than the existence of inbuilt oxygen to reduce the growth of smoke opacity [25].
Combustion characteristics
The performance and emission characteristics of diesel engine fueled by tamanu oil and tamanu oil with nanoparticles are mainly affected by combustion process. The combustion characteristics like in-cylinder gas pressure and net heat release rate are discussed in this section for the diesel, biodiesel blends and biodiesel with nano-additives.
In-cylinder pressure variation
It is clear from Fig. 15 that the diesel fuel produced high peak cylinder pressure compared to tamanu oil biodiesel blends and biodiesel with nano-additives. This is due to the higher calorific value of diesel fuel than that of biodiesel blends and its additives. The maximum peak cylinder pressure was 59.6 bar for diesel, whereas 58.6 bar, 58.2 and 57.7 for B10, B20 and B100 at full load. It is noticed that the combustion starts earlier for pure biodiesel and its blends with additives compared to diesel at all engine loads. This is due to the inherent characteristics like higher cetane number and excess oxygen content of tamanu oil. This improves the evaporation and atomization process of fuel which confines shorter ignition delay, and hence low peak cylinder pressure is obtained. Several researchers also obtained the same results in their studies [26]. Furthermore, the adding together of nano-additive with tamanu oil blends has shown slight rise in peak cylinder pressure compared to tamanu oil blends. The maximum peak pressures were 59.7, 59.2 and 58.6 bar for B10+Cu, B20+Cu and B100+Cu which are greater than B10, B20 and B100 at full load which is shown in Fig. 14. This confines that the presence of copper additive in biodiesel improves the oxidation reaction and leads to rise in peak pressure.
Net heat release rate (NHRR)
The heat release rate with respect to crank angle is shown in Fig. 16. The maximum heat release rate of biodiesel and their blends is lower to that of diesel, particularly, 38.64 J/deg CA, 36.07 J/deg CA and 35.41 J/deg CA for B10, B20 and B100 compared with 43.55 J/deg CA for diesel. This is due to the effect of the shorter ignition delay, lower heating value and weak premix combustion phase of biodiesel and their blends. On the other hand, the higher heat release rate of diesel is due to increased accumulation of fuel during the relatively longer delay period. As a result of the shorter delay period of biodiesel and their blends, maximum heat release rate occurs prior in comparison with neat diesel. On the other hand, the heat release rate during the phase of late combustion is slightly lower for biodiesel and their blends compared to that of diesel. This is because the biodiesel with superior oxygen content is adequate to ensure complete combustion of the fuel that is left over during the major combustion phase and continues to burn in the late combustion phase [27]. However, the addition of nanoparticles with biodiesel and their blends showed significant improvement in heat release rate particularly 5–6% compared to biodiesel and their blends at 100% load and 4–5% at 75% load. The differences in heat release rate without and with additive are shown in Table 3. The improvement in NHRR of biodiesel with additive is due to the enhanced oxidation reaction which took place inside the combustion chamber, and it is concluded that the presence of nano-metal additive would enhance the oxidation reaction as well as combustion reaction.
Energy and exergy analysis
Energy is a basic perception of thermodynamics and one of the most considerable aspects of engineering investigation. It is essential to identify the highest feasible performance of the various fuel modes which can offer crucial comparison parameters with base engine. These results will assist in reducing the available energy loss to enhance the overall engine performance [28, 29]. Exergy analysis technique has been extensively used in performance evaluation of different types of engines for identifying efficiencies and losses [30].
In this investigation, the first and second laws of thermodynamics were used to evaluate the quality and quantity of energy in a direct-injection single-cylinder diesel engine using diesel and tamanu oil biodiesel with their additives. Based on the experimental data, measurements of fuel, air and all the temperatures at relevant points were taken. Balances of energy and exergy rates for the above-mentioned engine were calculated, and then energy and exergy efficiencies were determined for each fuel blend and were compared with each other.
The energy input (Qin) of an IC engine is a chemical energy which is contained in its fuel and is given by
In an engine, the amount of input energy is converted into various following forms of energy.
Useful work output or shaft energy (Qb) is given by
Energy transferred to the cooling water (Qcw) is given by
Energy transferred to the exhaust gas (Qeg) is given by
Uncounted loss (Qun) due to friction, heat transfer to environment (radiation), working auxiliary parts, etc., is given by
The second law analysis indicates different forms of energy that have various levels of ability to do useful mechanical work. This ability to carry out useful mechanical work is defined as availability. Total available energy source is called as exergy.
In an IC engine, the exergy input (Ein), i.e., chemical availability of fuel converted into other exergy forms, is given by
Exergy for brake power (Eb) is given by available energy at the output shaft of the engine which is equal to shaft energy or useful work output (Qb).
Exergy for cooling water (Ecw) is given by
Exergy for exhaust gas (Eex) is given by
Destroyed exergy (Edes) is given by
Exergy efficiencies are useful for unique character means for utilizing energy resources. Exergy efficiencies also can be used to estimate the effectiveness of engineering measures taken to improve the performance of the engine. Efficiency of exergy indicates the percentage of energy that can be utilized. The second law efficiency or exergy efficiency (ηexergy) is the ratio of total exergy recovered from the system to the total exergy input into the system. The recovered availability includes Eshaft, Ecw and Eex.
An exhaustive investigation on the performance of the biodiesel–biodiesel blends with additives fuel was compared with neat diesel. This is a balance between the various terms of the second law analysis described by, namely, fuel exergy input, work exergy, exergy exchange through cooling water and exhaust exergy destroyed and exergy efficiency, as shown in Figs. 17 and 18.
The exergy efficiency increases at higher loads for all fuel blends. In particular, exergy efficiencies of blended fuels improve significantly when compared to low-load conditions. As a result of the improved combustion characteristics by additive at higher loads, the exhaust gas exergy and cooling exergy are increased. Besides, the shaft exergy of the fuels improved with an increased load. Consequently, when load is increased, the added cumulative exergies increased the exergy efficiency.
At medium loads of 25–75%, all fuel blends were tested in the engine and the exergy efficiency of biodiesel with its additives was higher compared to diesel mode. This is due to their lower energy content and combustion characteristics due to the low-temperature environment. However, at high loads, the exergy efficiency of diesel was higher than all other blends. The improvement of exergy efficiency of biodiesel and their blends with additive is due to enhanced oxidation and combustion reaction which resulted in effective utilization of fuel exergy into shaft exergy. So, it is concluded that additive helps in improving exergy efficiency.
Analysis of uncertainty
There are a number of operational and physical parameters that cause a little amount of uncertainty during measurement. Even with careful experimentation, the value of measured quantity differed from true value. This is due to the presence of error. Hence, complete error evaluation was carried out by means of an uncertainty analysis for the measured parameters. This analysis was carried out on the basis of the root-mean-square method [31, 32].
For the above, let R be the calculated quantity from n independent parameters (X1, X2… Xn) which were measured during the measurement. Hence, R = R(X1, X2 … Xn). Therefore, uncertainty limits for the measured parameters are X1 ± ∆X1, X2 ± ∆X2… Xn ± ∆Xn and the uncertainty limit for the calculated value is R ± ∆R.
In order to obtain the practical error limits for any calculated quantity based on several measured quantities, the magnitude of the error is calculated using root-mean-square method principle which is given by
The estimated uncertainty values of calculated parameters at different operating conditions are 0.5–1.4% for specific fuel consumption and 0.6–1.2% for brake thermal efficiency. The sensitivity of the various instruments used in this experimental study is given in Table 4.
Conclusions
The present investigation was carried out to study the effect of nano-copper additive on the performance, combustion and emission characteristics of diesel engine fueled with Calophyllum inophyllum methyl ester blends and were compared with diesel fuel. Based on the above discussion from the experimental study, the following conclusions were drawn:
-
The effect of copper nanoparticles with tamanu oil biodiesel fuel blends was found to improve the diesel engine performance characteristics in terms of higher brake thermal efficiency and lower engine brake specific fuel consumption. The improved performance is due to high surface area to volume ratio of nanoparticles which accelerates the combustion process.
-
The application of nanoparticles additive with biodiesel fuel blends enhances the engine combustion characteristics and reduces the emissions considerably. The existence of nanoparticles showed shorter ignition delays, higher cylinder gas pressure and net heat release rate. Addition of nanoparticle additives with biodiesel was found to be a better oxidizing agent of the fuels and augment its combustion characteristics.
-
Compared to diesel, significant reduction of HC, CO, NOx and unused O2 emissions was observed for fuels with additives. However, CO2 emissions were increased. Higher CO2 emissions and lower unused O2 emissions are evident for the better utilization of oxygen in the fuels which proved the improved combustion. This is due to the catalyst effect of nano-copper additives.
-
The improvement of exergy efficiency of biodiesel and their blends with additive is due to enhanced oxidation and combustion reaction which resulted in effective utilization of fuel exergy into shaft exergy. This is due to their lower energy content and combustion characteristics due to the low-temperature environment.
-
Finally, the nano-copper additive prepared by this electrochemical method was cheap and easy without any risk and also tamanu oil is easily available in the coastal region of South India. A significant improvement in the performance and reduction in emission characteristics proved that the nano-additive-blended biodiesel is commercially feasible in conventional engines without any engine modifications.
Abbreviations
- BP:
-
Brake power
- BSFC:
-
Brake specific fuel consumption
- BTE:
-
Brake thermal efficiency
- CR:
-
Compression ratio
- B10:
-
10% diesel & 90% biodiesel
- B10+Cu:
-
10% diesel & 90% biodiesel + copper nanoparticle
- Cu:
-
Copper
- HC:
-
Hydrocarbon
- NOx :
-
Oxides of nitrogen
- CO2 :
-
Carbon dioxide
- CO:
-
Carbon monoxide
- FFA:
-
Free fatty acid
- nm:
-
Nanometer
- mg:
-
Milligram
- KOH:
-
Potassium hydroxide
- NaOH:
-
Sodium hydroxide
- XRD:
-
X-ray diffraction
- SEM:
-
Scanning electron microscope
- C vf :
-
Calorific value of fuel (kJ kg−1)
- m a :
-
Mass flow rate of air (kg s−1)
- m f :
-
Mass flow rate of fuel (kg s−1)
- m w :
-
Engine water flow (kg s−1)
- C peg :
-
Specific heat of gas (kJ kg−1 K−1)
- C PW :
-
Specific heat of water (kJ kg−1 K−1)
- T iw :
-
Engine water inlet temperature (K)
- T ow :
-
Engine water outlet temperature (K)
- T a :
-
Ambient temperature (K)
- T og :
-
Exhaust gas outlet temperature (K)
- P eo :
-
Exhaust gas pressure outlet (bar)
- P a :
-
Ambient pressure (bar)
References
Ramadhas A, Jayaraj S, Muraleedharan C. Use of vegetable oils as IC engine fuels—a review. Renew Energy. 2004;29(5):727–42.
Kumar A, Sharma S. Potential non-edible oil resources as biodiesel feedstock: an Indian perspective. Renew Sustain Energy Rev. 2011;15(4):1791–800.
Ong H, Mahlia T, Masjuki H, Norhasyima R. Comparison of palm oil, Jatropha curcas and Calophyllum inophyllum for biodiesel: a review. Renew Sustain Energy Rev. 2011;15(8):3501–15.
Singh S, Singh D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: a review. Renew Sustain Energy Rev. 2010;14(1):200–16.
Atabani A, da Silva CA. Calophyllum inophyllum L.—A prospective non-edible biodiesel feedstock. Study of biodiesel production, properties, fatty acid composition, blending and engine performance. Renew Sustain Energy Rev. 2014;37:644–55.
Atabani A, Silitonga A, Ong H, Mahlia T, Masjuki H, Badruddin IA, et al. Non-edible vegetable oils: a critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew Sustain Energy Rev. 2013;18:211–45.
Tamilvanan A, Balamurugan K, Ponappa K, Kumar BM. Copper nanoparticles: synthetic strategies, properties and multifunctional application. Int J Nanosci. 2014;13(02):1430001–22.
Saxena V, Kumar N, Saxena VK. A comprehensive review on combustion and stability aspects of metal nanoparticles and its additive effect on diesel and biodiesel fuelled CI engine. Renew Sustain Energy Rev. 2017;70:563–88.
Sajith V, Sobhan C, Peterson G. Experimental investigations on the effects of cerium oxide nanoparticle fuel additives on biodiesel. Adv Mech Eng. 2010;2:581407.
Jeryrajkumar L, Anbarasu G, Elangovan T. Effects on nano additives on performance and emission characteristics of Calophyllim inophyllum biodiesel. Int J Chem Tech Res. 2016;9(4):210–9.
Balamurugan K, Tamilvanan A, Anbarasu M, Mohamed SA, Srihari SM. Nano-copper additive for reducing NOx emission in soya bean biodiesel-fuelled CI engine. J Biofuels. 2013;4(1):1–8.
Ganesh D, Gowrishankar G, editors. Effect of nano-fuel additive on emission reduction in a biodiesel fuelled CI engine. Electrical and Control Engineering (ICECE), 2011 Int Conf on; 2011: IEEE.
Vinukumar K, Azhagurajan A, Vettivel S, Vedaraman N. Rice husk as nanoadditive in diesel–biodiesel fuel blends used in diesel engine. J Therm Anal and Calorim. 2018;131(2):1333–43.
Mehta RN, Chakraborty M, Parikh PA. Nanofuels: combustion, engine performance and emissions. Fuel. 2014;120:91–7.
Imdadul H, Masjuki H, Kalam M, Zulkifli N, Rashed M, Rashedul H, et al. A comprehensive review on the assessment of fuel additive effects on combustion behavior in CI engine fuelled with diesel biodiesel blends. RSC Adv. 2015;5(83):67541–67.
Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol. 2005;86(10):1059–70.
Ayyasamy T, Balamurugan K, Duraisamy S. Production, performance and emission analysis of Tamanu oil-diesel blends along with biogas in a diesel engine in dual cycle mode. Int J Energy Technol Policy. 2018;14(1):4–19.
Dinesh K, Tamilvanan A, Vaishnavi S, Gopinath M, Mohan KR. Biodiesel production using Calophyllum inophyllum (Tamanu) seed oil and its compatibility test in a CI engine. Biofuels. 2016. https://doi.org/10.1080/17597269.2016.1187543.
Tamilvanan A, Balamurugan K, Ponappa K, Madhan Kumar B. Using response surface methodology in synthesis of ultrafine copper nanoparticles by electrolysis. Int J Nanosci. 2016;15(01&02):1650001.
Theivasanthi T, Alagar M, Nano sized copper particles by electrolytic synthesis and characterizations. Int J Phy Sci. 2011;16(15):3662–71.
Andrade RD, Faria EA, Silva AM, Araujo WC, Jaime GC, Costa KP, et al. Heat of combustion of biofuels mixed with fossil diesel oil. J Therm Anal Calorim. 2011;106(2):469–74.
Sahoo P, Das L, Babu M, Naik S. Biodiesel development from high acid value polanga seed oil and performance evaluation in a CI engine. Fuel. 2007;86(3):448–54.
Venkanna B, Reddy CV. Performance, emission and combustion characteristics of direct injection diesel engine running on calophyllum inophyllum linn oil (lionne oil). Int J Agric Biol Eng. 2011;4(1):26–34.
Kumar MV, Babu AV, Kumar PR. The impacts on combustion, performance and emissions of biodiesel by using additives in direct injection diesel engine. Alexandria Eng J. 2017;57(1):509–16.
Venkanna B, Venkataramana Reddy C. Direct injection diesel engine performance, emission, and combustion characteristics using diesel fuel, non-edible honne oil methyl ester, and blends with diesel fuel. Int J Energy Res. 2012;36(13):1247–61.
Nanthagopal K, Ashok B, Tamilarasu A, Johny A, Mohan A. Influence on the effect of zinc oxide and titanium dioxide nanoparticles as an additive with calophyllum inophyllum methyl ester in a CI engine. Energy Convers Manag. 2017;146:8–19.
Sahoo P, Das L. Combustion analysis of Jatropha, Karanja and Polanga based biodiesel as fuel in a diesel engine. Fuel. 2009;88(6):994–9.
Krishnamoorthi M. Exergy analysis of diesel engine powered by diesel-mustard biodiesel blend with diethyl ether as additive. J Chem Pharm Res. 2015;7(8):809–16.
Singh N, Kumar H, Jha M, Sarma AK. Complete heat balance, performance, and emission evaluation of a CI engine fueled with Mesua ferrea methyl and ethyl ester’s blends with petrodiesel. J Therm Anal Calorim. 2015;122(2):907–16.
Iortyer H, Bwonsi L. Energy and Exergy Analysis of a CI engine fuelled with biodiesel fuel from palm kernel oil and its blends with petroleum diesel. Int J Adv Eng Res Sci. 2017;4(7):87–97.
Varuvel EG, Mrad N, Tazerout M, Aloui F. Experimental analysis of bio-fuel as an alternative fuel for diesel engines. Appl Energy. 2012;94:224–31.
Paul A, Panua R, Debroy D. An experimental study of combustion, performance, exergy and emission characteristics of a CI engine fueled by Diesel-ethanol-biodiesel blends. Energy. 2017;141:839–52.
Acknowledgements
The authors would like to appreciate the support of Department of Mechanical Engineering, Kongu Engineering College, Erode, Tamil Nadu, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamilvanan, A., Balamurugan, K. & Vijayakumar, M. Effects of nano-copper additive on performance, combustion and emission characteristics of Calophyllum inophyllum biodiesel in CI engine. J Therm Anal Calorim 136, 317–330 (2019). https://doi.org/10.1007/s10973-018-7743-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7743-4