Abstract
Herein, we measured the enthalpy relaxation of three styrene oligomers with different molecular weights (styrene oligomer with Mw = 4.53 × 102: PSA-300, styrene oligomer with Mw = 5.89 × 102: PSA-500, and styrene oligomer with Mw = 1.01 × 103: PSA-1000) near their respective glass transition temperatures (Tg) using an adiabatic calorimeter. We determined the relaxation rates and the amounts of configurational enthalpy released from the temperature dependence of the temperature drift rates around Tg. Based on our experimental findings, we found the amounts of configurational enthalpy release per monomer unit to be 0.8, 3.5, and 1.6 kJ mol−1 for PSA-300, PSA-500, and PSA-1000, respectively. These values were 3.9–18 times larger than that of glycerol, which is a typical glass-forming liquid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The glassy state is considered to be a non-equilibrium thermodynamic state. At the glass transition temperature Tg, a transition occurs from a non-equilibrium thermodynamic state to an equilibrium thermodynamic state, which shows that the glass transition is essentially a time-dependent dynamic change. The glass transition is not a phase transition. Instead, it results from the freezing of molecular rearrangement motions [1]. It therefore depends on the thermal history of the cooling rate of the sample and on the timescale of the measurement. The glass transition temperature is generally defined as the temperature at which the relaxation time of rearrangement motions is 103 s [2, 3].
When a sample is slowly heated from a glassy state in which the molecular rearrangement motions are frozen, the timescale on which such rearrangements occur becomes sufficiently short so that relaxation and diffusive motions can be observed near the glass transition temperature Tg. These motions enable the relaxation of the non-equilibrium state toward the equilibrium one, and they are accompanied by a spontaneous exothermic release of heat from the sample. This phenomenon can be observed as an enthalpy change in a thermal measurement. In the adiabatic method, the sample is maintained in an adiabatic state and the enthalpy change accompanying the rearrangements of the molecules can be observed directly around Tg. From this feature, we can study the relaxation from the non-equilibrium state to the equilibrium glassy state from a thermodynamic point of view. With an adiabatic calorimeter, it is possible to measure the heat capacity with high precision and high accuracy and observe the enthalpy change even when the phenomenon has a considerably long relaxation time.
The principle of measuring the heat capacity with an adiabatic calorimeter is simple. The temperature is measured both before and after heating the sample, after which the heating energy is divided by the temperature difference. Measuring the temperature of the sample container at constant time intervals under the adiabatic condition is called a “temperature drift measurement.” In ordinary heat capacity measurements with an adiabatic calorimeter, temperature drift measurements are performed repeatedly and the sample is repeatedly heated in a controlled adiabatic state to measure the heat capacity. In the glass transition region, the sample undergoes spontaneous exothermic or endothermic changes so that an increase or decrease in temperature with time can be observed, irrespective of the fact that the temperature drift measurements are performed under adiabatic conditions.
These spontaneous exothermic or endothermic changes in the sample are observed as a temperature rise or a temperature drop of the whole measurement system, including the sample container.
Therefore, in the temperature drift measurement at around glass transition region, the change in temperature with respect to time, that is, temperature drift rate, means the energy transfer within the sample between the configurational energy and thermal vibration which determines the measured temperature. “Exothermic” and “endothermic” is the energy transfer from the configurational energy to the thermal vibration and vice versa, respectively.
To understand the dynamics of the glassy state [4,5,6,7,8,9,10,11,12,13], enthalpy change measurements have been performed in an adiabatic calorimeter for simple molecular glasses, including glycerol and 1-pentene, and for glassy crystals. However, the effects of the changes in molecular weight on the glassy dynamics around Tg have not yet been investigated. This study aims to explore the influence of the enthalpy relaxation of the amorphous state due to an increase in the repeating units.
Herein, we observed the enthalpy changes in the vicinity of the glass transition for styrene oligomers with different molecular weights. Based on the observations, we determined the total amount of configurational enthalpy released around Tg and compared it with the results obtained for previously reported molecular glasses.
Experimental
The three styrene oligomers used for the measurements were purchased from TOSOH Corporation. Their molecular weights were Mw = 4.53 × 102 (PSA-300), Mw = 5.89 × 102 (PSA-500), and Mw = 1.01 × 103 (PSA-1000), and the molecular weight distributions of these oligomers were Mw/Mn = 1.17 (PSA-300), Mw/Mn = 1.14 (PSA-500) and Mw/Mn = 1.16 (PSA-1000). All samples were atactic styrene oligomers, which never undergo crystallization.
We used a top-loading-type adiabatic calorimeter in conjunction with a refrigerator that was already installed in the laboratory. The degree of precision of this calorimeter is same as that of a similar type of calorimeter that has been reported in the literature [10]. The amount of each sample loaded in a cell was 4.20698 ± 0.00001 g for PSA-300, 4.44025 ± 0.00001 g for PSA-500, and 3.55279 ± 0.00001 g for PSA-1000. We charged the dead space of the cell (1 cm3) with helium gas (1.0 × 103 hPa at room temperature) to obtain good thermal contact between the cell and the sample.
After placing each sample in the apparatus, the sample was cooled from 300 to 160 K at a rate of 3.0 K min−1. We initiated the measurements of the enthalpy release at the lowest temperature. All three oligomers were measured at a temperature ranging from 160 to 300 K. For PSA-300, the enthalpy release was also measured on a sample that was cooled at a rate of 0.1 K min−1.
The measurements of the enthalpy change were performed by repeatedly measuring the temperature drift rate at which the sample temperature changed while it was heated from 160 to 300 K under adiabatic conditions. Around the glass transition region, we found that the spontaneous exothermic changes in the sample occurred below Tg due to relaxation from the thermodynamic non-equilibrium state to the equilibrium state. Subsequently, the spontaneous endothermic changes occurred above Tg because the relaxation time required by the sample to reach thermal equilibrium was longer than the heating time of the sample. The enthalpy release around Tg corresponded to these spontaneous exothermic and endothermic changes, which we observed as changes in the sample temperature over time under adiabatic conditions.
Results and discussion
Figures 1–3 show the temperature dependence of the spontaneous temperature drift rates around the glass transition for PSA-300, PSA-500, and PSA-1000, respectively. The temperature drift rate was the value of the slope obtained by linear fitting using 10 values of the temperature from 5 to 15 min after the energy input at each temperature was completed. This time was sufficiently long for achieving thermal equilibration in the cell in temperature regions free from the relaxation phenomena.
As the sample PSA-300 was cooled at a rate of 3.0 K min−1, we observed an exothermic temperature drift in the temperature range 185–219 K, followed by an endothermic drift from 219 to 240 K, as indicated by open circles in Fig. 1. In contrast, we only observed an endothermic temperature drift in the temperature range 200–235 K, as indicated by the closed circles in Fig. 1, when the sample was cooled gradually at a rate of 0.1 K min−1. These anomalies are the distinctive features of the glass transition [2, 4].
In Figs. 2 and 3, we observed an exothermic temperature drift, followed by an endothermic drift in the temperature range 190–250 K for PSA-500 and 245–285 K for PSA-1000.
From the temperature at which the temperature drift changed from an exothermic drift to an endothermic drift, we determined the calorimetric glass transition temperatures to be 219 K for PSA-300, 237 K for PSA-500, and 273 K for PSA-1000.
In the temperature range wherein an exothermic effect was observed in the temperature drift measurements, the sample was found to relax toward an equilibrium undercooled liquid via the release of excess enthalpy. This enthalpy corresponded to the configurational change in the styrene oligomers. We experimentally determined the temperature dependence and time dependence of the configurational enthalpy Hc(T, t) using the following equation:
where dHc/dt is the enthalpy relaxation rate. We evaluated this quantity by multiplying the spontaneous temperature drift rate by the heat capacity, including that of the sample cell. This heat capacity was simultaneously obtained with the enthalpy change measurement. Time increment (Δt = tafter − tbefore) is the difference between the time of the midpoint of the energy input before (tbefore) and after (tafter) the temperature drift measurement at a temperature. The time increment Δt was about 30 min. The origin of t is arbitrarily defined as the time at which the temperature is suitably low and no enthalpy relaxation occurs. The zero of Hc(T, t) was taken at the terminal point of the relaxation (equilibrium liquid) as seen in Figs. 4–6 later. This was possible because the samples reached the terminal point of the relaxation (equilibrium undercooled liquid) during the enthalpy release measurements; dHc/dt reached zero at 219 K for PSA-300, 237 K for PSA-500, and 270 K for PSA-1000, as shown in Figs. 1–3, respectively.
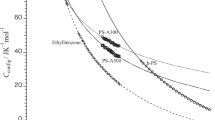
Figures 4–6 show the temperature dependence of the configurational enthalpies of PSA-300, PSA-500, and PSA-1000, respectively, obtained using Eq. (1). The horizontal segments of the step-like lines represent electrical heating, which was assumed to take infinitesimally short intervals. The vertical segments represent the amounts of the enthalpy changes. The dotted curve rising smoothly toward the right represents the configurational enthalpy of the equilibrium liquid. This curve was calculated from the heat capacity jump at the glass transition temperature. As stated above, the origin of Hc(T, t) was considered to be the temperature at which the sample reached the equilibrium state during the enthalpy change measurements. Figure 7 shows the temperature dependence of the configurational enthalpies for PSA-300, PSA-500, and PSA-1000. In this figure, the temperature was normalized by the respective value of Tg to conveniently compare the total amounts of configurational enthalpies for these styrene oligomers.
The total amount of configurational enthalpy released in each experiment was 0.8 ± 0.1 kJ mol−1 for PSA-300, 3.5 ± 0.2 kJ mol−1 for PSA-500, and 1.6 ± 0.1 kJ mol−1 for PSA-1000, where 1 mol is one monomer unit. The value for PSA-500 was 4.5 times larger than that for PSA-300 and 2.2 times larger than that for PSA-1000. From these results, we concluded that the structure of PSA-500 was much more disordered and strained than that of either PSA-300 or PSA-1000. The excess configurational enthalpy of these styrene oligomers did not exhibit a simple dependence on molecular weight. In principle, the number of molecular configurations increased with increasing molecular weight. The difference between the excess configurational enthalpy of PSA-500 and that of PSA-300 reflected the difference in the number of configurations because of the difference in the number of monomer units. On the contrary, we concluded that the excess configurational enthalpy of PSA-1000 was lower than that of PSA-500 due to the effect of entanglement. As the number of monomer units increased, this feature of the polymer became prominent in the sample. Inoue et al. reported that the results of the viscoelasticity measurements of PSA-1000 could be explained by the Rouse model [14]. Since the sample was physically and spatially more restricted with an increasing number of monomer units, the number of configurations available to the molecules decreased.
The corresponding configurational enthalpy changes for the molecular glasses butyronitrile [11], 1-pentene [12], and glycerol [13] were 0.8, 0.1, and 0.2 kJ mol−1, respectively. The excess configurational enthalpies of the styrene oligomers were comparable to or larger than those of the molecular glasses. This indicated that in comparison with molecular glasses, the styrene oligomer glass is in a non-equilibrium state that is farther from the equilibrium thermodynamic state.
Conclusions
In this study, we used an adiabatic calorimeter to measure the temperature drift rate in the vicinity of the glass transition temperature Tg for the styrene oligomers PSA-300, PSA-500, and PSA-1000. From the temperature dependence of the temperature drift rate and the heat capacity, including that of the sample cell, we evaluated the configurational enthalpy relaxation rate and the total amount of the configurational enthalpy released. From the temperature dependence of the temperature drift rate in the vicinity of the glass transition for the styrene oligomers, a spontaneous exothermic change was observed as the temperature increased and a spontaneous endothermic change was observed above the glass transition temperature. This result is same as that for the temperature dependence of the temperature drift rate obtained from the measurements of molecular glasses. The total amount of configurational enthalpy released was the largest for PSA-500, second largest for PSA-1000, and third largest for PSA-300. The amount of the configurational enthalpy change exhibited no simple molecular weight dependence, suggesting that multiple factors affected enthalpy relaxation in the vicinity of the glass transition. Comparing the amount of the configurational enthalpy released from the styrene oligomer with those previously reported for molecular glasses, we found that the configurational enthalpy release from the styrene oligomer was relatively higher. This indicated that in comparison with the molecular glasses, styrene oligomer glasses were frozen farther from the equilibrium thermodynamic state.
References
Tool AQ. Relation between inelastic deformability and thermal expansion of glass in its annealing range. J Am Ceram Soc. 1946;29:240–53.
Suga H, Seki H. Thermodynamic investigation on glassy states of pure simple compounds. J Non Cryst Solids. 1974;16:171–94.
Hodge IM. Enthalpy relaxation and recovery in amorphous materials. J Non Cryst Solids. 1994;169:211–69.
Suga H. Adiabatic calorimeter as an ultra-low frequency spectrometer: interplay between phase and glass transitions in solids. Thermochim Acta. 2001;377:35–49.
Matsuo T, Oguni M, Suga H, Seki S. Calorimetric study of the glassy state. XV. Thermodynamic study of pinacol hexahydrate and pinacol-d2 hexadeuterate. Bull Chem Soc Jpn. 1980;53:1493–500.
Matsuo T, Yamamuro O. Equilibrium and non-equilibrium transitions studied by adiabatic calorimetry. Thermochim Acta. 1999;330:155–65.
Okishiro K, Yamamuro O, Tsukushi I, Matsuo T, Nishikiori S, Iwamoto T. Calorimetric and dielectric studies on the water reorientation in the two-dimensional hydrogen-bond system of Cd(H2O)2Ni(CN)4·4H2O Crystal. J Phys Chem B. 1997;101:5804–9.
Oguni M, Matsuo T, Suga H, Seki S. Calorimetric study of glassy state. IX. Thermodynamic properties of stannous chloride dihydrate and dideuterate crystals. Bull Chem Soc Jpn. 1974;47:57–66.
Tsukushi I, Yamamuro O, Matsuo T. Structural relaxations and thermodynamic properties of molecular amorphous solids by mechanical milling. Progr Theor Phys Suppl. 1997;126:89–92.
Tsukushi I, Yamamuro O, Sadanami K, Nishizawa M, Matsuo T, Takeda K. Construction of a top-loading adiabatic calorimeter and enthalpy relaxation of glassy (1,3-propanediol)(0.5)(1,2-propanediamine)(0.5). Rev Sci Instrum. 1998;69:179–84.
Takeda K, Yamamuro O, Oguni M, Suga H. Thermodynamic characterization of vapor-deposited amorphous solid. Thermochim Acta. 1995;253:201–11.
Suga H. Thermodynamic aspects of glassy states. J Mol Liq. 1999;81:25–36.
Yamamuro O, Oishi Y, Nishizawa M, Matsuo T. Enthalpy relaxation of glassy glycerol prepared by rapid liquid quenching. J Non Cryst Solids. 1998;235:517–21.
Inoue T, Onogi T, Yao ML, Osaki K. Viscoelasticity of low molecular weight polystyrene. Separation of rubbery and glassy components. J Polym Sci B Polym Phys. 1999;37:389–97.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujimura, J., Nishiyama, E., Tsukushi, I. et al. Enthalpy relaxation of low molecular weight amorphous styrene oligomers measured with an adiabatic calorimeter. J Therm Anal Calorim 135, 2813–2817 (2019). https://doi.org/10.1007/s10973-018-7604-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7604-1