Abstract
During the development of the carbon fibres and oxidized fibres, the aim was to get a material with extraordinary mechanical properties. Nowadays, it becomes more and more important to make it also fire resistant. To achieve the fire resistance of the raw material PAN, a multistage technology process is needed in which its microstructure and flammability are changed. These special properties can be achieved by heat treatment, during which the microstructures of the fibres change at each step. The thermal transformation involves thermal stability. The thermal transformation can be followed by measuring the limited oxygen index (LOI), and compare it to traditional testing methods, such as infrared and Raman spectroscopy and elementary analysis. The results show that the oxygen index of the samples is a very sensitive and characteristic parameter of flammability. It seems a good correlation between the LOI and the structural parameters of the samples. The structural transformations are not linear but abrupt. Considering the temperature treatment ranges which are responsible for the increasing oxygen indices, three distinct areas can be determined. It has been confirmed by measurements that LOI can express the grade of stability. Based on spectroscopic measurements, it has also been confirmed that the oxidized fibres dramatically decompose when burning in enriched air with more than 50% oxygen content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon fibre-based products, due to their special properties, are also used in applications where heat and fire resistance must be ensured, e.g. in protective clothing including protective gloves, fire blankets and internal lining of vehicles. These carbon-based products and the carbon fibre itself are qualified, beside their mechanical and physical properties (fibre density, fibre diameter, tensile strength), by their oxygen index. The Fire Protection Testing Laboratory of the Institute of Fire Protection and Safety Engineering has a long-time experience in measuring the oxygen index of carbon fibres and oxidized fibres. In an earlier work, it was pointed out that the oxygen index is strongly dependent on the structure of the samples [1,2,3,4,5,6,7,8].
Microstructure of carbon and oxidized fibres and steps of their formation
The conversion of PAN to carbon fibres is normally made in 2 main stages [2, 6]:
-
1.
Stabilization
During this stage, a complicated process takes place, where not all the steps are detailed now. However, it is sure that the linear PAN-based precursor changes its structure to cyclic form. This change is depicted in Fig. 1a. The PAN-based polymer precursor is being stabilized by controlled low-temperature heating over the range 200–300 °C in air. The nitrile group in the fibres changes from linear –CN end groups to –C=N–C=N– groups in the cyclized structures (Fig. 1a).
Fig. 1 Main structural changes in process steps of polyacrylonitrile are: a stabilization, b carbonization [6]
-
2.
Carbonization and graphitization
The carbonization and graphitization of thermally stabilized fibres are carried out in an inert atmosphere containing gases such as nitrogen (N2) or argon. The polymer changes from a ladder to a stable ring structure, and the fibre changes colour from white, brown to black.
The carbonization and graphitization of the thermally stabilized fibres are a two-step process; in lower temperature range of 1500–1600 °C, carbonization takes place; and during the next step of the heat treatment in ranges above 1600–1800 °C and up to 3000 °C, the high-temperature graphitization is the main process, depending on the needed properties of the fibres (Fig. 1b).
The role of nitrogen in pyrolysis appears to be the most complicated. X-ray photoelectron spectroscopy (XPS) was used by Pels and Kapteijn [9] to investigate the fate of nitrogen functional forms present. The distribution of nitrogen functional forms changes with increasing severity of the pyrolysis conditions. Under mild pyrolysis conditions we understand, firstly unstable functionalities like pyridones, protonated pyridinic-N and N-oxides.
After severe pyrolysis, all nitrogen is eventually present in 6-membered rings located at the edges of the graphene layers as pyridinic-N or in the interior as quaternary-N. Upon exposure to the ambient, N-oxides of pyridinic-N can be formed. During pyrolysis, differences in nitrogen distribution of the char precursors have diminished. (The authors assume that nitrogen bonds play a major role in the non-flammability of carbon fibres.)
Oxidation mechanisms
The reactivity of the carbon fibre materials could be interpreted in three levels:
Atomic level it means the actual C + O2 = CO2. Or it can even mean both at the same time, if only those energy changes are examined that belong to those free atoms that are calculated from bonding energy. It could appear to be a simple, one-step process, but hundreds of literary references are investigating the real mechanisms which influence the micro- and macrostructures of the materials.
Microstructural level this determines what circumstances must be satisfied for the carbon atom to exist. It also defines the required cell units. These conditions are defined by the features of microstructures. At this level, the main control steps are chemisorption and diffusion.
Macrostructural level the determining role of macrostructure can be verified by measurements. Through different processing methods, oxidized or carbon fibre materials—with the same structure—get different flammability parameters. The long-term thermal resistance of the carbon fibres and the different processing ways are monitored by mass losses. Boehm and Benett’s results [10, 11] provide information about the role of the oxidized surface and the thermal stability of the functional groups which are present at the evaporation of carbon.
According to author Eckstein [12], there is a tight relationship between the mass loss and the carbon content of fibres. Among the different carbon fibres which were investigated, those with HTS type or made of PAN (has medium modulus) had the weakest mechanical properties. When the temperature is about 300 °C, the oxygen is bound to the surface of carbon material with chemisorption. In this way, the oxygen is stable, and it has a complex form which is the determining factor in the case of the actual oxidation of the carbon.
Boehm’s results [10] provide information about the role of the oxidized surface and about the thermal stability of the functional groups in the presence of the evaporation of carbon. Bennett and Johnson [11] monitored the chemisorption of oxygen with thermogravimetry (DTG), until the temperature reached 951 °C. These kinds of measurements are useful to follow the change and the size of the active surface.
The role of oxygen index in the burning classification
There are several methods to describe the flammability of various materials, ignition temperature, the flame propagation in different directions, smoke densities and fume temperatures. The flammability of materials can also be characterized by their minimal oxygen concentration required to maintain burning.
The majority of flammable materials is capable of combustion at normal oxygen levels (21 vol%), but there are materials that burn at lower or higher oxygen concentration, in comparison. Oxygen index (LOI—limited oxygen index) is not a commonly employed laboratory measurement method, despite being a significant parameter in the characterization of flammable materials. This method has originally been developed to describe the flammability of plastics, but in principle, it is applicable to all combustible solid materials. The LOI parameter is used for fire protection qualification of carbon fibres in the literature, and oxidized fibres and materials made from those.
The less LOI, the highest burnability or flammability. Conversely, the burning behaviour and tendency to propagate flame for a polymer with a limiting oxygen index greater than 20.95 will be reduced or even approach zero, after removal of the igniting source. Self-sustaining combustion is not possible if LOI > 100, since such values are not physically possible.
Nelson [13] investigated how the introduction of a fire-retardant changes the oxygen index of a material. For this purpose, it is useful to assign materials into experimentally meaningful groupings depending upon their oxygen index. The minimum level of flame retardancy required to increase the classification of a material can be calculated. From the preceding paragraph, the two obvious groupings are LOI < 20.95 and LOI > 100. We refer to materials satisfying these requirements as being flammable and intrinsically non-flammable, respectively. Several researchers have suggested that materials with a limiting oxygen index greater than 28 are generally self-extinguishing [10]. We describe materials satisfying 28.00 < LOI < 100 as being self-extinguishing. The threshold LOI = 20.95 is of great practical interest, and we define materials with a limiting oxygen index of 20.95 as being marginally stable. Fenimore [14] refers to materials that are between the marginally stable and self-extinguishing thresholds, i.e. 20.95 < LOI < 28, as being slow-burning.
Partly stable materials form a natural set for a quantification of the efficiency of fire-retardant mechanisms. We achieve this by finding the value of the relevant burning continuation parameter to increase the LOI of these materials to 28.0, the transition between slow-burning and self-extinguishing polymers, and to 100, the threshold for intrinsically non-flammable materials.
It should be realized that our classification of materials (flammable, slow-burning, self-extinguishing, intrinsically non-flammable) is specific to the limiting oxygen index test, i.e. a material that is self-extinguishing here is not necessarily self-extinguishing in another test method. The tenet in the limiting oxygen index is that the higher the value of the LOI, the safer the material. However, we emphasized that results from one test method do not necessarily agree with another [15,16,17,18]. The reasons for this were referred to in the opening paragraph.
Additional details of oxygen index methods and their applications, particularly to assessing the burning behaviour of textiles, are provided in the comprehensive review by Xu. et al. [19].
Experimental
The measurements were taken from the Szent István University, in the Fire Protection Testing Laboratory. The burning tests were made in standard equipment as described above. All the samples were tested before and after the combustion with infrared and Raman spectroscopy and elementary analysis.
Method of the measurement of the oxygen index
The precise descriptions of the measurement are regulated in standards (Plastics—Determination of flammability by oxygen index) and other international standards [20, 21].
The most important parts of the measuring instrument for the oxygen index are shown in Fig. 2.
Typical apparatus for the determination of oxygen index. Technical data of apparatus: FIRE Instrumentation and Research Equipment Limited, UK (ISO 4589 Part 2 Oxygen index test apparatus [20])
The parameters of the instrument which was used for the measurement are:
-
Oxygen analyser: range 0–100% O2
-
Repeatability ± 0.1% O2
-
Linearity: ± 0.1% O2
-
Flow: flow through column adjustable from 0 to 20 mL min−1.
The apparatus consists of five basic modules: (1) digital interface panel, (2) automatic conditioning system for sample environment, (3) electronic flow measurement device, (4) oxygen measurement system, (5) mixing chamber. Test column and sample holder assembly.
The size of the sample holder is a 60 × 160 mm, U-shaped, double-layered vertical metal frame, secured in a glass cylinder with its top end open. The ignition and the removal of the combustion products are made possible through this opening. The source of ignition is a 40-mm-long gas-flame as prescribed by standards. The specimen is ignited along the upper edge applying the flame for maximum of 15 s. Burning proceeds downwards against the flow of the pre-adjusted gas mixture. Nitrogen and oxygen content are adjustable as required.
For the purposes of this international standard [20], the following definition applies. Oxygen index: the minimum concentration of oxygen by percentage volume in a mixture of oxygen and nitrogen introduced at 23 ± 2 °C that will just support combustion of a material under specified test conditions. It is recommended that the first trials are performed at low (30–32%) oxygen concentration. Initially, the material does not show any ignition phenomena and is non-flammable. By increasing the oxygen content, initially only burning marks appear, then as the oxygen concentration increases, the length of the burnt area becomes greater.
Infrared and Raman spectroscopy analysis
Measurements for the structure investigations were considered important for two reasons. Measuring of the different spectra is a first step to determine the cyclization, stabilization and carbonization indices. On the other hand, the spectroscopic studies can follow the micro-level structural transformations in production steps identifying the most important groups (Table 1).
The rules of the most characteristic changes are [22]:
-
1.
The cyclization of hanging cyanide groups (–C=N) leads to ladder type structure.
-
2.
The dehydrogenation results in conjugated double bonds.
-
3.
During the oxidation, a carboxyl group (C=O) is created, and the loosely bound hydrogen escapes.
-
4.
The (–C≡N) and C=O/C–O groups which are hanging on C–H chain are being transformed accompanied by the decrease in absorbance at C–H locations (at approximately 2900 cm−1), due to the dehydrogenation.
-
5.
The C≡N binding peak at 2240 cm−1 decreases as a sign that the linear cyanide groups transform to cyclic structure.
In fact, a heterocyclic structure is formed with C=N, C=O bonds, besides the remaining C–H bonds.
The degree of transformations is also characterized by several parameters, e.g. by specifying a carbonization index and stabilization index. Stabilization index is the ratio of the stress at 180 °C in cooling, releted to that at the temperature where cooling started, of, to use as a parameter to evaluate the degree of modification in chemical structure in fibres by oxidation [22]. Carbonization index: it shows how well chemical structure of the PAN material has been transformed and how close it is to the crystalline graphite structure. It is taken into consideration the intensity of reflection peaks at 0.52 and at 0.35 nm [23].
Elementary analysis
The elementary analysis measurements were taken in the Central Chemical Research Institute in Budapest, on Elementar Vario EL III CHNOS Analyzer.
The tests were performed on fibre cables grouped by lower and higher density. They were combusted in different oxygen concentrations. The impact of combustion parameters on fibre structure, mass loss and elementary composition was investigated. The selected elements of the composition were the following: carbon, nitrogen, hydrogen.
Testing groups
Oxidized fibre (also known as PYRON fibre) samples produced in a commercial plant as a result of the treatment of a polyacrylonitrile-based precursor fibre (the raw material is commonly known as special acrylic fibre—SAF) in the presence of oxygen at 220–225 °C have been used in the present work.
Following the steps in the production technology, the test samples undergo changes in carbon content beginning with the raw material PAN up to the theoretical maximum at CF (with graphite-like structures). Table 2 shows the specific properties of these samples and the measured oxygen index.
The overview of analytic measurements is given in Table 3.
The samples can be grouped according to the performed analytic methods:
-
1.
Samples for infra and LOI analysis: polyacrylic-nitrile (PAN) as a raw material, and various forms from PAN depending on ascending treatment temperature (see Table 2). Identification as: PAN, OX1, OX2, OX3, LT, HT, CF. Samples were given by ZOLTEK Corp.
-
2.
Samples for elementary analysis: PN 1 tow (1.37 g cm−3, LOI 35, dtex: 1.7: product identification/manufacturer ID: PN37 TW, given by ZOLTEK Corp.) and PN 2 tow (1.41 g cm−3, LOI 61, dtex 2.2: product identification/manufacturer ID: PN41 TW, given by ZOLTEK Corp.) were combusted under following oxygen content: 21% (baseline), 30, 40, 50, 60, 70 and 80%. in the oxygen index test apparatus according to ISO 4589 Part 2. Number of samples is 7.
-
3.
Samples for infrared and elementary analysis: CF tow, fibre density of 1.78 g cm−3 with 98% carbon content, LOI over 90 (given by ZOLTEK Corp), were combusted under 90% oxygen content in the oxygen index test apparatus according to ISO 4589 Part 2. These samples were considered as the closest reference to pure carbon fibres.
Results and discussion
Influence of density on the oxygen index
Fibre density as an indicator of the transformation is the easiest and the most often measured parameter during the production. The samples with PN signature (trade name “PYRON” from company ZOLTEK) undergone similar treatment as the OX2 sample (Cf. Table 2). The density of fibre samples was measured according to (ASTM D-1505) standard for Density of Plastics by the Density-Gradient Technique. Figure 3 shows the oxygen index (LOI) as a function of the fibre density.
In the range of 1.34—1.42 g cm−3, linear correlation could be defined between the oxygen index and the density. Even the value of the density is exceeding 1.42 g cm−3, the tendency is changing, and the oxygen index will not grow by increasing the density value. A possible reason is the low air content between the fibres.
Influence of treatment temperature on the oxygen index
The changes were followed by the parameters carbon content, fibre density and oxygen index. The relationship of the most important parameters is shown in Fig. 4.
Comparing the measurements to external data (CF. Table 1) and plotted in Fig. 4, it can be observed that the characteristic parameters are changing depending on the temperature. Three distinct domains can be observed; they are:
-
(I) initial range: 20–250 °C,
-
(II) intermediate range: 250–800 °C,
-
(III) stable range of 800–1300 °C
The ranges are separated by abrupt changes. Heat treatment at 300 and 800 °C causes important changes in the properties indicated also by changes in oxygen index. Figure 4 shows the density and carbon content as a function of rising LOI values.
The substantial structural transformation starts at 300 °C, in same time with the increase in density. With the oxidized fibres, the trend continues, confirmed by flammability tests of different density fibres. In the plots, there are two jumps in parameters. One is between 700 and 800 °C. This is the temperature at which the major changes are completed. Higher temperatures contribute in a lesser extent to the increase in density, carbon content and LOI value. If we examine the changes as a function of the LOI, a jump is observed at the LOI > 50 value. It is at about 250 °C when PAN transforms to the oxidized fibre. It was possible to assign a specific value of oxygen index (LOI = 50) to this change.
Results of measurements by infrared and Raman spectroscopy
Studies of structure are based on the stabilization index and on the carbonization index [19], a qualifying parameter based on spectroscopic investigations. The best way to follow the conversion rate (the PAN fibre transformation to oxidized fibre) is to measure the decrease in linear cyanides (C≡N at 2240 cm−1) and the increase in related cyclic cyanides (C=N 1590 cm−1). The absorption signal of the linear and cyclic cyanides shows the conversion rate and indicates the oxidized fibre quality as well. To interpret the observed results so far, one has to know which structural changes occur in the process (Fig. 1). It is well known that the PAN forming linear cyanides (C≡N) in the stabilization step are being transformed into cyclic cyanides (–C=N–). This study shows that the transformation is not continuous, but staggered like the carbon content or the density increase.
Considering the microstructural parameters which are responsible for the increasing oxygen indices, three distinct areas can be determined (Fig. 5). The three domains are also indicated by the appearance (–C=N–) or disappearance of the functional groups (C≡N).
- Range. 1:
-
LOI 21–30, the cyclization is ending and the number of linear cyanides (–C=N) reaches its maximum value
- Range. 2:
-
LOI: 30–60, the remaining cyclic cyanides disappear, while the density increases. In this range, the structure becomes thermodynamically stable against the oxidation processes, which is confirmed by LOI values above 50
- Range. 3:
-
LOI: 60–90, the graphitization begins with a slight shrinkage of the fibres and a minimal decrease in fibre density could be also observed. This occurs when the cyano groups permanently disappear
Raman spectra, however, are suitable for the monitoring of graphitization. When it starts, the characteristic 1580 cm−1 first order and 1340 cm−1 s order peaks of the pure crystalline graphite appear.
The structural transformations of the PAN raw material are indicated by the quantitative change in the most common functional groups (C≡N groups decrease, C=N increase), the graphitization rate (Raman peak of graphite at 1580 cm−1), and changes in the fibre density and oxygen index. Note that the linear nitrile groups are changing gradually, while the cyclic nitrile groups are still present at quite high oxygen index values. The graphitization starts only when the nitrogen content is reduced or completely destroyed. This observation is consistent with the trends outlined in Fig. 6. Very high LOI values can be related to pure graphite structures only.
Influence of changes in elementary composition
For sake of comparison to the reference CF cable with 95% carbon content, the following two different samples were chosen for testing:
- PN1:
-
1.37 g cm−3, LOI 35 dtex: 1,7; product identification ID: PN37 TW;
- PN2:
-
1.41 g cm−3, LOI 61, dtex: 2,2; product identification ID: PN41 TW;
- CF:
-
1.78 g cm−3, LOI above 90
Procedure the samples were burnt in a standard LOI device (Fig. 2) at the following oxygen concentrations: 21, 30, 40, 50, 60, 70 and 80%. The reference CF cable was burnt only at oxygen concentration above 80%, as it is well known that it has a high LOI value.
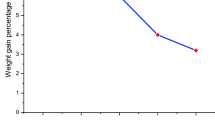
Results the mass loss after burning was determined by weighing. The results are summarized in Table 4. Figures 6 and 7 show the results in a diagram.
At 50% oxygen concentration, the structure starts to change drastically, with a simultaneous mass loss observed. Above the oxygen content of 50%, the incineration of hydrogen shows the greatest mass loss, PN1 sample: 2.38–1.70%, and PN2 sample: 3.05–1.35% in change. The remainder is most likely the residual moisture content.
Conclusions
The parameters: carbon content, density and LOI, are structure dependent and their values change as the consequence of the drastic structural change due to heat treatment.
We found out that all the microstructural parameters affect the combustibility, and these parameters are related to each other as well. A correlation can be found between oxygen index, fibre density, carbonization index, stabilization index and the carbon content. The taken measurements confirm that the LOI values are not a linear function of the structural transformations but abrupt changes can be detected.
- Range. 1:
-
LOI 21–30, end of cyclization
- Range. 2:
-
LOI: 30–60, the structure becomes thermodynamically stable
- Range. 3:
-
LOI: 60–90, the graphitization process takes place
It could be shown that the flammability range is dependent on the decrease or increase of linear (–C≡N) and cyclic (–C=N–) groups.
It has been confirmed by measurements that LOI can express the grade of stability. Based on spectroscopic measurements, it has been also confirmed that the oxidized fibres dramatically decompose when burning in enriched air with more than 50% oxygen content. Materials with LOI > 50 possess the necessary thermodynamic stability against combustion. These materials are characterized by slow, flameless burning in solid-phase as the easily removable functional groups on surface no longer exist.
References
Singer L. Overview of Carbon Fiber Technology, Material Technology Center Newsletter, Southern Illinois University at Carbondale, Spring; 1994.
Tokarsky EW, Diefendorf RJ. High performance carbon fibers. Polym Eng Sci. 1975;15(3):150.
Chiao TT, Chiao CC, Sherry RJ. Lifetimes of fiber composites under sustained tensile loading. In: Proceedings of the 1977 international conference on fracture mechanics and technology; 1977.
Feldman LA. High temperature creep effects in carbon yarns and composites. In: Proceedings of the 17th biennial conference on carbon. American Carbon Society, 1985, p. 393.
Diefendorf RJ. Carbon/graphite fibers, composites. Engineered materials handbook, vol. 1. Metals Park: ASM International; 1987. p. 51.
Tálos J, Zoltek R. A pyron oxidált szál a műszaki textíliák egyik fontos alapanyaga. Magyar Textíltechnika. 2006;LIX./1:9–11.
Kerekes Z. Ph.D. Dissertation Thesis, Szent István University, Ybl Miklós Faculty, 2016, p. 150.
Kerekes Z, Beda L. The oxygen index of oxidized fibres and influencing factors. Annu News. 2003;1:50–4.
Pels JR, Kapteijn F. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon. 1995;33(11):1641–53.
Boehm HP. Surface oxides on carbon and their analysis: a critical assessment. Carbon. 2002;40(2):145–9.
Bennett SC, Johnson DJ. Structural characterization of a high modulus carbon fibre by high-resolution electron microscopy and electron diffraction. Carbon. 1976;14:170–2.
Eckstein H. The oxidation of carbon fibers in air between 230 & #xB0;C and 375 & #xB0;C. Fibre Sci Technol. 1981;14:129–56.
Nelson MI, Sidhu HS, Weber RO, Mercer GN. A dynamical systems model of the limiting oxygen index test. ANZIAM J. 2001;43(1):105–17.
Fenimore CP. Candle-type test for flammability of polymers. In Lewin M, Atlas SM, Pearce EM, editors. Flame-retardant polymeric materials, vol. 1. New York: Plenum; 1975. p. 371–97.
Balázs LG, Czoboly O, Kopecskó K, Ribeiro IJS. Effect of coating to the properties of hemp fibre and hemp fibre reinforced concrete. In: Innovative Concrete Technology in Practice: the 11th central European congress on concrete engineering, p. 363. Hainburg, Austria, 2015.10.01–2015.10.02. Wien: Austrian Society for Construction Technology, 2015. pp. 264–7.
Qin XH. Structure and property of electrospinning PAN nanofibers by different preoxidation temperature. J Therm Anal Calorim. 2010;99:571–5. https://doi.org/10.1007/s10973-009-0126-078.
Kenawy IMM, Hafez MAH, Lashein RR. Thermal decomposition of chloromethylated poly(styrene)-PAN resin and Its complexes with some transition metal ions. J Therm Anal Calorim. 2001;65:723–36. https://doi.org/10.1023/A:1011999325998.
Burkanudeen A, Krishnan GS, Murali N. Thermal behavior of carbon fiber precursor polymers with different stereoregularities. J Therm Anal Calorim. 2013;112:1261–8. https://doi.org/10.1007/s10973-012-2706-7.
Xu JZ, Tian CM, Ma ZG, et al. Study on the thermal behaviour and flammability of the modified polyacrylonitrile fibers. J Therm Anal Calorim. 2000;63:501–6. https://doi.org/10.1023/A:1010181618305.
MSZ 10200:1989 és ISO 4589 (1985): Plastics—determination of flammability by oxygen index.
ASTM 2863 (2001): Standard test method for measuring the minimum oxygen concentration to support candle-like combustion of plastics (oxygen index), Plastics—determination of flammability by oxygen index.
Ogawa H, Saito K. Oxidation behavior of polyacrylonitrile fibers evaluated by new stabilization index. Carbon. 1995;33(6):783–8.
Donnet J, Wang TK, Peng J. Carbon fibers, 3rd ed, Revised and Expanded, Chapter 2.2.3, Marcel Dekker; 1996. p. 94.
Acknowledgements
The author acknowledge the help by the Hungarian Institute for Forensic Sciences, Department of Physical Chemistry—Morphology and Inorganic Analytics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kerekes, Z., Lublóy, É. Effect of thermal transformation and stability on the flammability of PAN precursors-based carbon fibres. J Therm Anal Calorim 133, 1075–1084 (2018). https://doi.org/10.1007/s10973-018-7136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7136-8