Abstract
Enzymatic hydrolysis is one of the major steps involved in the conversion of sugarcane bagasse into ethanol. Pretreatments break down macrostructures in order to improve the enzyme access to the targeted glycosidic bonds. This study reports on the use of thermoanalytic techniques together with other different techniques for the verification of the structural and morphological changes occurred in sugarcane bagasse subjected to acid and alkaline pretreatments. The techniques evaluated differences in the BET and BJH surface areas, diameter and pore volume investigated by porosimetry, scanning electron microscopy and wettability. Thermal analysis (TG/DTG and DTA) was also used to evaluate the thermal degradation of hemicelluloses, cellulose and lignin contents that remained in the samples after pretreatments. The results show that chemical pretreatments were effective in the degradation of lignocellulosic samples and significant morphological changes occurred after the pretreatments. Acid and alkaline pretreatments caused an increase in the surface area, diameter and volume of pores. Wettability also revealed important effects regarding surface changes of the biomasses. In summary, all tested pretreatments were effective to chemically degrade the macrostructures of sugarcane bagasse that hinder enzymatic hydrolysis in, for instance, the second-generation ethanol production.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass is the fourth largest energy source worldwide after coal, petroleum and natural gas [1], as it provides approximately 10.2% (equivalent 50.3 EJ per year) of the annual global total primary energy supply (TPES) [2]. Recent reports have revealed that global biomass technical potentials could supply as much as four times the current global needs, i.e., up to 1500 EJ [3,4,5]. According to the Food and Agriculture Organization of the United Nations [6], sugarcane is among the most important crops in the world, which include maize, rice and wheat, potatoes, sugar beet and soybeans.
A typical Brazilian sugarcane mill converts 500 tones h−1 in Brazil [7,8,9], placing this country among the top sugarcane producers in the world. Each tone of processed sugarcane generates around 140 kg of bagasse (fibrous material remaining after juice extraction). Between 60 and 90% of this material is used by its own industry as a fuel to generate power and heat [7,8,9]. Bagasse sugarcane is constituted of three main parts—cellulose, hemicellulose and lignin—which together sum up to 90% of the total mass. Xylose is the main carbohydrate found in the hemicellulose fraction, representing around 80% of the total sugar [10, 11]. On the other hand, extractives such as grease, gum, starch, alkaloids, resin and essential oils range from 1.7 up to 7.5% of the total mass [12, 13]. The sugarcane bagasse can be used to increase the ethanol production via the second-generation conversion pathway [8].
Lignocellulose is the most abundant biomass that can be converted into liquid fuels by enzymatic hydrolysis and/or microbial fermentation. Considering that sugarcane bagasse is a lignocellulosic substrate, therefore water insoluble, the heterogeneous reactions involved in biomass conversion processes require direct physical contact between enzymes and substrates (i.e., cellulose and hemicellulose) [13,14,15,16]. Thus, pretreatments are necessary to enhance biomass digestibility by changing biomass structural features. Theoretically, if the role of structural features determining digestibility is modeled, it may be possible to predict the enzymatic digestibility of lignocellulose and design more effective pretreatments [14].
Lignocellulosic materials predominantly contain a mixture of carbohydrate polymers (cellulose and hemicellulose), lignin, extractives and ashes [15, 16]. Lignin and hemicellulose play an important role in the rate and yield of the enzymatic hydrolysis. Results reported in the literature have shown that the saccharification of the cellulose increases with the removal of hemicellulose and lignin [15, 17].
The key inhibitory role of lignin is attributed to combination between the nonspecific adsorption of enzyme on the surface, the inaccessibility to cellulose, due to sterical impediment and difficult the degradation enzymatic of lignin [18]. Due to these factors, the biomass pretreated can be converted into fuel and chemicals. Pretreatment of lignocellulosic biomass is a common step to remove hemicelluloses and lignin, reduce the cellulose crystalline and increase their porosity [19].
Nitsos et al. [20] cited several methods and processes that have been investigated and reported in the literature for the pretreatment of lignocellulosic biomass. These include physical methods—milling; chemical methods—acid, alkaline, oxidative, ozone and organosolv pretreatment; mechanical methods based on dry ball-milling of substrates previously impregnated with dilute inorganic acids; physicochemical methods—as steam explosion, ammonia fiber explosion (AFEX), SO2 explosion, supercritical CO2, hydrothermal treatment; and biological methods, which utilize wood-degrading fungi for the removal of hemicellulose and lignin.
Several studies on the digestibility of lignocellulosic biomasses have been focused in the conversion of hemicellulose/cellulose to ethanol, methane and hydrogen [21, 22].
Chemical pretreatments tend to act in a particular way, either modifying cellulose crystallinity or removing cellulose and lignin from the plant matrix [22]. Acid pretreatment, using mainly sulfuric acid, and hydrothermal methods, based on the autocatalytic action of acetic acid released by the hydrolytic cleavage of acetyl groups, have shown to be effective in the improvement of the enzymatic hydrolysis of cellulose [23].
The main issue in the pretreatment of lignocellulosic materials with diluted acid is to solubilize the hemicellulosic sugars and keep as much cellulose as possible in the remaining pretreated solid substrate that may be enzymatically hydrolyzed [24]. Alkaline treatments that usually employ NaOH (sodium hydroxide) and Ca(OH)2 (calcium hydroxide) tend to increase both pore volume and surface area due to the dismantling of the highly ordered cellulose structure, which leads to a decrease in the crystallinity degree and cellulose polymerization. Such processes also cause a breakdown of lignin–carbohydrate linkages, as well as perturbations in the lignin structure [11, 18].
According to Guo and Catchmark [25], different origins and treatments are responsible for the complexity and variability of cellulosic structures. The knowledge of the surface area and porosity is important for the understanding of the structure, formation and degradation of cellulose, which can be used for the bioethanol production. Fan et al. [26] demonstrated that the surface area of a biomass was important in the enzymatic hydrolysis of the cellulose due to the direct physical contact between cellulases enzyme and the surface of the cellulose, which was decisive for the hydrolysis of the glycosidic bonds. The authors also showed that a large surface area and pore volume guarantee sufficient adsorption of the cellulases to the cellulose surface.
Considering that the use of in natura sugarcane bagasse to prepare hemicellulose hydrolysis can result in a product with high content of microbial activity inhibitor compounds, the composition of this raw material was evaluated before and after the chemical treatment. This study focuses on the use of thermal analysis and other techniques, such as porosimetry, scanning electron microscopy (SEM images) and wettability for the evaluation of sugarcane bagasse samples in both in natura and after chemical pretreatments. Thermogravimetric analysis (TG/DTG and DTA) was applied for a semiquantitative and qualitative analysis of the hemicelluloses, cellulose and lignin content in the biomasses. In addition, the morphology, pores diameter and volume of such pretreated biomasses were investigated by scanning electron microscopy (SEM images), BET surface area (Brunauer, Emmett and Teller) and BJH (Barrett, Joyner and Halenda) [27, 28]. The contact angle (wettability) was measured and used to compare the effects among substrate and the several pretreatments. The results are important for a better understanding on how pretreatment methods affect the macrostructures of the biomasses, which is essential for the design of fast and efficient subsequent enzymatic hydrolysis processes.
Materials and methods
Substrate
The sugarcane bagasse samples used in the experiments were provided by Raizen Group (São Paulo State, Brazil) from the 2011–2012 harvest. Prior to the pretreatment and analysis, the samples were dried at 45 °C for 48 h and stored in plastic bags at room temperature until use [10, 18].
Acid and alkaline pretreatments
Sugarcane bagasse was initially hydrolyzed with diluted H2SO4 (1% v/v in water) for 40 min at 120 °C. The pressure was kept at 1.05 bar, and a 1:16 solid-to-liquid ratio (g of bagasse mL−1 of solution) was used. This set of conditions was previously optimized for hemicellulose removal from sugarcane bagasse. The bagasse solid fraction was separated from the hydrolysate by filtration and abundantly washed with tap water to eliminate acid excess before oven drying at 60 °C for 24 h. A second pretreatment step for bagasse delignification followed, using NaOH solutions with increasing concentrations (1, 2, 3 and 4% w/v at 120 °C for 40 min). Four pretreated bagasse samples were then obtained by filtration, thorough washing until a neutral pH was reached and solid drying in an oven for more 24 h at 60 °C [10, 18].
Thermal analysis (TG/DTG and DTA)
The moisture, hemicelluloses, cellulose, lignin and ash content in in natura and chemically pretreated sugarcane bagasse samples were determined by thermoanalytical techniques, in particular thermogravimetric analysis (TG), derivative thermogravimetry (DTG) and differential thermal analysis (DTA) [1, 29]. TG enables a semiquantitative determination of such contents in the biomasses [30].
After chemical pretreatments, the samples were washed in running water for the removal of impurities and sieved by ASTM standard sieves so that different size fractions could be yielded. The average particle size selected was 0.35 mm. Non-isothermal thermogravimetric experiments (TG/DTG and DTA) were carried out in Shimadzu analyzers, TGA-50H and DTA-51 models. The experimental conditions used in both TG and DTA experiments are described as follows: synthetic air with flow rate of 100 mL min−1, heating rate of 10 °C min−1 from room temperature up to 800 °C, sample mass of 10.0 ± 0.5 mg and alumina crucible. Tests were carried out in duplicate, and the mean values and standard deviations were considered (Table 1).
Surface area and porosity analysis (ASAP)
Porosimetry with nitrogen gas adsorption at 77 K was performed in an ASAP 2020 Micromeritics porosimeter. Tests were performed in duplicate, and a sample mass of 1.5 ± 0.2 g was used. The samples were previously dried in an oven for approximately 24 h at 60 °C.
The degas conditions and analyses are described below:
-
1.
Degas conditions: heating rate of 10 °C min−1 from room temperature up to 50 °C under vacuum restricted evacuation rate of 10 mmHg s−1 to 1 mmHg. The evacuation was changed to non-restricted vacuum at the same evacuating rate until 10 μmHg and remained under these conditions for 30 min. The heating rate was same (10 °C min−1) up to 70 °C, and the samples remained under these conditions for 240 min.
-
2.
Analysis conditions: the samples started in vacuum restricted at an evacuation rate of 5 mmHg s−1 up to 5 mmHg. The evacuation was changed to non-restricted vacuum at the same evacuation rate until 10 μmHg, and the samples remained under these conditions for 6 min. A programming of P/Po was applied for 43 points (26 adsorption and 17 desorption). P/Po is the pressures ratio (namely relative pressure), and its maximum value reached was 0.994—(P) is the applied pressure, and (Po) is the vapor saturation pressure of the nitrogen gas adsorbed.
Wettability or contact angle (CA)
Wettability measurements were taken by an Easy Drop System—DO 4010 model (Goniometer), by Krüss German with a DSA—Drop Shape Analysis computational program, at room temperature and controlled pressure. A drop volume of 8 µL with deionized water was deposited onto the substrates by the circle fitting method (indicated for rough surfaces). The images were acquired in duplicate, and the first measurement (drop) was saved with ± 3° accuracy, immediately after the drop placement on the surface to avoid the perturbation by evaporation process [31, 32].
Scanning electron microscopy (SEM images)
The morphological and structural analyses of the in natura and chemically pretreated (acid and alkaline medium) sugarcane bagasse samples were conducted on scanning electronic microscope equipment, LEO 440 model. All samples used were fixed on an aluminum support and submitted to a gold metallization process. The cross section obtained by the fracture (plunged into liquid nitrogen) of the fibers was also evaluated.
Results and discussion
Thermal analysis
Figures 1–3 show the TG, DTG and DTA curves, respectively, for in natura sugarcane bagasse samples and samples chemically pretreated with dilute solutions of acid (1% H2SO4) and alkali (1, 2, 3 and 4% NaOH).
Figure 1 shows the TG curves in which two thermal degradation steps and one dehydration event can be observed. The moisture release occurs at approximately 110 °C. The first step, i.e., the largest amount of weight loss, is characterized by the thermal decomposition of hemicellulose and cellulose, both at the same stage, around 200–320 °C, respectively. This step is known as “devolatilization” in the biomass combustion process and is explained by the release of volatile materials, mainly CO2 and H2O compounds [33]. Finally, the second step is related to the lignin content and its decomposition temperature ranges between 325 and 450 °C. This decomposition range is characterized by the carbonization of lignocellulosic materials and char formation [34].
Ramajo-Escalera et al. [34] and Munir et al. [35] showed that the behavior of the TG curves for sugarcane bagasse and other in natura biomass samples was similar to that of the TG curves demonstrated here. In the thermal decomposition process of biomasses, the dominant step of mass loss is related to the hemicellulose and cellulose degradation [35]. These constituents are long-chain glucose polymers of high thermal stability [36].
Samples chemically treated with all concentrations of NaOH exhibited the same behavior of the thermal decomposition of the in natura samples, i.e., three main steps of mass loss. However, a significant difference in the TG curve profile between 200 and 550 °C could be observed for the sample pretreated with sulfuric acid.
The alterations observed in the TG curve for samples pretreated with acid may be due to the destruction of molecular structures and linkages of the cell walls in the samples. After the acid pretreatment, the lignocellulosic structure may be more amenable to anaerobic digestibility and hemicelluloses solubilization, which makes cellulose more accessible for a subsequent enzymatic treatment [21].
During the dilute acid pretreatment, once the hemicelluloses have been partially converted into soluble sugars, complex lignocellulosic chains are broken, which implies that the compacted recalcitrant structure of the sugarcane bagasse has been at least substantially weakened [37]. According to Chen et al. [37], the profile of the TG/DTG curves for the samples pretreated with dilute acid revealed the destruction of the lignocellulosic structure.
Such an interpretation is based on other studies [21, 38, 39]. Sanchez and Cardona [38] reported that a complex lignocellulosic chain is broken in order to reduce the crystallinity degree of cellulose and increase the fraction of amorphous cellulose, which is the most suitable form for an enzymatic attack. According to Hendriks and Zeeman [21] and Zheng et al. [39], the hemicellulose removal process by dilute acid treatment has a positive effect, as it increases the degradability of the cellulose by an enzymatic access.
The DTG results in Fig. 2 show a double peak around 310 °C for in natura samples due to the thermal degradation of hemicellulose prior to cellulose [34, 37]. This double peak is more pronounced than that in alkaline solution pretreated samples, evidencing the breakdown of the lignocellulosic structure by means of the chemical treatment, as previously described. The three peaks of the in natura samples observed at 310, 330 and 460 °C correspond to the thermal degradation of hemicellulose, cellulose and lignin, respectively.
However, these peaks were not identified for dilute acid-treated samples, possibly due to the disruption of lignocellulosic constituents. For samples pretreated in alkali solutions, two main peaks can be observed at around 330 and 430 °C, and it is worth mentioning that for these samples there is no shoulder, which is commonly present in the in natura samples. Such an absence may have been caused by the solubilization of hemicellulose, leaving only cellulose and lignin remain in the sample. Moreover, the mass loss rate tends to increase in function of the increase in the alkaline concentration solutions.
The DTA curves in Fig. 3 show enthalpic events (exothermic and endothermic) that occur during the thermal degradation. The exothermic and endothermic peaks represent the thermal degradation steps related to organic components, volatile materials and macromolecular structural changes [40, 41]. In all samples, only the first step is an endothermic event, related to the moisture content. The two others are exothermic events. An interesting point observed is the amplitude of the peaks. For example, in the DTG curves the stage related to hemicellulose and cellulose decomposition showed a higher and narrow peak (≈ 330 °C), while in the DTA curves the peaks were lower and wider. A contrary behavior is observed for the lignin degradation from 400 up to 430 °C, i.e., lower and higher DTG peaks and pronounced DTA peaks.
The intensity of the peaks, mainly related to the lignin decomposition (350–550 °C), differs in each treatment. For in natura and acid-treated samples, no significant difference was observed.
The data shown in Table 1 were obtained from the TG curves. Except for the moisture and ash contents, those were obtained by proximate analysis. Thermal breakdown process occurs under inert atmosphere and therefore reflects the combustion of volatiles released during the partial decomposition of organic materials.
The moisture content was slightly affected by pretreatments, except for samples treated with 2% NaOH, which showed 3.3% of moisture content, possibly due to the moisture intrinsic removal of the lignocellulosic materials fibers associated with the highly hydrophilic nature of the biomasses [30]. For other samples, the moisture content ranged from 4.0 up to 5.7%, and as the alkaline solution concentration increased, the moisture content decreased.
The term holocellulose is used to designate the sum of hemicellulose and cellulose contents [42]. In this study, the thermal degradations of both components are considered at the same step, because they are virtually indistinguishable. In contrast, the lignin thermal degradation step could be distinguished in all cases.
Hemicelluloses and lignin were removed from the lignocellulosic structure, which releases more cellulose for the process. A semiquantitative analysis of the TG curves showed that the samples treated with alkaline solution concentrations (2 and 4% NaOH) provided the best results in terms of retaining higher holocellulose contents (75.2 and 82.0%) and lower lignin content (17.1 and 6.3%), respectively.
The final residue is the ash in the biomass, and in this study, the ash content ranged from 4.2 up to 7.2%, depending on the treatment. The ash content over 7.0% for treated samples can be attributed to the presence of alkaline metals and nonmetals, such as sodium and sulfur [43]. Factors, such as differences in cultivation method, different sugarcane varieties and/or hybrids, soil composition, fertilizers, and climate [35, 44, 45], which contribute to a high ash content, have been reported in the literature.
Surface area and porosity analysis
The adsorption and desorption isotherms of nitrogen were obtained for in natura and chemically pretreated sugarcane bagasse samples, as shown in Fig. 4.
According to Koo et al. [46], lignocellulosic materials show pores, which may also be generated by chemical and mechanical treatments. The authors state that the generation of pores increases both the specific surface area and the enzyme access into the interstices of the substrate.
In all samples, little or almost no hysteresis was observed. According to the profiles shown, the adsorption and desorption isotherms were classified as H3 type. Hysteresis occurs due to the pores geometry and indicates the evaporation process (desorption) was unlike the condensation process (adsorption) of nitrogen within the pores [27, 47].
All the sugarcane bagasse samples of this study showed low hysteresis in comparison with other biomasses, such as tucumã seed and rice husk [48].
According to IUPAC (International Union of Pure and Applied Chemistry), H3-type hysteresis is observed in aggregate fine particles whose pores have assumed a gap format [49]. Such hysteresis can be explained by a higher packing and a more rigid cellulosic structure, which result in lower porosity [25, 28].
The IUPAC also classifies the pore size into three categories: micropores (< 2 nm), mesopores (between 2 and 50 nm) and macropores (> 50 nm) [47].
Specific surface areas of native cellulosic plants, such as spruce, wheat straw, flax and dried hemp, are lower than 1.0 m2 g−1 [50]. In this study, the SBET of the in natura sugarcane bagasse samples was approximately 0.63 m2 g−1 and the pores were smaller (< 10 nm).
After 1% H2SO4 treatment, the surface area of the sugarcane bagasse samples was 81% higher than that of the in natura samples. Similar results were described by Koo et al. [46] and Wiman et al. [51], who showed that this change in the surface area is due to the modification in the lignin structure. Such changes improve the degradation of the cellulosic matrix, generating pores and consequently facilitating the enzyme accessibility [50].
The limited structure of the cell wall available to the action of nitrogen gas was enhanced, due to exposition of its inner tissues by acid treatment [50].
Regarding the samples pretreated with alkaline solutions, the increase in their surface areas resulted from defibrilization, which is a disorganization of the structure of the fibers. Such a pretreatment is important because it may lead to an improvement in the enzymatic accessibility [46, 51]. However, no effect was observed in the samples treated with 1% NaOH, possibly due to the migration of the water formed during the process to the pore walls and because most of the nitrogen molecules did not enter the smallest pores during the analysis [52].
Porosity analysis
Table 2 shows the porosity characteristics of the in natura and chemically pretreated sugarcane bagasse samples. Although BET and BJH areas are usually considered for a surface area evaluation, here we considered only the BET method. The BJH method was used for estimating the diameter and volume pores.
Table 2 also shows additional information on the diameter (DBJH) and volume of pores (VBJH) calculated by the BJH desorption method. The diameter of the pores ranged from 146.4 up to 230.5 Ǻ, while their volume ranged between 2.1 and 6.2 Ǻ. Desorption relative pressure values are used because they correspond to a more stable thermodynamic condition [53]. A proportional increase in both acid and alkaline treatments was also observed in the adsorbed quantity (QA) of nitrogen atoms on the surface of the samples (Fig. 4 and Table 2).
Figure 5 shows the pore diameter distribution for in natura and chemically pretreated sugarcane bagasse samples, calculated by the BJH desorption method. Mesopores, which are characterized by pores of 15 up to 30 Å, prevailed in all samples. The removal of hemicellulose by acid treatment increased approximately 81% the surface area and 27% the pore diameter (mesopores) in relation to in natura samples. Similar effects were also observed by Hendriks and Zeeman [21] and Yu et al. [50].
In the transition region between mesopores (lower than 500 Å) and macropores (higher than 500 Å), the amount of pores increases in the alkaline pretreated samples. The pretreated samples with 4% NaOH reached maximum values of approximately 6 m2 g−1 Å−1 of nitrogen adsorption, which indicates that the alkaline solution treatment was more efficient for the increase in the specific surface area [50]. A smaller surface area of some biomasses can also be indicative of few pores [47].
Figure 6 shows the pores volume distribution of in natura and chemically pretreated sugarcane bagasse samples, calculated by the desorption BJH method. All samples showed a high concentration of pores on their surfaces, with a major concentration of mesopores in the region between 15 and 30 Å. In comparison with in natura samples, the acid pretreatment was more efficient to increase the pores volume and possibly favored a higher exposure of the internal spaces of the samples [50].
The total pores volume for in natura and chemically pretreated sugarcane bagasse samples is shown in Fig. 6. The pores volume obtained from the maximum peak in the differential distribution curves ranged from 1.3 up to 2.1 × 10−5 cm3 g−1. The magnitude order of such a distribution is similar to those reported in the literature [25, 50]. However, the pretreatments caused the removal of amorphous regions (hemicellulose and lignin) and the loss of the lignocellulosic structure in comparison with in natura samples [25, 46].
Wettability or contact angle (CA)
Wettability is defined as a surface condition that determines either how fast a liquid will wet the surface or whether the surface will repel it [54]. It is important for the evaluation of a good adhesion force between a solid and a liquid. In this technique, the cellular structure of lignocellulosic materials is penetrated by a liquid in order to establish an intimate contact between the substrate surface and the liquid molecules. When CA value is equal or close to zero, a perfect wetting of the surface occurs. Liquids do not wet the surfaces with CA higher than 90° [31, 54], which indicates a stronger interaction between the liquid and its molecules.
Wettability measurements provide information on the crystallographic structure of the surface, which is associated with variations in its contact with different types of liquids [26]. This technique is commonly used in the characterization of thin carbon films or carbonaceous materials (micro, nano, boron-doped diamond and carbon nanotubes, and others) [55,56,57,58]. In this study, the wettability technique was applied for the evaluation of the effects of the acid and alkaline pretreatments on the lignocellulosic material surfaces.
The presence of some coatings or treatments may alter the wettability characteristics of the original solid due to the modifications in the solid surface free energy, which impacts on contact angles. Such treatments may cause modifications on the substrate surfaces, leaving them hydrophilic or hydrophobic [59].
The CA values of some in natura biomass samples range between 90 and 100° (hydrophobic characteristic) [60], which indicates weak interactions between crystalline cellulose and liquid component. Surfaces of in natura biomasses with hydrophobic characteristics can exhibit a major resistance to acid and alkaline treatments, explained by the recalcitrant nature of the biomass cell wall [61]. The natural roughness showed in some plants may also influence wettability, resulting in a decrease in CA [59].
Table 3 shows the contact angle values obtained for in natura and chemically pretreated sugarcane bagasse samples. The adhesive forces between a liquid and a solid are an energetic parameter of interaction between the liquid molecules and solid surface and allow the liquid drop to spread on the surfaces, leading to low CA [62, 63].
Figure 7a–f shows the images obtained from the wettability technique. For in natura samples (Fig. 7a), the CA around 90° confirms the hydrophobic character for in natura lignocellulosic materials and is partly due to the presence of extractives [60].
The hydrophobic characteristic is also observed in the sample treated with 1% H2SO4 (Fig. 7b) due to high CA = 112°. The possible explanation for the CA increase in comparison with the in natura samples is the smoothing of the irregularities in the surface topography caused by acid and alkaline treatments [64]. However, this behavior can also be explained by the presence of lignin, which has a hydrophobic nature and was exposed to the surface due to chemical treatments [59].
The 33% increase in the lignin content in the sample treated with 1% H2SO4 is indicative that the lignin was released from the lignocellulosic structure.
The hydrophilicity for the samples treated with 1 and 4% NaOH (Fig. 7c, f) can be observed by means of CA = 0° and indicates complete wettability. This behavior suggests that alkaline treatments with 1 and 4% NaOH would greatly facilitate the interaction of the liquid with the surface of the pretreated sugarcane bagasse and a further accessibility of the enzymes into the substrate in an enzymatic hydrolysis.
However, alkaline treatments with 2 and 3% NaOH showed a hydrophobic character with CA = 102° and 124°, respectively (Fig. 7d, e), which indicates these samples may be resistant to a further enzymatic hydrolysis.
Although the wettability could be used to predict the enzymatic hydrolysis, no correlation between wettability and lignin content was established. A higher lignin content cannot explain the hydrophobic characteristic of the sample treated with 1% with H2SO4, since this sample contains approximately 33% of lignin, which is higher than the content all other pretreated samples.
Scanning electron microscopy
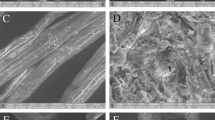
SEM micrographics of the in natura and chemically pretreated samples are shown in Figs. 8 and 9 for the surfaces and cross section, respectively.
The in natura samples in Fig. 8a show elongated parallel fibers, massive beams and piths. Such characteristics are in agreement with those showed by Rezende et al. [11] and Driemeir et al. [65]. A swirl shape structure has been developed, possibly due to the blades during the milling process.
After 1% H2SO4 treatment, as shown in Fig. 8b, there is a change in the morphology of the in natura samples, mainly due the absence of elongated fibers.
In this study, a region of piths was observed for the sample treated with 1% H2SO4, but differently than our findings, Rezende et al. [11] detected the presence of small holes on the pretreated sample surfaces. On the other hand, our results are consistent with those of Sanchez and Cardona [38], who observed the destruction of sugar beet pulp (SBP) fibers by acid pretreatment. The removal of inner parts of the cells by acid pretreatment increased the structural changes of the lignocellulosic matrix. The authors also verified that there occurred an increase in the enzyme accessibility to the cellulose in the pretreated samples with dilute acid. Such a pretreatment enhanced the degradability of the SBP fibers.
For the samples treated with alkaline solutions (Fig. 8c–f), the SEM images show a peeling of fiber bundles. Such a behavior was also reported by Hendriks and Zeeman [21], who considered that a polysaccharides loss is the main factor for this effect.
Thin fiber bundles remain free and are better seen in the samples with 2% NaOH (Fig. 8d). Figure 8e (3% NaOH) shows a large amount of detached fibers, which indicates a reduction in both size and mechanical properties due to the alkaline treatment [66]. According to Fig. 8f (4% NaOH), the elongated fiber bundles are separated in a tube shape, presence of broken ends and residual material along of the fibers. Rocha et al. [23] observed that regions more or less affected by chemical pretreatments can be found in any part of the samples. In these regions, the fibers are less affected by treatments than the piths.
The in natura samples in Fig. 9a exhibit a highly porous structure, known as honeycomb [67]. The empty cavity is known as lumen and exists in the cell wall of the biofibers. The presence of this lumen decreases the bulk density of the biofiber [68].
In a pretreatment with 1% H2SO4 (Fig. 9b), the fibers exhibited a collapsed and distorted cell wall structure, but the tissue integrity was maintained to some extent. Samples treated with 1 and 2% NaOH (Fig. 9c, d) showed a compressed cell structure with some small pores in the fibers.
Figure 9e, f, corresponding to samples treated with 3 and 4% NaOH, shows the occurrence of melting and peeling of the lignocellulosic structure. These effects are due to the increase in the pretreatment severity with higher alkali solution concentrations [46, 66]. In strong alkali solution concentrations, the peeling of end groups, as well as alkaline hydrolysis and dissolved polysaccharides decomposition, can occur. This peeling is an advantage for a later conversion, because lower molecular weight compounds are formed as a result of the high alkali concentration. The risk of degradation and carbon loss through CO2 releases also increases [21].
A delignification process gives the cell wall structure a more fragile appearance in comparison with the in natura samples [11, 50]. However, this appearance is questionable, because an important aspect of the alkaline pretreatment is the cellulosic structure change to a visually denser and thermodynamically more stable form in comparison with in natura cellulose [21].
Conclusions
Sugarcane bagasse samples underwent treatments by dilute acid and alkaline solutions for the understanding of their effects on the biomass lignocellulosic structure, regarding their subsequent enzymatic hydrolysis. Techniques to evaluate the physical–chemical properties of the in natura and chemically pretreated samples were used.
According to the TG/DTG and DTA curves, the sugarcane bagasse samples pretreated with alkaline solutions exhibited a similar thermal profile, unlike dilute acid pretreatment. The acid and alkaline solutions caused modifications in the sugarcane bagasse samples due to the breakage of linkages of the lignocellulosic structures. In comparison with in natura samples, the alkaline treatments produced an increase in the percentages of hemicellulose and cellulose and a decrease in the lignin content. For the samples treated with sulfuric acid, the percentages of such compounds, respectively, decreased and increased in the lignocellulosic structures. These conditions were created due to the solubilization of the compounds. The alkaline pretreatment showed another important aspect: the change in the cellulose structure to form another structure visually denser and of questionable fragility.
The SEM micrographics of the surfaces showed that the samples pretreated with dilute acid suffered corrosive effects on their surfaces and the amount of piths was increased due to the destruction of the cell structures. Samples treated with alkaline solutions showed separation, peeling, fibers swelling and removal of surfaces material. According to the SEM micrographics of the cross section of sugarcane bagasse fibers, morphological changes, namely swelling, deformation, melting, compressed samples and peeling pores, occurred in their fibers after acid and alkaline treatments.
Regarding porosity and in comparison with in natura samples, treatments with 2 and 4% NaOH showed an increase in their specific surface areas, diameter and volume of pores. All these aspects facilitate the subsequent enzymatic hydrolysis.
According to the wettability results, the in natura samples and the samples treated with 1% H2SO4, 2 and 3% NaOH showed a hydrophobic character, while samples treated with 1 and 4% NaOH revealed a superhydrophilic character and are supposed to easily suffer enzymatic hydrolysis. The effects of the treatments on the sample surfaces are in accordance with the contact angles formed, i.e., samples with a superhydrophilic character may result from a partial destruction of the lignocellulosic material. A higher exposure of the lignin content was observed in the lignocellulosic matrix for the hydrophobic samples possibly due to the polysaccharides hydrolysis.
The best pretreatment conditions found were those for samples treated with 2 and 4% NaOH, which showed a significant reduction in the lignin content and an increase in the hemicellulose and cellulose content in comparison with in natura sugarcane bagasse samples. Lignin content, together with the specific surface area and crystallinity degree, is the most important factors in the susceptibility of cellulose to enzymatic hydrolysis.
The results show the importance of a full understanding of the lignocellulosic surface structures of chemically treated sugarcane bagasse samples, as it is the necessary basis for the development of fast and efficient enzymatic hydrolysis. This is a sine qua non condition for the viability of large-scale second-generation processes of biofuels production.
References
Mothé CG, Miranda IC. Characterization of sugarcane and coconut fibers by thermal analysis and FTIR. J Therm Anal Calorim. 2009;97:661–5.
Menon V, Rao M. Trends in bioconversion of lignocellulose: biofuels, platform chemicals and biorefinery concept. Prog Energy Combust Sci. 2012;38:522–50.
Sartori MMP, Florentino HO, Basta C, Leão AL. Determination of the optimal quantity of crop residues for energy in sugarcane crop management using linear programming in variety selection and planting strategy. Energy. 2001;26:1031–40.
Satori MMP, Florentino HO. Energy balance optimization of sugarcane crop residual biomass. Energy. 2007;32:1745–8.
Slade R, Saunders R, Gross R, Bauen A. Energy from biomass: the size of the global resource. Centre for Energy Policy and Technology: Imperial College, London; 2011. p. 120.
Food and Agriculture Organization of the United Nations. FAO statistical yearbook. 2013. http://www.fao.org. Accessed 28 June 2017.
Silva CG. Renewable energies: choosing the best options. Energy. 2010;35:3179–93.
Cortez LAB, Leal MRLV, Nassar AM, Moreira MMR, Feldman S, Taube-Netto M, Silva A. Necessidade de terras para a produção de etanol no Brasil. In: Bioetanol de cana-de-açúcar: P&D para produtividade e sustentabilidade. São Paulo: Blucher; 2010. p. 301–16.
Leal MRLV. Evolução tecnológica do processamento da cana-de-açúcar para etanol e energia elétrica. In: Bioetanol de cana-de-açúcar: P&D para produtividade e sustentabilidade. São Paulo: Blucher; 2010. p. 561–76.
Rezende CA, et al. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels. 2011;4:1–18.
Yu H, et al. Comparative study of alkaline hydrogen peroxide and organosolv pretreatments of sugarcane bagasse to improve the overall sugar yield. Bioresour Technol. 2015;187:161–6.
Masarin F, et al. Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnol Biofuels. 2011;4:1–10.
Betancur GJV, Pereira N Jr. Sugarcane as feedstock for second generation ethanol production. Part I: diluted acid pretreatment optimization. Electron J Biotechnol. 2010;13:1–9.
Chang VS, Holtzapple MT. Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol. 2000;84–86:5–37.
Fingueret J, Meirelles AJA, Guirardello R, Costa AC. Fermentation, hydrolysis, and distillation. In: Biomass for energy. São Paulo: University of Campinas; 2008. p. 433–73 (in Portuguese).
Taherzadeh MJ, Karimi K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources. 2007;2:472–99.
Mohan D, Pittman CU Jr, Steele PH. Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuel. 2006;20:848–89.
Rabelo SC. Evaluation and optimization of pretreatments and enzymatic hydrolysis of the sugarcane bagasse for second generation ethanol production. Ph.D. thesis, School of Chemical Engineering, University of Campinas; 2010. p. 450 (in Portuguese).
Lei H, Cybulska I, Julson J. Hydrothermal pretreatment of lignocellulosic biomass and kinetics. J Sustain Bioenergy Syst. 2013;3:250–9.
Nitsos CK, Matis KA, Triantafyllidis KS. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. Chem Sustain Chem. 2013;6:110–2.
Hendriks ATWM, Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol. 2009;100:10–8.
Banerjee G, et al. Rapid optimization of enzyme mixtures for deconstruction of diverse pretreatment/biomass feedstock combinations. Biotechnol Biofuels. 2010;3:1–15.
Rocha GJM, Martin C, Soares IB, Maior AMS, Baudel HM, Abreu CAM. Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenergy. 2011;35:663–70.
Cara C, Ruiz E, Oliva JM, Sáez F, Castro E. Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharification. Bioresour Technol. 2008;99:1869–76.
Guo J, Catchmark JM. Surface area and porosity of acid hydrolyzed cellulose nanowhiskers and cellulose produced by Gluconacetobacter xylinus. Carbohydr Polym. 2012;87:1026–37.
Fan LT, Lee YH, Beardmore DR. The influence of major structural features of cellulose on rate of enzymatic hydrolysis. Biotechnol Bioeng. 1981;23:419–24.
Webb PA, Orr C. Analytical methods in fine particle technology. 1st ed. Norcross: Micromeritics Instrument Corporation; 1997. p. 301.
Gregg SJ, Sing KSW. Adsorption, surface area and porosity. 2nd ed. Cambridge: Academic Press; 1982. p. 303.
Brown ME. Introduction to thermal analysis—techniques and applications. 1st ed. New York: Chapman and Hall; 1988. p. 211.
Sasmal S, Goud VV, Mohanty K. Characterization of biomasses available in the region of North-East India for production fuels. Biomass Bioenergy. 2012;45:212–20.
Azevedo AF, Matsushima JT, Vicentin FC, Baldan MR, Ferreira NG. Surface characterization of NCD films as a function of sp2/sp3 carbon and oxygen content. Appl Surf Sci. 2009;255:6565–70.
Krüss Information Database. Installation and operation V1-02 Equipment handbook. Hamburg: Krüss; 2005. p. 167.
Demirbas A. Combustion characteristics of different biomass fuels. Prog Energy Combust Sci. 2004;30:219–30.
Ramajo-Escalera B, Espina A, García JR, Sosa-Arnao JH, Nebra SA. Model-free kinetics applied to sugarcane bagasse combustion. Thermochim Acta. 2006;448:111–6.
Munir S, Daood SS, Nimmo W, Cunliffe AM, Gibbs BM. Thermal analysis and devolatilization kinetics of cotton stalk, sugarcane bagasse and shea meal under nitrogen and air atmospheres. Bioresour Technol. 2009;100:1413–8.
Yang H, Yan R, Chen H, Lee DH, Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–8.
Chen WH, Tu YJ, Sheen HK. Impact of dilute acid pretreatment on the structure of bagasse for bioethanol production. Int J Energy Res. 2010;34:265–74.
Sanchez OJ, Cardona CA. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol. 2008;99:5270–95.
Zheng Y, Lee C, Yu C, Cheng YS, Zhang R, Jenkins BM. Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl Energy. 2013;105:1–7.
Mansaray KG, Ghaly AE. Thermal degradation of rice husks in nitrogen atmosphere. Bioresour Technol. 1998;65:13–20.
Diaz PR, Semet VZ. Studies on thermal decomposition and combustion mechanism of bagasse under non-isothermal conditions. Thermochim Acta. 1985;93:349–52.
Guimarães JL, Frollini E, Silva CG, Wypych F, Satyanarayana KG. Characterization of banana, sugarcane bagasse and sponge gourd fibers of Brazil. Ind Crop Prod. 2009;30:407–15.
Jenkins BM, Baxter LL, Miles TR Jr, Miles TR. Combustion properties of biomass. Fuel Process Technol. 1998;54:17–46.
Nassar MM, Ashour EA, Wahid SS. Thermal characteristics of bagasse. J Appl Polym Sci. 1996;61:885–90.
Hu S, Jess A, Xu M. Kinetic study of Chinese biomass slow pyrolysis: comparison of different kinetic models. Fuel. 2007;86:2778–88.
Koo BW, Kim HY, Park N, Lee SM, Yeo H, Choi IG. Organosolv pretreatment of Liriodendron tulipifera and simultaneous saccharification and fermentation for bioethanol production. Biomass Bioenergy. 2011;35:1833–40.
San Miguel G, Domínguez MP, Hernández M, Sanz-Pérez F. Characterization and potential applications of solid particles produced at a biomass gasification plant. Biomass Bioenergy. 2012;47:134–44.
Pécora AAB, Ávila I, Lira CS, Cruz G, Crnkovic PM. Prediction of combustion process in fluidized bed based on particles physical-chemical properties of biomass and their hydrodynamic behaviors. Fuel Process Technol. 2014;124:188–97.
Rouquerol F, Rouquerol J, Sing K. Adsorption by powders and porous solids: principles, methodology and applications. 1st ed. London: Academic Press; 1999. p. 467.
Yu CT, Chen WH, Men LC, Hwang WS. Microscopic structure features changes of rice straw treated by boiled acid solution. Ind Crop Prod. 2009;29:308–15.
Wiman M, Dienes D, Hansen MAT, Van der Meulen T, Zacchi G, Lidén G. Cellulose accessibility determines the rate of enzymatic hydrolysis of steam-pretreated spruce. Bioresour Technol. 2012;126:208–15.
Piccolo C, Wiman M, Bezzo F, Lidén G. Enzyme adsorption on SO2 catalyzed steam-pretreated wheat and spruce material. Enzyme Microb Technol. 2010;46:159–69.
Allen T. Particle size measurement. 5th ed. London: Chapman and Hall; 1997. p. 525.
Baharoğlu M, Nemli G, Sari B, Bardak S, Ayrilmiş N. The influence of moisture content of raw material on the physical and mechanical properties, surface roughness, wettability, and formaldehyde emission of particleboard composite. Compos B Eng. 2012;43:2448–51.
Ostrovskaya L, Perevertailo V, Ralchenko V, Saveliev A, Zhuravlev V. Wettability of nanocrystalline diamond films. Diam Relat Mater. 2007;16:2109–13.
Zhou Y, et al. Control over the wettability of amorphous carbon films in a large range from hydrophilicity to super-hydrophobicity. Appl Surf Sci. 2006;253:2690–4.
Kaibara Y, Sugata K, Tachiki M, Umezawa H, Kawarada H. Control wettability of the hydrogen-terminated diamond surface and the oxidized diamond surface using and atomic force microscope. Diam Relat Mater. 2003;12:560–4.
Pinzari F, Ascarelli P, Cappelli E, Mattei G, Giorgi R. Wettability of HF-CVD diamond films. Diam Relat Mater. 2001;10:781–5.
Maximova N, Österberg M, Laine J, Stenius P. The wetting and morphology of lignin adsorbed on cellulose fibers and mica. Colloid Surf A. 2004;239:65–75.
Heiss-Blanquet S, Zheng D, Ferreira NL, Lapierre C, Baumberger S. Effect of pretreatment and enzymatic hydrolysis of what straw on cell wall composition, hydrophobicity and cellulase adsorption. Bioresour Technol. 2011;102:5938–46.
Himmel ME, et al. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315:804–7.
Eustathopoulos N, Nicholas MG, Drevet B. Wettability at high temperatures. 1st ed. Oxford: Pergamon; 1999. p. 419.
Novikov NV, Khandozhko SI, Perevertailo VM, Ostrovskaya LY, Gontar AG, Loginova OB. The wettability of aC: H films by solution of different physicochemical compositions. Diam Relat Mater. 1998;7:1263–6.
Fuentes CA, et al. Wetting behavior and surface properties of technical bamboo fibers. Colloid Surf A. 2011;380:89–99.
Driemeier C, Oliveira MM, Mendes FM, Gómez EO. Characterization of sugarcane bagasse powders. Powder Technol. 2011;214:111–6.
Cao Y, Shibata S, Fukumoto I. Mechanical properties of biodegradable composites reinforced with bagasse fiber before and after alkali treatments. Compos A Appl Sci. 2006;37:423–9.
Zabaniotu A, Stavropoulos G, Skoulou V. Activated carbon from olive kernels in a two-stage process: industrial improvement. Bioresour Technol. 2008;99:320–6.
Vilay V, Mariatti M, Taib RM, Todo M. Effect of fiber surface treatment and fiber loading on the properties of bagasse fiber-reinforced unsaturated polyester composites. Compos Sci Technol. 2008;68:631–8.
Acknowledgements
The authors gratefully acknowledge CAPES (DS00011/07-0) and FAPESP (2010/20681-4 and 2012/00639-2) for the financial support, Thermal Engineering and Fluids Laboratory (LETeF) from University of São Paulo (USP), Dra. Adriana Faria de Azevedo (National Institute for Space Research—INPE) for the Wettability analysis, and Angela Pregnolato Giampedro for the English language review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cruz, G., Santiago, P.A., Braz, C.E.M. et al. Investigation into the physical–chemical properties of chemically pretreated sugarcane bagasse. J Therm Anal Calorim 132, 1039–1053 (2018). https://doi.org/10.1007/s10973-018-7041-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7041-1