Abstract
Urtica dioica L. has been used by Brazilian population as an herbal medicine to treat several diseases, such as respiratory allergies. Thus, this study aimed to characterize the U. dioica plant drug samples in different particle sizes and its extract by thermogravimetry (TG), differential thermal analysis (DTA) and pyrolysis coupled to gas chromatography and mass spectrometry (Pyr–GC/MS). The plant drug samples were named UD42, UD100, UD200 and UD400 according to mesh. The extracts were dried by spray dryer at 160 °C and oven-drying at 42 °C. The all samples were submitted to dynamic TG in two different atmospheres (nitrogen and air), TG isothermal in the air and DTA in the nitrogen. The kinetic parameters were determined by Ozawa model and Arrhenius equation. The molecules degradable at 250, 350 and 450 °C were identified by Pyr–GC/MS. The dynamic TG curves showed five decomposition steps for all samples and six for extracts in the nitrogen. In the air showed six decomposition steps for all samples. The sample with small-sized particles (UD400) had greater loss of the total mass, take less time for degradation of 5% of the mass at 30 °C, showed lower enthalpy and activation energy than the others plant drug samples. The dry extract through spray dryer showed higher thermal stability than extract dried in oven-drying. Were identified 54 different molecules in all plant drug samples and 42 in extracts. TG, DTA and Pyr–GC/MS are useful auxiliary tools to characterize vegetable raw material for the production of phytotherapeutic drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Herbs and other plant products with therapeutic properties as Urtica dioica L. have been used for respiratory allergies treatment as an effective alternative in worldwide, with special emphasis in development countries [1]. Phytotherapeutic products minimize certain issues of drug ineffectiveness that have been used for the treatment of allergic rhinitis and asthma due to their complex chemical composition and few risks of side effects [2, 3].
Urtica dioica L. (Urticaceae) is a widespread perennial plant that grows commonly in the temperate zones of America, Europe, Asia and parts of Africa [4]. In the northeastern Brazil, it is known as “urtiga” or “queimadeira,” due to the presence of formic acid and histamine in the chemical composition of the plant [5], which causes inflammation and burning sensation on the human skin [6,7,8]. For this reason, it has been undervalued by most communities [9]. However, the plant has been used as an herbal medicine to treat several diseases, such as respiratory allergies (allergic rhinitis and asthma), inflammatory conditions, benign prostatic hyperplasia and hypoglycemia [10,11,12,13]. Several secondary metabolites that modulate expressions of transcription factors and key pro-inflammatory molecules involved in the process of developing respiratory allergies such as flavonoids, alkaloids and coumarins have been recorded from U. dioica [14,15,16,17,18]. So, the extensive use of this plant as an herbal medicine in Brazil requires the evaluation of the quality according to the ANVISA (agency that regulate the registration of herbal medicine in Brazil) [19].
The crushing of the plant raw material and the granulometry powder control are important and critical steps in the process of plant drugs production [20, 21], while in the technological development of phytotherapeutic drugs, the extracts have been submitted to several drying techniques to guarantee the physical and chemical stability of the intermediate product. The drying techniques for a specific extractive plant solution involve some factors, such as mass of solvent or water to be removed, stability of the active substances contained in the product and physicochemical characteristics preferred in the final product [22, 23]. Spray dryer and drying oven are commonly used techniques for drying vegetable extracts, because they offer a dry product suitable for technological production of phytotherapeutic drugs [24,25,26].
The thermal characterization of the dry extracts and plant drugs is very relevant in the evaluation of the quality of the raw material [27, 28]. The thermogravimetry (TG) and differential thermal analysis (DTA) are the most commonly used thermoanalytical techniques to characterize the dry extracts and plant drugs, in raw material production, for analyzing thermal decomposition profile and kinetic parameters, as follows: reaction order (n), activation energy (Ea), frequency factor (A) and degradation constant [29, 30]. Pyrolysis coupled to gas chromatography and mass spectrometry (Pyr–GC/MS) has been used together with TG and DTA to identify molecular fragments degradable resulting from the temperature processes [31,32,33].
In our best knowledge, there is no available information in the literature about quality evaluation of plant drug and extracts of U. dioca as raw material for the technological development of phytotherapeutic drugs.
Thus, in the present research study we characterized the U. dioica plant drug samples in different particle sizes and extracts from the same plant dried by spray dryer and drying oven, using TG, DTA and Pyr–GC/MS.
Materials and methods
Plant material and powder preparation
The plant material of U. dioica was collected in João Pessoa city, Paraíba, Brazil (07°06′54″S, 34°51′47″W), during 2016. The plant material was dehydrated in an oven at 40 °C and was subsequently milled and pulverized.
The different particle sizes were separated through a sieve set with four distinct meshes (42, 100, 200 and 400, corresponding to 441, 149, 74 and 37 μm, respectively). Therefore, the plant drug samples were named UD42, UD100, UD200 and UD400 according to mesh. The material obtained by each mesh was stored in a frosted polyethylene container, properly sealed and protected from light and humidity at room temperature.
Preparation of hydroethanolic extract
Four hundred grams of plant powder was subjected to maceration in 4000 mL hydroethanolic solution (ethanol 50%) for 5 days at room temperature under stirring sporadic. The mixture was filtered using dry cotton followed by filter paper. In order to determine the dry residue, the fluid extract was placed in a drying oven at 105 °C for 2 h and then cooled in a desiccator. There was obtained 23.35% of dry residue, determined by the following formula:
The extracts were submitted into the spray dryer and oven-drying. The spray-dried filtrate was prepared from a suspension containing 20% of colloidal silicon dioxide. During the atomization procedure, the mixture was mixed with a magnetic stirring bar. The drying temperature was 160 °C, and the pump flow was 8 mL min−1. Another fluid extract was dried in oven-drying with air circulation at 42 °C. The dry extract was stored at room temperature in plastic containers with lid, to minimize possible changes in the material, such as agglomeration caused by water absorption and oxidation, until further use. The dry extract through spray dryer was named extract (S. dryer), and the extract dried in oven-drying was named extract (O. drying).
TG dynamic
The dynamic TG curves were acquired using a Shimadzu thermobalance, model TGA-60, using an alumina crucible. Rising temperature experiments were conducted in the temperature range 40–900 °C, at heating rate of 10 °C min−1 in the nitrogen atmosphere (50 mL min−1 flow) and 5, 10, 20 and 40 °C min−1 in air atmosphere (20 mL min−1 flow), with a sample mass of 3.000 ± 0.001 mg, in both tested atmospheres. The apparatus was calibrated with calcium oxalate monohydrate. The data were analyzed using the TASYS (TA-60) software from Shimadzu and Origin Pro 8.0.
TG isothermal
The TG-isothermal curves were carried out over 240 min in air atmosphere at different temperatures: 200, 210, 220, 230 and 240 °C for all U. dioica plant drug samples; 130, 140, 150, 160 and 170 °C for samples of U. dioica extract (S. dryer) and 160, 170, 180, 190 and 200 °C for samples of extract (O. drying), according to dynamic TG profiles in air atmosphere. The apparatus was calibrated with calcium oxalate monohydrate. The data were analyzed using the TASYS (TA-60) software from Shimadzu and Origin Pro 8.0. The degradation constant was calculated using the Arrhenius equation, while the degradation time of 5% of the mass at room temperature (30 °C) was calculated based on the equation of the zero order.
Kinetic parameters determination
The degradation kinetic parameters of U. dioica material, namely reaction order (n), activation energy (Ea) and frequency factor (A), were determined by dynamic and isothermal TG measurements in air atmosphere (20 mL min−1), using the Ozawa model (TGA Kinetic Analysis Application—TA60WS-Shimadzu corporation, Japan) under heating rates of 5, 10, 20 and 40 °C min−1 and Arrhenius equation (lnK = lnA–Ea/RT), respectively.
Differential thermal analysis
DTA curves of the samples were recorded using a Shimadzu differential thermal analyzer model DTA-50 in the nitrogen atmosphere (50 mL min−1 flow) at a heating rate of 10 °C min−1, up to the temperature of 900 °C. The samples mass was 3.000 ± 0.002 mg, which was analyzed by its typical transition phases, using the TA-60WS software (Shimadzu, Japan). The apparatus was calibrated through the melting points of the standards zinc and indium. The DTA curves were plotted using Origin Pro 8.0 (OriginLab Research).
Pyrolysis coupled to gas chromatography and mass spectrometry
Pyr–GC/MS was used together with TG and DTA to identify the molecular fragments degradable resulting from the temperature processes. The pyrolyzer (Shimadzu, Pyr-4A) was directly interfaced with a gas chromatograph and mass spectrometer (Shimadzu, GCMS-QP5050A). A capillary column with stationary phase phenyl/dimethylpolysiloxane (5:95) (with 30 m length, 0.25 mm internal diameter and 0.25 μm particle size) was used. The gas chromatograph was interfaced with the mass spectrometer, which was configured to scan a mass range from m/z 50 to 450. Helium was used as a gas carrier at a flow rate of 1.7 mL min−1 and a split ratio of 1:5. Ionization was effected by electron impact (EI) at 70 eV. The chromatographic run was performed using a temperature ramp at rate 10 °C min−1, starting at 70 up to 300 °C, for 28 min. A fraction of the samples in the platinum crucible was introduced in the pyrolyzer preheated at temperatures of 250, 350 and 450 °C, separately for each experiment. These temperatures were selected according to the dynamic TG curves of the samples under a nitrogen atmosphere (50 mL min−1) at 10 °C min−1. The molecular fragments were identified through mass–charge ratio using GCMS Real Time Analysis and GCMS Postrun Analysis softwares. The identification of the compounds was made by comparing their mass spectra with the Wiley/NBS library reference data (6th Edition for Class-5000).
Results and discussion
TG dynamic
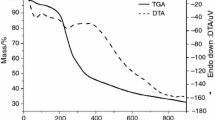
In the nitrogen atmosphere, the dynamic TG curves showed five decomposition steps for all plant drug samples (Fig. 1a) and six for all extract samples (Fig. 1b).
In plant drug samples (UD42, UD100, UD200 and UD400), the first decomposition step occurred in the range of 35.0–107.0 °C, referring to the volatiles, mainly water, corresponding to 5.29, 4.65, 4.60 and 4.85%, respectively, and the main step represents the macro- and microcompound degradations, which occur in the temperature ranges of 300.6–368.9, 300.4–368.2, 300.2–369.5 and 300.6–368.7 °C, corresponding to mass losses of 30.35, 36.57, 36.42 and 36.08% for UD42, UD100, UD200 and UD400, respectively. The total mass losses during the five steps in the nitrogen atmosphere were 75.04, 77.38, 76.98 and 77.90% for UD42, UD100, UD200 and UD400, respectively. The results showed that the UD400 sample had greater loss of the total mass and the amount of the sample-UD400 residue was greater than the others.
In a sample of U. dioica extract dried in a spray dryer, the main step represents the macro- and microcompound degradations occurring in the temperature ranges of 323.8–478.7, having lost 19.35% of the mass. For dry extract in oven-drying, the main step of mass loss was recorded between 315.9 and 368.1, having lost 30.12%. The total mass losses during all decomposition steps were 49.72 and 87.31% for dry extract through the spray dryer and oven-drying, respectively. The extract dried in spray dryer showed greater amount of residue than extract (O. drying) (Fig. 1b) suggesting that this extract presents greater physical stability [25, 26], probably due to its higher level of dehydration than extract dried in oven-drying.
In the synthetic air, the dynamic TG curves showed six decomposition steps for all samples (Figs. 2a–d, 3a–d). Table 1 shows the values of mass degradation in plant drug and extract samples. For plant drug samples, the temperature range of the main step occurred between 270.4 and 398.6 °C with a maximum mass loss for UD400, corresponding to 35.92%, at heating rate of 5 °C min−1; at 10 °C min−1 occurred between 276.8 and 366.0 °C with a maximum mass loss for UD400, corresponding to 33.06%; at 20 °C min−1 occurred between 285.5 and 388.9 °C with a maximum mass loss for UD200, corresponding to 37.21%; and at 40 °C min−1 occurred between 300.7 and 398.8 °C with a maximum mass loss for UD400, corresponding to 37.98% of the total mass. In the main step of mass loss, the dynamic TG curves showed similarity in all samples (Fig. 2a–d). This coincident temperature range could be attributed to the similar degradation of macro- and microcomponents, likely to the loss by evaporation. However, it was observed that the UD400 sample had greater loss of the total mass, the same that was observed under a nitrogen atmosphere.
For extract dried through the spray dryer, the temperature range of the main step occurred between 341.8 and 454.3 °C, having lost 24.94% of the mass, and for extract dried in oven-drying, the main step of mass loss occurred between 298.5 and 358.0, having lost 35.89%, at heating rate of 5 °C min−1. At 10 °C min−1, the main step occurred between 365.5 and 485.9 °C for extract (S. dryer) and 312.8–370.2 °C for extract (O. drying), having lost 23.79 and 32.25% of mass, respectively. At 20 °C min−1, the main step occurred between 380.0 and 496.6 °C for extract (S. dryer) and 326.3–382.0 °C for extract (O. drying), having lost 23.28 and 30.60% of mass, respectively. And at 40 °C min−1, the main step occurred between 398.1 and 501.7 °C for extract (S. dryer) and 351.0–405.8 °C for extract (O. drying), having lost 22.59 and 31.76% of mass, respectively. The dynamic TG curves of extract (S. dryer) showed different thermal decomposition characteristics compared to the dry extract through oven-drying (Fig. 3a–d). Extract (S. dryer) showed higher thermal stability, because it showed greater amount of residue and lower total mass loss than extract (O. drying). Similar results were observed in dynamic TG curves under a nitrogen atmosphere.
TG isothermal
Figure 4 represents the TG-isothermal curves for plant drug and extract of U. dioica. The degradation time of 5% of the mass at 30 °C and thermal degradation constant at different temperatures, for plant drug and extract samples, are shown in Table 2. We have observed that the greatest mass loss occurred at high temperatures for all evaluated samples (Table 2) which means that the majority part of the substances present in U. dioica’s raw material degrade at high temperatures.
The thermal degradation constant determined by Arrhenius equation evidenced larger values for UD400 than other plant drug samples and slightly larger values for dry extract through oven-drying as compared to the dry extract through the spray dryer. In this context, UD400 is more unstable than all plant drug samples and extract (O. drying) is more unstable than extract (S. dryer). The result is corroborated through the values that determine the validity of the raw material, in which the required time for the degradation of 5% of the sample mass at room temperature (30 °C) was 1.352, 1.226, 1.227 and 1.073 years for UD42, UD100, UD200 and UD400, respectively, and 0.953 and 0.866 years for extract (S. dryer) and extract (O. drying), respectively.
Kinetic parameters
The results obtained by Ozawa kinetic model and Arrhenius equation application showed the zero order for all samples evaluated by the derived method (Table 3). Reaction order parameter obtained was evident that the main decomposition process step, which was submitted to kinetic calculation, occurs by the mass loss which could be due to the vaporization without decomposition or by the molecules degradation process, forming substances with gas characteristics. The activation energy values (Ea) were different between the different U. dioica samples in the synthetic air atmosphere. In plant drug samples, it was found that the activation energy is higher in samples with large particles size, with the following decreasing order: UD42, UD100, UD200 and UD400 which means that UD42 sample needs more energy to promote the enthalpic events, as shown in Table 4. Similar result was observed in plant drug from Poincianella pyramidalis (Tul.) [34]. The dry extract through spray dryer showed lower activation energy than dry extract via oven-drying. In this case, the material of UD400 and extract (S. dryer) samples is more available to interact with the heat and required less energy to initiate its chemical degradation process.
Differential thermal analysis
DTA curves showed three exothermic peaks for all herbal medicine samples (Fig. 5a) and two in the extract samples (Fig. 5b) described in terms of temperature and enthalpy (Table 4) showing that there are some chemical compounds present in the plant drug which were not extracted. The peaks provide differences in terms of released heat between the samples. In all samples, the second peak occurred near to 450 °C and presented higher values of enthalpy than the first and second.
In samples of U. dioica plant drug, it was observed that the values of enthalpy are greater in samples with larger particles size. The difference in thermal behaviors which were observed between the different particle size samples suggests that the UD400 sample characterized by smaller particle size showed low heat of decomposition than others. In U. dioica, extract samples were observed that dry extract in spray dryer presented lower enthalpy in the first event and greater in the second event comparing with extract (O. drying). According to the data analysis, it is possible to specify each sample considering the released heat.
Pyrolysis coupled to gas chromatography interfaced with mass spectrometry
Pyr–GC/MS evidenced some of the main molecular fragments released during thermal degradation at different temperatures. The TG data, in the nitrogen atmosphere, showed significant mass losses to the plant drug and extracts of U. dioica in the temperature range of 250–450 °C at 10 °C min−1. Considering these TG results and the mass loss percentages, the samples were submitted to the pyrolysis process at 250, 350 and 450 °C, as shown in Fig. 6.
The molecules identified as degradation products through retention time when evaluated semiquantitatively in relation to their peak areas demonstrate percentage variations between different samples for all measured temperatures. Table 5 shows the main molecules released during the stages of thermal degradation and its relative area percentages, analyzed by library of Wiley 6th Edition. To calculate the percentage of the relative area of peaks matching to molecules was considered the sum of the areas of all peaks analyzed as 100%.
In all plant drug samples, we identified 54 different molecular fragments. Of these, the main compounds identified were oleic acid (C18H34O2) at 16.49 min, palmitic acid (C16H32O2) at 16.57 min, linoleic acid (C18H34O2) at 18.28 min, methyl commate (C31H50O4) at 25.35 min and vitamin E (C29H50O2) at 27.27 min at the temperature of 250 °C (Fig. 6a). At 350 °C were identified 2,6 dimethoxyphenol (C8H10O3) at 9.32 min, oleic acid (C18H34O2) at 16.44 min, palmitic acid (C16H32O2) at 16.55 min, linoleic acid (C18H34O2) at 18.23 min, 8,11,14-eicosatrienoic acid (C20H34O2) at 18.37 min, stearic acid (C18H36O2) at 21.57 min, 2,6,10,14,18,22 tetracosahexaene (C30H50) at 23.89 min and methyl commate (C31H50O4) at 25.35 min (Fig. 6b). At 450 °C were identified acetic acid (C2H4O2) at 3.63 min, 2-furanmethanol (C5H6O2) at 3.96 min, formic acid (C2H4O2) at 4.17 min, phenol-2-methoxy (C7H8O2) at 5.52 min, limonene (C10H16) at 6.10 min, oleic acid (C18H34O2) at 16.49 min, palmitic acid (C16H32O2) at 16.59 min, linoleic acid (C18H34O2) at 18.25 min, stearic acid (C18H36O2) at 21.57 min, methyl commate (C31H50O4) at 25.35 min and flavone 4′-OH, 5-OH, 7-di-o-glucoside (C27H30O15) at 26.46 min (Fig. 6c).
In all extract samples, we identified 42 different molecular fragments. Of these, the main compounds identified were oleic acid (C18H34O2) at 16.06 min, palmitic acid (C16H32O2) at 16.54 min and linoleic acid (C18H34O2) at 17.69 min and vitamin E (C29H50O2) at 27.23, at the temperature of 250 °C (Fig. 6a). At 350 °C were identified 2,6 dimethoxyphenol (C8H10O3) at 9.46 min, oleic acid (C18H34O2) at 16.05 min, linoleic acid (C18H34O2) at 17.67 min, palmitic acid (C16H32O2) at 20.41 min and stearic acid (C18H36O2) at 21.49 min (Fig. 6b). At 450 °C were identified 2-furanmethanol (C5H6O2) at 3.96 min, phenol-2-methoxy (C7H8O2) at 5.52 min, oleic acid (C18H34O2) at 16.06 min, linoleic acid (C18H34O2) at 17.66 min, palmitic acid (C16H32O2) at 20.41 min, stearic acid (C18H36O2) at 21.45 min and flavone 4′-OH, 5-OH, 7-di-o-glucoside (C27H30O15) at 26.38 min (Fig. 6c).
As the temperature at which the sample was submitted increases, more molecules were identified, which confirms the profile of the mass loss observed in the TG analysis. These molecules remain intact in lower temperature (250 °C) to higher (450 °C) which can be related to the loss by volatilization, established in the main step thermal decomposition showed for thermogravimetric curves and for the kinetic study previously reported, which was of zero order, confirming the presence of molecules that do not degrade, but undergo a transition phase from solid to gas, occurring a vaporization. However, the linoleic acid, oleic acid and palmitic acid were observed in all samples tested at 250, 350 and 450 °C. The Pyr–GC/MS results showed that the U. dioica plant drug samples have more molecules degradable than extracts samples, confirming that not all chemical compounds present in the plant drug were extracted, as verified in the DTA data.
Conclusions
Through the thermal analysis, we have recorded that the mass loss of U. dioica vegetable material depends on atmosphere, heating rate and physicochemical characteristics of the sample. The sample with small-sized particles (UD400) had greater loss of the total mass, take less time for degradation of 5% of the mass at room temperature, showed lower heat of decomposition and activation energy than the others plant drug samples which suggest that the UD400 sample needs less energy to promote the enthalpic events. The dry extract through spray dryer showed higher thermal stability than extract (O. drying) and take longer time for degradation of 5% of the mass at room temperature. The plant drug samples have more molecules degradable than U. dioica extract, at 250, 350 and 450 °C. TG, DTA and Pyr–GC/MS are useful auxiliary tools to characterize vegetable raw material for the production of phytotherapeutic drugs.
Abbreviations
- A :
-
Frequency factor
- DTA:
-
Differential thermal analysis
- E a :
-
Activation energy
- EI:
-
Electron impact
- K :
-
Rate constant
- m/z :
-
Mass-to-charge ratio
- N :
-
Reaction order
- Pyr–GC/MS:
-
Pyrolysis coupled to gas chromatography interfaced with mass spectrometry
- R :
-
Gas constant (8.314 J K−1 mol−1)
- RT:
-
Retention time
- T :
-
Temperature
- TG:
-
Thermogravimetry
- UD:
-
Urtica dioca
References
Araújo KRM, Kerntopf MR, Oliveira DR, Menezes IRA, Brito Júnior FE. Medicinal plants in the treatment of respiratoty diseases in childhood: a view from popular knowledge. Rev Rene. 2012;13:659–66.
Silveira PF, Bandeira MAM, Arrais PSD. Farmacovigilância e reações adversas às plantas medicinais e fitoterápicos: uma realidade. Braz J Pharmacogn. 2008;18:618–26.
Pedrosa RC, Yunes RA, Cechinel Filho V. Fármacos e fitoterápicos: a necessidade do desenvolvimento da indústria de fitoterápicos e fitofármacos no Brasil. Quim Nova. 2001;24:147–52.
Bisht S, Snehlata B, Bisht NS. Urtica dioica (L.): an undervalued, economically important plant. Agric Sci Res J. 2002;2:250–2.
Anderson BE, Miller CJ, Adams DR. Stinging nettle dermatitis. Am J Contact Dermat. 2003;14:44–6.
Safari VZ, Ngugi MP, Orinda G, Njagi EM. Anti-pyretic, Anti-inflammatory and analgesic activities of aqueous leaf extract of Urtica Dioica (L.) in Albino Mice. Med Aromat Plants. 2016;5:237.
Shailajan S, Hande H, Singh D, Tiwari B. Estimation of ursolic acid from Urtica dioica L. using validated HPTLC method. J Appl Pharm Sci. 2014;4:92–5.
Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136–9.
Safari VZ, Ngugi MP, Orinda G, Njagi EM. Anti-pyretic, Anti-inflammatory and analgesic activities of aqueous leaf extract of Urtica Dioica (L.) in Albino Mice. Med Aromat Plants. 2016;5:237.
Farahpour MR, Khoshgozaran L. Antinociceptive and anti-Inflammatory activities of hydroethanolic extract of Urtica dioica. Int J Biol Pharm. 2015;4:160–70.
Ayers S, Roschek B Jr, Williams JM, Alberte RS. Pharmacokinetic analysis of anti-allergy and anti-inflammation bioactives in a nettle (Urtica dioica) extract. Online J Pharmacol Pharmacokin. 2008;5:6–21.
Akbay P, Basaran AA, Undeger U, Basaran N. In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother Res. 2003;17:34–7.
Bnouham M, Merhfour FZ, Ziyyat A, Mekhfi H, Aziz M, Legssyer A. Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia. 2003;74:677–81.
Al-Tameme HJ, Hadi MY, Hameed IH. Phytochemical analysis of Urtica dioica leaves by fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry. J Pharmacogn Phytother. 2015;7:238–52.
Said AAH, Otmani IS, Derfoufi S, Benmoussa A. Highlights on nutritional and therapeutic value of stinging nettle (Urtica dioica). Int J Pharm Pharm Sci. 2015;7:8–14.
Farhan SA, Faraj M, Al-Shemari HH, Jassim AKMN. Study of some Urtica dioica L. Leaves components and effect of their extracts on growth of pathogenic bacteria and identify of some flavonoids by HPLC. Al-Mustansiriya J. Sci. 2012;23:79–86.
Otles S, Yalcin B. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci World J. 2012;3:1–12.
Pinelli P, Ieri F, Vignolini P, Bacci L, Baronti S, Romani A. Extraction and HPLC Analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. J Agric Food Chem. 2008;56:9127–32.
Brasil. Agencia Nacional de Vigilância Sanitária. Resolução-RDC no 26, de 13 de maio de 2014. Dispõe sobre o registro de medicamentos fitoterápicos e o registro e a notificação de produtos tradicionais fitoterápicos. Diário Oficial da União. Brasília, DF; 2014.
Correia LP, Santana CP, Medeiros ACD, Macêdo RO. Sideroxylon obtusifolium herbal medicine characterization using pyrolysis–GC/MS, SEM, and different thermoanalytical techniques. J Therm Anal Calorim. 2016;123:993–1001.
Wesołowski M, Konieczynski P. Thermoanalytical, chemical and principal component analysis of plant drugs. Int J Pharm. 2003;262:29–37.
Machado RD. Desenvolvimento tecnológico e caracterização de extratos vegetais obtidos a partir das raízes de Piper umbellatum L. (Piperaceae). Universidade Federal de Goiás, Goiânia. 2014.
List PH, Schimidt PC. Phytopharmaceutical technology. Boca Raton: CRC; 1989.
Silva RMF, Gomes TCBL, Albuquerque MM, Silva Junior JOC, Barbosa WLR, Rolim Neto PJ. Approach on different drying processes employed for obtaining dry extracts from medicinal plants. Rev Bras Plantas Med. 2012;14:103–9.
Oliveira PC, Conceição EC, Oliveira PA, Nascimento MV, Costa EA, De Paula JR, Bara MTF. Obtaining a dry extract of Pterodon emarginatus (Fabaceae) fruits by spray-drying. J Pharm Res. 2012;5:641–5.
Souza KCB, Petrovick PR, Bassani VL, Ortega GG. The adjuvants Aerosil 200 and Gelita-Sol-P influence on the technological characteristics of spray-dried powders from Passiflora edulis var. flavicarpa. Drug Dev Ind Pharm. 2000;26:331–6.
Oliveira MA. Análise Térmica Aplicada A Fármacos E Formulações Farmacêuticas Na Indústria Farmacêutica. Quim Nova. 2011;34:1224–30.
Corrigan OI. Thermal analysis of spray dried products. Thermochim Acta Irlanda. 1993;248:245–58.
Oliveira AH, Moura EA, Pinto MF, Procópio JVV, Souza VG, Souza FS, Macêdo RO. Thermal characterization of raw material pentoxifylline using thermoanalytical techniques and Pyr–GC/MS. J Therm Anal Calorim. 2011;106:763–6.
Procópio JVV, Souza VG, Costa RA, Correia LP, Souza FS, Macêdo RO. Application of thermal analysis and pyrolysis coupled to GC/MS in the qualification of simvastatin pharmaceutical raw material. J Therm Anal Calorim. 2011;106:665–70.
Correia LP, Procópio JVV, Santana CP, Santos AFO, Cavalcante HMM, Macêdo RO. Characterization of herbal medicine with different particle sizes using pyrolysis GC/MS, SEM and thermal techniques. J Therm Anal Calorim. 2013;111:1691–8.
Wang L, Wang C, Pan Z, Sun Y, Zhu X. Application of pyrolysis–gas chromatography and hierarchical cluster analysis to the discrimination of the Chinese traditional medicine Dendrobium candidum Wall. ex Lindl. J Anal Appl Pyrol. 2011;90:13–7.
Oliveira EJ, Alvarez EDA, Lima NGPB, Macêdo RO. Usefulness of pyrolysis coupled to gas chromatography/mass spectrometry for evaluating the reproducibility of commercial samples of Cymbopogon citratus Stapf., Poaceae. Braz J Pharmacogn. 2010;20:93–9.
Guimarães GP, Santos RL, Brandão DO, et al. Thermoanalytical characterization of herbal drugs from Poincianella pyramidalis in different particle sizes. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-016-6076-4.
Acknowledgements
The authors are grateful to Programa de Estudantes-Convênio de Pós-Graduação (PEC-PG) of the CAPES/CNPq, Brazil, for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cuinica, L.G., Macêdo, R.O. Thermoanalytical characterization of plant drug and extract of Urtica dioica L. and kinetic parameters analysis. J Therm Anal Calorim 133, 591–602 (2018). https://doi.org/10.1007/s10973-018-6986-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6986-4