Abstract
Lanthanum ferrite, LaFeO3 (LF), has raised considerable interest since it can be used in many applications such as solid-oxide fuel cell electrode, sensor material (H2O and ethanol) and catalyst. Since the conventional ceramic route of synthesis has some disadvantages, mainly related to an exaggerated grain growth, LF has been prepared by different methods including combustion synthesis, sol–gel, hydrothermal processes, polymerizable complex method and mechanochemistry. As concerns this last method, a problem occurs due to the moisture sensitivity of La2O3. To overcome the problem, we used lanthanum acetate sesquihydrate [La(CH3COO)3·1.5H2O] and iron (II) oxalate dehydrate [FeC2O4·2H2O] as precursors. The mechanism of the solid-state reactions in the mixtures has been studied by TG–DSC and XRPD. Synthesis of LaFeO3 has been realized by annealing the mechanically activated mixtures for 3 h at temperatures between 500 and 800 °C. While LF prepared at 500 °C < T < 600 °C has an amorphous character, LF obtained at T ≥ 600 °C is free from carbonaceous impurities as it is shown by FT-IR and TG measurements. The specific area of the LaFeO3 powders obtained starting from the mechanically activated mixture is decreasing by increasing the annealing temperature. On the contrary, the annealing on samples of physical mixture at temperatures up to 800 °C only yields a mixture of LaFeO3, La2O3 and Fe2O3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the perovskite oxides (general formula ABO3), the lanthanum ferrite, LaFeO3 (LF), has raised considerable interest since it can be used in many applications due to its resistance to high temperatures. So, for example, LaFeO3 can be employed as solid-oxide fuel cell electrode [1], as sensor material for the detection of humidity and alcohol [2] and as catalyst [3, 4].
Several methods have been worked out for LF synthesis. The conventional ceramic route has some disadvantages mainly related to an exaggerated grain growth [5, 6].
Therefore, LF has been prepared by different methods including combustion synthesis [7], sol–gel [8], hydrothermal processes [9] and polymerizable complex method [10, 11].
Such “Soft Chemistry” approaches present some problems essentially linked to the use of expensive and oft not environment-friendly, starting reagents. Besides, another considerable issue lies with experimental conditions such as temperature, pH and chemical composition of the reacting system that have to be carefully and continuously monitored.
Mechanochemical activation has been used during the last decades as a powerful tool for the preparation of metastable crystalline and amorphous phases, and nanostructured materials that cannot be obtained through conventional methods [12, 13]. The effect of conveying mechanical energy to a sample powder can be described under three fundamental reasons: (1) shortening of reaction times; (2) reduction in the high temperatures usually required for developing solid-state reactions; (3) possibility of preparing materials with special properties.
In previous works [14–17], we studied the formation of different ternary oxides (BaTiO3, YFeO3, CaSnO3 and NiFe2O4) from a combination of mechanical and thermal treatment.
In the present work, we report on a further study of the mechanothermal synthesis of LaFeO3. As lanthanum and iron precursors, we used, respectively, lanthanum acetate sesquihydrate [La(CH3COO)3·1.5H2O], that has a hedge over lanthanum oxide since it is not hygroscopic, and iron (II) oxalate dehydrate [FeC2O4·2H2O]. By studying the reactions taking place in the mixtures during heating, we set up a synthesis procedure that combines mechanical and thermal treatment of the mixtures. Finally, we characterized the products formed in the mixtures annealed at different temperatures with different techniques (XRPD, TG, FT-IR, SEM and nitrogen porosimetry).
Experimental
The starting chemicals used were FeC2O4·2H2O (purity 99.9%—Aldrich Chimica, Italy) and La(CH3COO)3·1.5 H2O (purity 99.9%—Alfa Aesar–Germany).
Physical mixtures of composition ratio La/Fe = 1.0 were prepared by weighing the appropriate amounts of the two precursors and by stirring them in acetone suspension for 3 h. Then, the solvent was allowed to evaporate at room temperature overnight.
The mechanically activated mixtures were prepared by dry milling lots of 1 g of physical mixtures: the powders were put into zirconia jars (12.5 ml) of a planetary mill (Pulverisette 7 by Fritsch, Germany) with 5 zirconia balls (12 mm diameter; the mass ratio between the milling balls and the sample powder was 11:1). The mill was operated at 650 rpm rotation speed for 6 h.
TG/DSC measurements were performed with a TG/DSC simultaneous analyser (Q600, TA Instruments Inc. USA) connected to a computer fitted with an appropriate software. Samples of ≈ 50 mg of both physical and milled mixtures were placed in an alumina pan and heated at 10 K min−1 (under air flow of 100 mL/min) from 25 up to 800 °C where a constant mass value is reached.
Samples of both milled and physical mixture have been heated in tube furnace (static air, 10 K min−1) up to temperatures between 500 and 800 °C in steps of 50 °C with isothermal stage of 3 h at the end of each heating ramp.
X-ray powder diffraction (XRPD) patterns have been taken to determine the phases that were formed during the annealing. The relevant patterns were recorded with an X-ray powder diffractometer (Bruker D5005) in step-scan mode (CuKα radiation, step width 0.015°, 2 s/step, 40 kV, 40 mA, 2ϑ° = 15–50).
Diffuse reflectance (DR) FT-IR spectra have been recorded on samples of milled mixture heated up to different temperatures by a Nicolet spectrometer (iS10, Nicolet, USA). The spectra collected with DR have been recorded on samples dispersed in KBr (≈ 5% by mass in KBr): 128 scans have been coadded at 4 cm−1 resolution and ratioed against 128 scans collected on samples of pure KBr (99+%, Sigma-Aldrich, Italy).
The specific surface area of the milled mixtures after annealing under different conditions was determined by N2 adsorption (BET method). The nitrogen adsorption curve was recorded by a Sorptomatic 1990 (Thermo Electron Corporation, operating with the static volumetric principle). The correction for the volume of the sample was introduced by measuring the He sorption.
SEM measurements were taken using a Zeiss EVO MA10 (Carl Zeiss, Oberkochen, Germany) at an acceleration voltage of 20 kV and 8.5-mm working distance on gold-sputtered samples.
Results and discussion
Thermal behaviour of the mixtures
Figure 1 shows the XRPD patterns of both the physical and the activated mixture. As it can be seen, the peaks of the two precursors disappear in the activated mixture as a result of the mechanical treatment that induces amorphization.
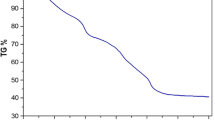
Figure 2 shows the TG–DSC–DTG signals recorded on a sample of the milled mixture.
The first stage of mass loss process ends (≈ 175 °C) at a residual mass of 87.46 ± 0.19% (mean value from 5 independent measurements) that is in a reasonable agreement with the value of residual mass expected for the total dehydration of the mixture (87.94%). Under the first stage of mass loss, an endothermic DSC peak is present that corresponds to the endothermic dehydration process. The DTG curve shows two peaks: the first one ends at ≈ 260 °C and corresponds to a residual mass value (76.93 ± 0.36%) that is ≈ 1% higher than the value expected for the formation of the mixture La(CH3COO)3 + (1/2)Fe2O3(75.70%). The DSC counterpart is an exothermic peak whose enthalpy cannot be evaluated as such a peak is not well separated from the subsequent one much more intense exothermic DSC peak. The following DTG peak is much more intense than the previous one, and it is accompanied by a strong exothermic DSC peak. The mass value at the end of this peak is 52.39 ± 0.55% (T ≈ 500 °C). Such a value is sensibly lower than the value of the residual mass expected (54.83%) at the end of reaction (1):
Clearly, the two stages of the mass loss process involve, besides Fe(II) oxalate decomposition and La acetate combustion, a partial decomposition of La oxycarbonate to La oxide. The total enthalpy under the two exothermic DSC peak is ΔexoH = − 1512 ± 78 J g−1.
The last stage of mass loss ends at ≈ 700 °C at a residual mass of 46.08 ± 0.32% that shows a good agreement with the value expected for the formation of a mixture (1/2) La2O3–(1/2) Fe2O3 (46.41%).
For the sake of comparison, a TG–DSC run has also been performed on a sample of physical mixture. Figure 3 shows the TG–DSC–DTG signals recorded on this sample.
The first stage of mass loss shows a final mass value at the end of the first stage (86.00 ± 0.25%) that is sensibly lower than that expected (87.94%) for the complete dehydration of the mixture. Clearly, the first stage of mass loss contains, besides the dehydration of the precursors, a share of the FeC2O4 decomposition. The DSC counterpart under these stages is an endothermic peak.
By higher temperature, only a DTG peak is present, contrarily to what happens in the activated mixture. At the end of such a peak (≈ 350 °C), the residual mass value is 50.42 ± 0.32% that is sensibly lower than the value expected for reaction (1). In the case of the physical mixture too, the process of combustion includes the oxycarbonate decomposition to La(III) oxide and to a larger extent than it is the case with milled mixture. The DSC counterpart under such a stage of mass loss is, as with the milled mixture, a double exothermic peak, but with inverted relative intensities. Moreover, it can be observed that the heat released under such a double exothermic peak is much higher (Δexo H = − 3000 ± 97 J g−1). This points out to the fact that different processes are going on within the two types of mixture. The final stage of mass loss ends at ≈ 780 °C with a residual mass of 45.83 ± 0.22% that shows a fair agreement with the value expected for the formation of a mixture (1/2)La2O3–(1/2) Fe2O3 (46.41%).
It seems that the main difference between the TG–DSC runs on the mixtures is observed in the enthalpy of the exothermic DSC peak under the main mass loss stage. However, the most striking difference on what happens by heating the two types of mixture can be observed in Fig. 4, which shows the XRPD patterns of the residual of both physical and mechanically activated mixtures recovered at the end of the runs performed up to 800 °C.
The only diffraction peaks of the residual of the mechanically activated mixture (a) are those characteristic of lanthanum ferrite LaFeO3 (orthorhombic JCPDS-00-037-1493). The situation is completely different as concerns the residual of the physical mixture (b) that, besides some less intense peaks of LaFeO3, shows the peaks characteristic of Fe2O3 and of La(OH)3 that forms by the interaction between unreacted La2O3 and air moisture.
Synthesis of LaFeO3
Figure 5 shows the XRPD patterns of samples of milled mixtures heated up to different temperatures (from 500 to 800 °C in steps of 50 °C; isothermal stage of 3 h).
In all the samples, but that heated up to 500 °C, only the peaks of LaFeO3 are present. In the sample heated up to 500 °C, the peaks are very broad and point out to the formation of amorphous LaFeO3. The collected experimental evidence shows that LaFeO3 can be obtained by short annealing (3 h) of the mechanically activated mixture at temperatures as low as 550 °C.
The same thermal schedule (with isothermal stages of 3 h at 550–800 °C in steps of 50 °C) has been applied to samples of physical mixture. The XRPD patterns of the different samples after the annealing are shown in Fig. 6. It can be seen that, although the peaks of LaFeO3 are present starting from the patterns of the mixture annealed at 600 °C, the most intense peaks up to temperatures as high as 800 °C are those characteristic of the two component oxides (La2O3 and Fe2O3). The obtained results suggest that, starting from the activated mixture, LaFeO3 forms directly as the precursors thermally decompose, while the situation is different when the synthesis is attempted starting from a physical mixture. In this case, the oxide mixture forms from the thermal decomposition of the precursors in the same temperature range as it is the case with the milled mixtures, but it reacts slowly, leading to a final product that is a mixture La2O3-Fe2O3-LaFeO3.
Characterization of LaFeO3 synthesized from mechanically activated mixtures
It is reported in the literature [18] that the samples of LaFeO3 prepared by different routes can be contaminated by carbonate which likely forms from the interaction of unreacted La oxide with atmospheric carbon dioxide or with the presence of undecomposed La oxycarbonate formed during the combustion process of the acetate.
To assess whether such a contamination is present in the samples obtained by annealing the milled mixture, the IR spectra of these very same samples have been recorded. The spectra of the samples annealed at temperatures up 650 °C are shown in Fig. 7.
It can be observed that bands at 1600–1300, 1059 and 844 cm−1, suggesting the presence of La2O(CO3)2 [18], are present only in the IR spectra of the activated mixture annealed for 3 h at 500 and 550 °C. The spectra of the sample annealed at T ≥ 600 °C do not longer show these peak but only the doublet at 570 and 430 cm−1 that is due to the stretching of Fe–O bonds in LaFeO3 [18]. Therefore, according to the IR evidence, a thermal treatment of 3 h at 600 °C it is enough to yield carbonate-free LaFeO3 powder when starting from the mechanically activated mixture.
Such information has been checked by TG measurements performed on the samples of milled mixture annealed for different times at different temperatures. The samples, after the annealing, have been heated up to 900 °C, and the residual mass values attained at the end of the runs are reported in Table 1.
From the obtained results, it can be concluded that the reaction has not been completed yet after the 3-h annealing at 500 and 550 °C: indeed the relevant samples show a larger mass loss in the TG runs performed on them. The opposite is true by higher annealing temperatures (T ≥ 600 °C): the relevant samples show a negligible mass loss in the TG runs performed on them after they have been annealed. However, it has to be noted that the reaction can be completed also at lower temperatures provided that the mixture is kept at these temperatures (500 and 550 °C) for longer times. Indeed, 83 h (at 500 °C) and 24 h (at 550 °C) are needed to yield samples that in the relevant TG run show residual masses near to those obtained after the annealing performed at T ≥ 600 °C. The XRD patterns of these mixtures confirm that LaFeO3 is the only phase present in the mixtures annealed for 3 h at T ≥ 600 °C and for 83 h (500 °C) and 24 h (550 °C).
The specific surface area has been determined on samples of LaFeO3 obtained from the milled mixture annealed at 500 °C (83 h), 550 °C (24 h), and at all the temperatures from 600 up to 800 °C (3 h annealing).The results are reported in Table 2, showing that the specific surface area decreases by increasing the annealing temperature.
This result is confirmed by SEM micrographs taken on samples of milled mixture annealed for 3 h at 600 °C (Fig. 8a), 700 °C (8b) and 800 °C (8c). They show that, by increasing the annealing temperature, the share of the fine grains present in the annealed mixtures decreases.
Conclusions
-
1.
The mechanism of the solid-state reactions in the La(CH3COO)3·1.5H2O–FeC2O4·2H2O mixtures has been studied by TG–DSC. The mass loss processes taking place in the milled and in the physical mixture are quite similar. What is different is the exothermic enthalpy change under the main mass loss process. Furthermore, XRPD shows that LaFeO3 is the product formed when starting from milled mixtures, while a mixture of LaFeO3 and the forming oxides (La2O3–Fe2O3) is the product obtained when starting from a physically prepared mixture;
-
2.
The synthesis of LaFeO3 can be accomplished from reaction between La2O3 and Fe2O3 formed by the thermal decomposition of the milled mixture of the precursors. Such a decomposition/solid -state reaction is realized by 3-h annealing at temperatures as low as 600 °C;
-
3.
The LaFeO3 is free from carbonaceous impurities when synthesized at T ≥ 600 °C. However, the compound can be synthesized at temperatures as low as 500 °C provided it is maintained at these temperatures for longer times. The specific surface area of the samples decreases with increasing temperature.
References
Kumar M, Srikanth S, Ravikumar B, Alex TC, Das SWK. Synthesis of pure and Si-doped LaGaO3, LaFeO3 and LaCoO3 and Sr-Mg-doped LaGaO3 for ITSOFC application using different wet chemical routes. Mater Chem Phys. 2009;113:803–15.
Song P, Wang Q, Zang Z, Yan Z. Synthesis and gas sensing properties of biomorphic LaFeO3 hollow fibers templated from cotton. Sens Actuators. 2010;B147:248–54.
Chandradass J, Hyeon Kim K. Nano-LaFeO3 powder preparation by calcining an emulsion precursors. Mater Chem Phys. 2010;122:329–32.
Parida KM, Reddy KH, Martha S, Dars DP, Biswale N. Fabrication of nanocrystalline LaFeO3: an efficient sol-gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int J Hydrog Energy. 2010;35:12161–8.
Idrees M, Nadcem M, Atif M, Siddique M, Mehmood M, Hassan MM. Origin of colossal dielectric response in LaFeO3. Acta Mater. 2011;59:1338–45.
Belessi VC, Trikalis PN, Ladavos AK, Bakas TV, Pomonis PJ. Structure and catalytic activity of La1−xFeO3 (x = 0, 0.05, 0.10, 0.15, 0.20, 0.25, 0.35) for the NO + CO reaction. Appl Catal A. 1999;177:53–68.
Wang Y, Zhu J, Zhang L, Jang X, Lu L, Wang X. Preparation and characterization of perovskite LaFeO3 nanocrystals. Mater Lett. 2006;60:1767–70.
Rajendran M, Bhattacharya AK. Nanocrystalline orthoferrite powders: synthesis and magnetic properties. J Eur Ceram Soc. 2006;26:3675–9.
Kemeng J, Hongxing D, Jiguang D, Song L, Shaohua X, Han W. Glucose assisted hydrothermal preparation of porous LaFeO3 for toluene combustion. J Solid State Chem. 2013;199:164–70.
Popa M, Franti J, Kakihama M. Lanthanum ferrite nanopowders obtained by the polymerizable complex method. Solid State Ion. 2002;154–155:437–45.
Andoulsi R, Horchani-Naifer K, Ferid M. Preparation of lanthanum ferrite powder. Ceramica. 2012;58:126–30.
Sorescu M, Tiantang X, Burnett JD, Aitken JA. Investigation of LaFeO3 perovskite growth mechanism through mechanical ball milling of La and Fe oxides. J Mater Sci. 2011;46:6709–19.
Cristobal AA, Botta PM, Bercoff PG, Porto Lopez JM. Mechanosynthesis and magnetic properties of microcrystalline LaFeO3 using different iron oxides. Mater Res Bull. 2009;44:1036–40.
Berbenni V, Marini A, Bruni G. Effect of mechanical milling on solid state formation of BaTiO3 from BaCO3–TiO2 (rutile) mixtures. ThermochimicaActa. 2001;374:151–8.
Berbenni V, Milanese C, Bruni G, Girella A, Marini A. Synthesis of YFeO3 by thermal decomposition of mechanically activated mixtures Y(CH3COO)3·4H2O–FeC2O4·2H2O. ThermochimicaActa. 2011;521:218–23.
Berbenni V, Milanese C, Bruni G, Girella A, Marini A. Synthesis of calcium metastannate (CaSnO3) by solid state reactions in mechanically activated mixtures calcium citrate tetra hydrate [Ca3(C6H5O7)2·4H2O]—tin(II) oxalate (SnC2O4). Thermochim Acta. 2015;608:59–64.
Berbenni V, Milanese C, Bruni G, Marini A. The combined effect of mechanical and thermal energy on the solid-state formation of NiFe2O4 from the system 2NiCO3·3Ni(OH)2·4H2O–FeC2O4·2H2O. Thermochim Acta. 2008;469:86–90.
Sivakumar M, Gedanken A, Zhong W, Jiang YH, Du YW, Brukental I, Bhattacharya D, Yeshurun Y, Novik I. Sonochemical synthesis of nanocrystalline LaFeO3. J Mater Chem. 2004;14:764–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berbenni, V., Bruni, G., Milanese, C. et al. Synthesis and characterization of LaFeO3 powders prepared by a mixed mechanical/thermal processing route. J Therm Anal Calorim 133, 413–419 (2018). https://doi.org/10.1007/s10973-017-6878-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6878-z