Abstract
Titanium and its alloys are used in production of implants such as knee and hip prostheses due to their superior properties. Ti–Nb–Zr ternary alloys are preferred over other metallic implant materials due to the presence of non-toxic elements, high corrosion resistance, good biocompatibility, and proper mechanical properties. The aim of this work is to investigate the effect of zirconium addition on α → β phase transformation, microstructure, and mechanical behavior of Ti–16Nb alloy. In doing so, Ti–16Nb–xZr (x: 0, 5, 10, 15 mass%) alloys are produced by powder injection molding, which offers advantages such as low cost, net shape, and easy production of complicated parts for implant fabrication. X-ray diffraction analysis and scanning electron microscope images showed that zirconium behaves as a β stabilizer and according to differential thermal analysis, and it decreases α to β transition temperature approximately 30 °C. It is also revealed that increasing zirconium content caused finer microstructure and hardness of the alloy was raised from 336 HV0.5 to 412 HV0.5 while elastic modulus remains approximately steady between 103 and 110 GPa. It is concluded that Ti–Nb–Zr alloys have been found to be a good alternative to known metallic implant materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For hard tissue replacement, titanium and its alloys are widely used owing to their high corrosion resistance, low density, good biocompatibility, comparatively low elastic modulus, and high strength [1]. Alloy development studies for implant materials mainly focus to the reach to closest properties to the bone. High strength and low elastic modulus properties are required for load bearing implant applications as well as biocompatibility and corrosion resistance [2, 3]. Ti–Ni alloys and Ti–6Al–4V alloys are widely used for biomedical applications, but it has been observed that these alloys have long-term ignition and carcinogenic effects due to release of toxic elements ions [4, 5]. Also Ti–Ni and Ti–6Al–4V alloys exhibit excessively higher elastic modulus compared to the bone (10–30 GPa) [6], and this difference resulting in a stress shielding problem and leads to poor osseointegration and loosening of the implant [7]. In recent years, to overcome these drawbacks, there has been focus on the development of higher corrosion-resistant beta titanium alloys with lower elastic modulus and non-toxic elements (niobium, zirconium, tantalum, etc.) [8].
Titanium alloys have two stable phases, α phase (HCP) at lower temperatures, β phase (BCC) at higher temperatures. In case of quenching or heat treatment, several metastable phases (α ı, α ıı, w) can be formed and β phase has the lowest elastic modulus among these phases [9]. Elastic modulus of the alloy is reduced by the addition of β stabilizer elements such as niobium and non-toxic elements such as zirconium, tantalum, and tin generally used as an alloy element to improve mechanical properties and corrosion resistance as well [10,11,12]. Studies in the literature have reported that zirconium enhances the strength of the Ti–Nb alloys by grain refinement and solid solution hardening [13,14,15,16]. Also some recent works revealed that zirconium increases β phase stability and corrosion resistance [17, 18].
The powder injection molding method is a relatively new kind of manufacturing which allows the rapid and easy production of complex shaped parts [19] and commonly used for metallic implant production [20, 21]. Although titanium alloys such as Ti6Al4V has been extensively studied in the literature and reviewed by the previous researchers [22,23,24], it is not possible to see studies about powder injection molding (PIM) processed Ti–Nb alloys except a few articles [9, 15, 25,26,27,28] and only one of them focuses to effects of zirconium as alloying element [15]. In this study effects of zirconium, as a β stabilizer, on the properties of PIM Ti–16Nb alloy were investigated. In order to do this, four kinds of Ti–Nb alloys were produced by using PIM method. One of the alloys was zirconium free, whereas other three alloys contains zirconium among 5–15 mass%. Rheological, microstructural, and mechanical properties of the produced alloys were analyzed and discussed.
Experimental
Properties of the raw powder are given in Table 1.
Samples made from Ti–16Nb–(0, 5, 10, 15 mass%) Zr were produced by PIM process. Firstly, the powders were mixed in a Turbula mixer for 2 h. After, mixed elemental powders were blended with a multicomponent binder system in a specially designed mixer at 190 °C for 40 min. The feedstock was included 55 vol% alloy powder and 45 vol% binder. The multicomponent binder system consisted of 69 mass% paraffin wax, 20 mass% carnauba wax, 10 mass% polypropylene, and 1 mass% stearic acid. The polypropylene in the binder system provides strength to protect the shape of the molded sample while paraffin wax and carnauba wax develops moldability by lowering the viscosity of feedstock. Stearic acid acts as lubricant. Rheological behaviors of the prepared feedstocks were measured by using a Physica MCR51 rotational viscometer.
Feedstock was molded by an injection molding device which its barrel and nozzle heated to 145 °C. Injection pressure was 8 MPa and holding time was 30 s. The debinding process was applied in two steps. In the first step, the molded samples were held in heptane at 60 °C for 8 h, then thermal debinding process was conducted by waiting 2 h at 200–400–600 °C under the Argon atmosphere(heating rate 2 °C min−1). In order to understand thermal debinding behavior of the binders, thermal gravimetry (TG) analyses were carried out. According to TG analysis results (Fig. 1), all of the binders decompose under the thermal debinding temperature except polypropylene. It is predicted that residual polypropylene will be decomposed during sintering at higher temperatures. So it was therefore concluded from the TG analysis of the binders that applied thermal debinding process is proper for eliminating the binders. Just after, pre-sintering is performed under the Argon atmosphere at 900 °C, for 1 h. Finally, sintering process was conducted in a vacuum (3.4 × 10−6 mbar) atmosphere at 1500 °C for 4 h. In the sintering process, 10 °C min−1 heating rate was used up to 1200 °C and then 5 °C min−1 heating rate was used between 1200 °C and the sintering temperature. The cooling rate was selected as 10 °C min−1. Detailed information about debinding–sintering cycle is given elsewhere [9]. Comparative sizes of the green (Fig. 2a) and sintered (Fig. 2b) specimens are given in Fig. 2. It should be noted that the length of the reference pen is approximately 145 mm.
After sintering, densities of sintered samples were measured according to Archimedes’ water immersion method and then relative densities were calculated.
Samples for metallographic examination were prepared by cutting from the cross sections of specimens. Kroll solution (6 mL nitric acid + 2 mL HF + 92 mL distilled water) was used as etchant. A scanning electron microscope (SEM) (Jeol JSM 6060LV) coupled with an energy–dispersive spectroscopy (EDS) IXRF 5000EDX) was used for investigating the microstructural features. X-ray diffraction (XRD) analyses were conducted by using a RIGAKU D/Max 2200 XRD analyzer while β phase ratio was calculated by using PANalytical Empyrean XRD analyzer equipped with computer software. Carbon content measurements of the alloys were accomplished through LECO-CNHS 628 analyzer. Microhardness measurements were carried out under a load of 0.5 kgf and elastic modulus of the alloys measured by using a CSM Instruments Nanoindentation tester equipped with Berkovich-type diamond indenter at a maximum applied load of 80 mN. Also differential scanning calorimetry (DSC) analysis was used to examine the β–α phase transformation with the addition of Zr. DSC was performed at a heating rate of 2 °C min−1 in the SETARAM Labys SDT Q600 Simultaneous Thermal Analyzer device under the Argon atmosphere.
Results and Discussion
For a successful PIM application, the metal injection molding feedstock should exhibit a pseudoplastic flow property [29]. In order to ensure appropriate flow of feedstock, viscosity should be less than 1000 Pa s [30, 31]. Suitable binder/metal powder ratio and appropriate molding parameters can be determined by rheological study. Thereby, faults due to improper molding temperatures can be avoided. Figure 3 shows the viscosities of feedstock at different shear rates and temperatures (145–155–165 °C). It is clearly seen from Fig. 3 that viscosity decreased with increasing shear rate. So this kind of behavior of the powder binder component can be termed as pseudoplastic flow. It is well known that during metal injection molding process, a sudden drop in viscosity and increase in shear rate at the injection molding temperature is desired [32,33,34]. It is inferred from the rheology analysis that chosen injection molding temperature (145 °C) is proper for feedstock viscosity. It was therefore concluded that using rheology made it possible to achieve smooth production by obtaining proper powder/binder ratio and molding parameters. Similar viscosity–shear rate curves are observed in other work about titanium-based alloys produced by PIM [35, 36].
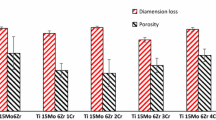
There was no shape distortion seen in the samples after sintering (Fig. 2). The average shrinkage between the green and sintered parts is 18 ± 0.5 and 19 ± 0.5% for the length and diameter, respectively. According to a recent study [37], shrinkage of the powder injection molded parts may be between 10 and 30%. Sintering parameters and metal powder/binder ratio change the shrinkage level. According to previous works about Ti–Nb PIM system, increased binder/powder ratio lead to the higher shrinkage levels. For example, Zhao et al. [28] detected 11–12% shrinkage rate with the use of 31% binder. In the work of Bidaux et al. [27] 13–14% shrinkage rate occurred by using % 40 binder. Higher metal powder charge provides lower shrinkage and easier dimension tolerance control. However, loading higher amounts of metal powders is also unacceptable because it leads to too high feedstock viscosity and results in the failure of injection molding [32].
Porosity of 0.92–2.75% was calculated for sintered alloys at 1500 °C (Fig. 4). It is known that, density changes depending on sinter parameters, diffusion coefficient of alloy elements and particle size. Similar density results (2–6% porosity) were obtained from the alloys with comparable chemical compositions [25, 38]. The porosity values attained in this study are compatible with the human cortical bone [39]. It can be seen that porosity increases from 0.92 to 2.75% as the zirconium is added to the Ti–16Nb alloy. It is suggested that lower diffusion coefficient of the Zr in Ti lead to lower density values [40]. Such kind of behavior of the Zr was also determined from Nagaram et al. [15] in their study about Ti–22Nb alloy.
For understanding morphological and microstructural developments during PIM and thermal processes, SEM images were taken after each stage of PIM. It is worth noting that zirconium addition to the base alloy has no effect on the appearance of the powders neither before nor after debinding and pre-sintering processes. SEM image of the Ti16Nb15Zr alloy after injection molding and pre-sintering is given in Fig. 5a, b, respectively. SEM images of the powder injection molded blend (Fig. 5a) showed that process did not caused plastic deformation. It is postulated that since the affinity of zirconium to oxygen is higher than for titanium, higher oxygen content is detected on zirconium powders (Table 2) [41]. Furthermore, oxygen content of the niobium powders is lower than that of other alloying elements due to its lower affinity to oxygen. It may be concluded from the EDS analysis results of the powder injection molded compacts that binders caused oxygen and carbon contamination of the powders. It should be noted that C and O ratio were reduced to 0–1% after debinding. Carbon content of the alloys was measured as 1.0512, 0.58615, 0.51953, and 0.61984 mass% for Ti16Nb, Ti16Nb5Zr, Ti16Nb10Zr, and Ti16Nb15Zr, respectively. It is postulated that polymer-based binders stick and cover the surfaces of the metal powders and a little amount of binder stays intact after debinding (Fig. 1) and residual binder causes C and oxygen contamination even after debinding. Figure 5b shows that neck formation between particulates occurred during pre-sintering. Zhao et al. [25] reported similar microstructural development in their study about PIM process of Ti–Nb alloys.
XRD analysis of the produced alloys revealed that the amount of the β phase shows a trend of increasing with increasing amount of the zirconium (Fig. 6). Also it was confirmed from the XRD analysis that all of the alloys comprised of α + β phases. It is clearly proved that zirconium acts as a β stabilizer for Ti–Nb alloys and facilitates transformation from α to β. It is also worth pointing out that there is a decrease in the angle of the beta phase peaks with increasing amount of zirconium, such result indicating an increase in the lattice parameter [8]. Amount of the increase in the lattice parameter will be discussed in later parts of this paper.
SEM images of the sintered samples (Fig. 7) confirm the presence of α and β phases, and there was no other phases detected. It is clearly seen from the SEM images that both of the Ti16Nb and Ti16Nb15Zr alloys exhibit lamellar structure. As noted previously in the literature, brighter colored regions on the SEM images of the Ti–Nb alloy represent niobium rich β phase while darker regions depict α phase [8, 13, 25, 39]. It is obviously seen from Fig. 7b that zirconium addition into Ti–16Nb causes increase in brighter regions (β phase) on microstructure, so it is determined that SEM images proves the XRD analysis results. According to the result of semiquantitative XRD analysis, the addition of 15 mass% Zr to the Ti–16Nb alloy increased the β phase ratio from 25 to 54% in microstructure (Fig. 8). Zirconium addition into the Ti16Nb alloy caused to grain refinement, and such behavior of the alloy is consistent with the findings of Zhou et al. [42]. Although reason for grain size reducing effect of the zirconium is not clearly explained, it is postulated that newly formed particles can prevent grain growth. Martins et al. [40] stated that stability of the β phase is increased with the addition of zirconium to the Ti–Nb-based alloys.
In order to evaluate the influence of zirconium on sintered samples’ phase transformation behaviors, differential thermal analysis (DTA) were carried out, and effect of Zr as a β stabilizer was confirmed (Fig. 9). Although the allotropic conversion temperature of pure titanium from α to β is about 882 °C [43, 44], it is known that some elements such as niobium and tantalum lower this temperature. Endothermic peaks are seen at 483 and 457 °C for Ti–16Nb and Ti–16Nb–15Zr alloy, respectively (Fig. 9). DTA data indicated a light drop in β-transus temperature (~ 30 °C) with increasing zirconium content, and this decrease is attributed to the effect of zirconium in stabilizing the β phase, since zirconium is considered to be the β-eutectoid forming element in titanium alloys. Yu et al. [42] reported that α–β transition temperature of the Ti25NbXZr alloy decreased from 480 to 418 °C with the addition of Zr. Malek et al. [17] stated that it is possible to decrease the transformation temperature from 513 to 451 °C by alloying the Ti35Nb with Zr.
It is apparent that lattice parameters of the α and β phase increased with increasing Zr content (Fig. 10a, b). Atomic diameters of titanium, niobium, and zirconium elements are 0.145, 0.147, and 0.160 nm, respectively. It is conceivable that substitutional solid solution of zirconium element increases lattice parameters due to its atomic size [42]. Thus, along with the addition of zirconium, it is thought that the carbon solubility also increases with the expansion of the crystal lattice. Nagaram et al. [15] reported similar results about the effects of the zirconium addition to the crystal lattice parameters.
Figure 11 summarizes the mechanical properties of sintered alloys. It is clearly visible that the hardness increased as zirconium is added to Ti–16Nb alloy. This increment is attributed to the solid solution hardening which causes local crystal lattice distortions and hinders dislocation movements. Also, it is suggested that the reduction in grain size contributes to the increment of strength. In some studies in the literature, it has been reported that zirconium addition increases the hardness and strength of Ti–Nb alloys due to the mentioned reasons [14, 15]. The hardness values in this study are consistent with the literature (300–400 HV) [16, 45]. The addition of zirconium did not have a significant effect on the elastic modulus of the alloy. Although the solid solution hardening and grain size reduction have an effect of enhancing elastic modulus, improving the stability of β phase, and increasing in lattice parameters, it is clearly seen that the elastic modulus remains approximately constant. In the Ti–22Nb–xZr study in which the PIM method was used in literature, the elastic modulus remained constant with the Zr addition [15]. As a result, the increment in strength was achieved without increasing the elastic modulus by addition of zirconium in dense material. Lower elastic modulus values are achieved compared with titanium [46] and Ti6Al4V [47] which is widely used in biomedical application. But the elastic modulus values are still higher than that of the bone. In order to overcome this problem, it is advised that materials with higher porosity could be produced or Ti-based hydroxiapaptite-reinforced composite materials should be considered.
Conclusions
Ti–16Nb–(0, 5, 10, 15)Zr alloys were successfully produced by PIM and from the work completed in this study the following conclusions have been drawn:
-
Low porosity values (0.92–2.72%) were obtained in sintered (1500 °C) alloys, and addition of the zirconium to the base alloy led to the higher porosity.
-
Rheology measurements showed that viscosity of feedstock at injection temperature was appropriate for producing PIM Ti–Nb alloy.
-
β stabilizing effect of Zr for Ti–Nb alloys was proved by using XRD, SEM, and DTA analyses.
-
Transformation temperature of α to β decreased ~ 30 °C by using Zr as β stabilizer.
-
In parallel with the increase in the amount of zirconium, the grain size decreased and the amount of lattice parameters and β phase increased.
-
It was shown that hardness of the base alloy increased to 412 HV from 336 HV.
-
The addition of zirconium to the base alloy has no significant effect on the elastic modulus and elastic modulus stays steady at the band of 103–100 GPa. However, the elastic modulus values are lower than that of pure titanium and commonly used Ti6Al4V alloy.
-
It was concluded that produced alloys are good alternatives to commonly used Ti-based biomaterials.
References
Kim DG, Woo KD, Kang DS, Lee T, Lee MH. Fabrication and biocompatibility evaluation of porous Ti–Nb-based biomaterials with space holder by rapid sintering. Mater Res Innov. 2015;19:S1-301–4. https://doi.org/10.1179/1432891715Z.0000000001491.
Sridhar TM, Vinodhini SP, Kamachi Mudali U, Venkatachalapathy B, Ravichandran K. Load-bearing metallic implants: electrochemical characterisation of corrosion phenomena. Mater Technol. 2016;31:705–18. https://doi.org/10.1080/10667857.2016.1220752.
Nicoara M, Raduta A, Locovei C, Buzdugan D, Stoica M. About thermostability of biocompatible Ti–Zr–Ta–Si amorphous alloys. J Therm Anal Calorim. 2017;127:107–13.
Ou K, Weng C, Lin Y, Huang M. A promising of alloying modified beta-type titanium–niobium implant for biomedical applications: microstructural characteristics, in vitro biocompatibility and antibacterial performance. J Alloys Compd. 2017;697:231–8. https://doi.org/10.1016/j.jallcom.2016.12.120.
Bolzoni L, Ruiz-Navas EM, Gordo E. Evaluation of the mechanical properties of powder metallurgy Ti–6Al–7Nb alloy. J Mech Behav Biomed Mater. 2017;67:110–6. https://doi.org/10.1016/j.jmbbm.2016.12.005.
Xu Y, Xiao Y, Yi D, Liu H, Wu L, Wen J. Corrosion behavior of Ti–Nb–Ta–Zr–Fe alloy for biomedical applications in Ringer’s solution. Trans Nonferrous Met Soc China. 2015;25:2556–63. http://www.sciencedirect.com/science/article/pii/S1003632615638754.
Geetha M, Singh AK, Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants: a review. Prog Mater Sci. 2009;54:397–425. http://www.sciencedirect.com/science/article/pii/S0079642508001126.
Moraes PEL, Contieri RJ, Lopes ESN, Robin A, Caram R. Effects of Sn addition on the microstructure, mechanical properties and corrosion behavior of Ti–Nb–Sn alloys. Mater Charact. 2014;96:273–81. http://www.sciencedirect.com/science/article/pii/S1044580314002551.
Yılmaz E, Gökçe A, Findik F, Özkan Gülsoy H. Characterization of biomedical Ti–16Nb–(0–4)Sn alloys produced by powder injection molding. Vacuum. 2017;142:164–74. http://linkinghub.elsevier.com/retrieve/pii/S0042207X16310612.
Biesiekierski A, Lin J, Li Y, Ping D, Yamabe-Mitarai Y, Wen C. Investigations into Ti–(Nb,Ta)–Fe alloys for biomedical applications. Acta Biomater. 2016;32:336–47. http://www.sciencedirect.com/science/article/pii/S1742706115302488.
Behera M, Raju S, Mythili R, Saroja S. Study of kinetics of α ⇔ β phase transformation in Ti–4.4 mass% Ta–1.9 mass% Nb alloy using differential scanning calorimetry. J Therm Anal Calorim. 2016;124:1217–28.
Ibrahim MK, Hamzah E, Saud SN, Nazim EM, Iqbal N, Bahador A. Effect of Sn additions on the microstructure, mechanical properties, corrosion and bioactivity behaviour of biomedical Ti–Ta shape memory alloys. J Therm Anal Calorim. 2017; http://springerlink.bibliotecabuap.elogim.com/10.1007/s10973-017-6636-2.
Kim JI, Kim HY, Inamura T, Hosoda H, Miyazaki S. Shape memory characteristics of Ti–22Nb–(2–8)Zr(at.%) biomedical alloys. Mater Sci Eng A. 2005;403:334–9. https://doi.org/10.1016/j.msea.2005.05.050.
Zhang J, Sun F, Hao Y, Gozdecki N, Lebrun E, Vermaut P, et al. Influence of equiatomic Zr/Nb substitution on superelastic behavior of Ti–Nb–Zr alloy. Mater Sci Eng A. 2013;563:78–85. https://doi.org/10.1016/j.msea.2012.11.045.
Nagaram AB, Ebel T. Development of Ti–22Nb–xZr using metal injection moulding for biomedical applications. Key Eng Mater. 2016;704:334–42. https://doi.org/10.4028/www.scientific.net/KEM.704.334.
Sungtong W, Khantachawana A. Effect of Zr addition on mechanical properties of Ti–Nb–Zr alloys for biomedical applications. Adv Mater Res. 2012;463–464:841–4. http://www.scientific.net/AMR.463-464.841.
Málek J, Hnilica F, Veselý J, Smola B, Kolařík K, Fojt J, et al. The effect of Zr on the microstructure and properties of Ti–35Nb–XZr alloy. Mater Sci Eng A. 2016;675:1–10. https://doi.org/10.1016/j.msea.2016.07.069.
Abdel-Hady M, Fuwa H, Hinoshita K, Kimura H, Shinzato Y, Morinaga M. Phase stability change with Zr content in B-type Ti–Nb alloys. Scr Mater. 2007;57:1000–3. https://doi.org/10.1016/j.scriptamat.2007.08.003.
German RM, Bose A. Injection molding of metals and ceramics. Metal Powder Industries Federation; 1997. https://books.google.com.tr/books?id=jXINAAAACAAJ.
Abdullahi AA, Nahar N, Azuddin M, Choudhury IA. 1.16 net-shape microfabrication technique by Micrometal Powder Injection Molding A2—Hashmi, MSJ BT—Comprehensive Materials Finishing. Oxford: Elsevier; 2017. p. 466–503. http://www.sciencedirect.com/science/article/pii/B9780128035818091621.
Aslam M, Ahmad F, Yusoff PSMBM, Altaf K, Omar MA, M.German R. Powder injection molding of biocompatible stainless steel biodevices. Powder Technol. 2016;295:84–95. http://www.sciencedirect.com/science/article/pii/S0032591016301322.
German RM. Progress in titanium metal powder injection molding. Materials (Basel). 2013;6:3641–62. https://doi.org/10.3390/ma6083641.
Niinomi M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci Technol Adv Mater. 2003;4:445–54. https://doi.org/10.1016/j.stam.2003.09.002.
German R. Titanium powder injection moulding: a review of the current status of materials, processing, properties and applications. Powder Inject Mould Int. 2009;3:21–37.
Zhao D, Chang K, Ebel T, Nie H, Willumeit R, Pyczak F. Sintering behavior and mechanical properties of a metal injection molded Ti–Nb binary alloy as biomaterial. J Alloys Compd. 2015;640:393–400. https://doi.org/10.1016/j.jallcom.2015.04.039.
Bidaux JE, Closuit C, Rodriguez-Arbaizar M, Carreno-Morelli E. Metal injection moulding of Ti–Nb alloys for implant application. Eur Cells Mater. 2011;22:32.
Bidaux J-E, Closuit C, Rodriguez-Arbaizar M, Zufferey D, Carreño-Morelli E. Metal injection moulding of low modulus Ti–Nb alloys for biomedical applications. Powder Metall. 2013;56:263–6. http://www.maneyonline.com/doi/abs/10.1179/0032589913Z.000000000118.
Zhao D, Chang K, Ebel T, Qian M, Willumeit R, Yan M, et al. Microstructure and mechanical behavior of metal injection molded Ti–Nb binary alloys as biomedical material. J Mech Behav Biomed Mater. 2013;28:171–82. https://doi.org/10.1016/j.jmbbm.2013.08.013.
Huang B, Liang S, Qu X. The rheology of metal injection moulding. J Mater Process Technol. 2003;137(1–3):132–7. http://www.sciencedirect.com/science/article/pii/S0924013602011007.
Nor NHM, Muhamad N, Ismail MH, Jamaludin KR, Ahmad S, Ibrahim MHI. Flow behaviour to determine the defects of green part in metal injection molding. Int J Mech Mater Eng. 2009;4:70–5.
Hausnerova B, Marcanikova L, Filip P, Saha P. Rheological characterization of powder injection moulding using feedstock based on aluminium oxide and multicomponent water-soluble polymer binder. In: Recent advances in fluid mechanics and heat and mass transfer—Proceedings of 9th IASME/WSEAS international conference on fluid mechanics and aerodynamics. FMA’11, proceedings of 9th IASME/WSEAS international conference on HTE’11. 2011. p. 245–50. https://www.scopus.com/inward/record.uri?eid=2-s2.0-83655197611&partnerID=40&md5=469550809b07714c39878dd27974b712.
Li Y, Li L, Khalil KA. Effect of powder loading on metal injection molding stainless steels. J Mater Process Technol. 2007;183:432–9. https://doi.org/10.1016/j.jmatprotec.2006.10.039.
Li Y, Huang B, Qu X. Viscosity and melt rheology of metal injection moulding feedstocks. Powder Metall. 1999;42:86–90. http://www.tandfonline.com/doi/full/10.1179/pom.1999.42.1.86.
Gülsoy HÖ, Özgün Ö, Bilketay S. Powder injection molding of Satellite 6 powder: Sintering, microstructural and mechanical properties. Mater Sci Eng A. 2016;651:914–24. http://www.sciencedirect.com/science/article/pii/S0921509315306481.
Lin D, Chung ST, Kwon YS, Park SJ. Preparation of Ti–6Al–4V feedstock for titanium powder injection molding. J Mech Sci Technol. 2016;30:1859–64. https://doi.org/10.1007/s12206-016-0343-y.
Jamaludin KR, Muhamad N, Yulis SYM. Metal injection moulding (MIM) feedstock preparation with dry and wet mixing: a rheological behavior investigation. In: Advances in mechanical, manufacturing and materials engineering. 2008. p. 76–93.
Loh NHH, German RMM. Statistical analysis of shrinkage variation for powder injection molding. J Mater Process Technol. 1996;59:278–84. https://doi.org/10.1016/0924-0136(95)02158-2.
Kafkas F, Ebel T. Metallurgical and mechanical properties of Ti–24Nb–4Zr–8Sn alloy fabricated by metal injection molding. J Alloys Compd. 2014;617:359–66. http://linkinghub.elsevier.com/retrieve/pii/S0925838814017617.
Bousson V, Bergot C, Meunier A, Barbot F, Parlier-Cuau C, Laval-Jeantet A-M, et al. CT of the middiaphyseal femur: cortical bone mineral density and relation to porosity. Radiology. 2000;217:179–87. http://pubs.rsna.org/doi/10.1148/radiology.217.1.r00se11179.
Perez RA, Nakajima H, Dyment F. Diffusion in α-Ti and Zr. Mater Trans. 2003;44:2–13. https://doi.org/10.2320/matertrans.44.2.
Ivasyshyn OM, Savvakin DH. Synthesis of zirconium- and titanium-based alloys with the use of their hydrides. Mater Sci. 2016;51:465–74. https://doi.org/10.1007/s11003-016-9863-y.
Zhou Y, Li Y, Yang X, Cui Z, Zhu S. Influence of Zr content on phase transformation, microstructure and mechanical properties of Ti75–xNb25Zrx (x = 0–6) alloys. J Alloys Compd. 2009;486:628–32. https://doi.org/10.1016/j.jallcom.2009.07.006.
Sharma B, Vajpai SK, Ameyama K. Microstructure and properties of beta Ti–Nb alloy prepared by powder metallurgy route using titanium hydride powder. J Alloys Compd. 2015;656:978–86. https://doi.org/10.1016/j.jallcom.2015.10.053.
Moffat DL, Kattner UR. The stable and metastable Ti–Nb phase diagrams. Metall Trans A. 1988;19:2389–97. https://doi.org/10.1007/BF02645466.
Santos DR, Pereira MS, Cairo CAA, Grac MLA, Henriques VAR, Graça MLA, et al. Isochronal sintering of the blended elemental Ti–35Nb alloy. Mater Sci Eng A. 2008;472:193–7. https://doi.org/10.1016/j.msea.2007.03.075.
Han MK, Kim JY, Hwang MJ, Song HJ, Park YJ. Effect of Nb on the microstructure, mechanical properties, corrosion behavior, and cytotoxicity of Ti-Nb alloys. Materials (Basel). 2015;8:5986–6003. https://doi.org/10.3390/ma8095287.
Sidambe AT. Biocompatibility of advanced manufactured titanium implants: a review. Materials (Basel). 2014;7:8168–88. https://doi.org/10.3390/ma7128168.
Acknowledgements
The authors are thankful to the Sakarya University BAPK for their financial support of the Project (BAPK-2016-09-08-009). One of the authors (A.G.) would like thank to Miss. Hazal ERDOĞAN, from Western Sydney University, Australia, for her efforts on editing the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yılmaz, E., Gökçe, A., Findik, F. et al. Assessment of Ti–16Nb–xZr alloys produced via PIM for implant applications. J Therm Anal Calorim 134, 7–14 (2018). https://doi.org/10.1007/s10973-017-6808-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6808-0