Abstract
We used Fourier-transform nuclear magnetic resonance (NMR) spectroscopy to investigate the temperature dependences of the chemical shift and resonance frequency observed with magic-angle spinning NMR and static NMR, respectively, to confirm a high-temperature behavior of NH4H2PO4. The hydrogen bonds in both O–H–O between two PO4 groups and N–H–O between NH4 and PO4 were distinguished, and the changes occurring in the chemical shift and resonance frequency near the characteristic temperature TP are related to changes in the atomic positions. The experimental results of thermogravimetric analysis conducted to interpret the high-temperature phenomena without the critical change around TP are consistent with a phase-transition-like phenomenon at TP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
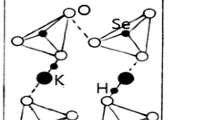
Several dihydrogen phosphate salts, MH2PO4, are of technological interest because they exhibit ferroelectric (M = K, Rb, Cs) or antiferroelectric (M = NH4) properties at low temperature [1,2,3]. MH2PO4 compounds have also been suggested to be suitable for fuel-cell applications because they undergo a superionic phase transition at high temperatures [4,5,6,7,8,9,10,11,12,13]. NH4H2PO4, a member of the KH2PO4 family, shows a high-temperature phase transformation at the characteristic temperature T p = 430 K [14]. In addition, NH4H2PO4 undergoes a paraelectric-to-antiferroelectric phase transition at T C = 148 K [15]. In the paraelectric phase, the crystal is tetragonal with the space group I42d, whereas in the antiferroelectric phase, it is orthorhombic with the space group P212121. The structure of this paraelectric phase is shown in Fig. 1 [16, 17]. Two types of hydrogen bonds linking PO4 ions and NH4 ions were reported; in NH4H2PO4, hydrogen bonds exist in both O–H–O between two PO4 groups and N–H–O between NH4 and PO4 ions [18,19,20,21].
The high-temperature behavior of NH4H2PO4 has been investigated in several studies. However, there are many discrepancies in the high-temperature phase transition temperature T P. Viswanath and Miller [22] found a sharp increase in the conductivity at 430 K and attributed it to a structural phase transition. They also confirmed this transition by infrared spectroscopy, differential scanning calorimetry (DSC), and thermogravimetric analysis (TG). Torijano et al. [23] observed using TG that, when heating NH4H2PO4 powder above 430 K, a rapid mass loss occurs starting at approximately 463 K, which is attributed to the thermal dehydration of the sample. In addition, Park et al. [24] reported that the mechanisms of electrical conductivity in H-bonded crystals must include thermal dehydration as well as ionic transport, because it is known that many H-bonded crystals show surface instabilities such as thermal dehydration at high temperature. The study on the surface transformation of hydrogen-bonded crystals at high temperatures and topochemical nature was discussed by Lee [25]; the hydrogen-bonded crystals near temperature T P were sensitive to changes in the conditions at the surface and seem to be due to an onset of thermal decomposition at the surface. On the other hand, Eichele and Wasylishen [1] conducted a 31P nuclear magnetic resonance (NMR) study of powder and single-crystal NH4H2PO4. Recently, NMR measurements have been used to investigate the molecular motions of the hydrogen bond in N–H–O between NH4 and PO4, hydrogen bond in O–H–O between two PO4 groups, and 31P ions in PO4 groups to confirm a high-temperature behavior of NH4H2PO4 [7]. Although many researchers have studied these crystals both experimentally and theoretically, the mechanism of high-temperature phase transformation is still not completely understood.
In this study, the geometric structure of the hydrogen bond in N–H–O between NH4 and PO4, hydrogen bond in O–H–O between two PO4 groups, 31P ions in PO4 groups, and 14N ions in NH4 groups of NH4H2PO4 was investigated by the chemical shift and resonance frequency by magic-angle spinning (MAS) NMR and static NMR. The mechanisms of phase transition were elucidated by measuring the temperature dependences of the chemical shift and resonance frequency for 1H, 14N, and 31P ions by using Fourier-transform NMR spectroscopy. On the basis of the results, we discussed a phase-transition-like phenomenon at the characteristic temperature T P.
Experimental
NH4H2PO4 single crystals were grown by the slow evaporation of an aqueous solution. The single crystals obtained here had tetragonal shapes and were colorless.
TG was carried out under N2 atmosphere on Du Pont 910 equipment. The samples were heated at a rate of 10 °C min−1.
Solid-state NMR experiments in a rotating frame were performed using a Bruker 400 MHz NMR spectrometer at the Korea Basic Science Institute, Western Seoul Center. The 1H MAS NMR and 31P MAS NMR experiments were performed at Larmor frequencies of ω o/2π = 400.13 and ω o/2π = 161.98 MHz, respectively. Powdered samples were placed in the 4-mm MAS probe. The MAS rate was set to 10 kHz for 1H and 31P MAS to minimize the spinning sideband overlap. The widths of the π/2 pulses for 1H and 31P were 5 and 25 μs, respectively.
In addition, the 14N NMR spectra of the NH4H2PO4 single crystals in the laboratory frame were measured using a Unity INOVA 600 NMR spectrometer at the Korea Basic Science Institute, Western Seoul Center. The static magnetic field was 14.1 T, and the Larmor frequency was set to ω 0/2π = 43.342 MHz. The 14N NMR experiments were performed using a solid-state echo sequence: 4.5 μs–τ (12 μs)–4.5 μs–τ (12 μs). The NMR measurements were obtained in the temperature range of 180–440 K. Unfortunately, the chemical shift and resonance frequency could not be measured above 440 K because the NMR spectroscopy did not have adequate temperature control at high temperature. All the sample temperatures were maintained at constant values by controlling the helium gas flow and heater current, which yielded an accuracy of ±0.5 K.
Results and discussion
TG was used to determine whether these high-temperature transformations are structural phase transitions or chemical reactions. Figure 2 shows the resulting curve of NH4H2PO4 at heating rate of 10 °C min−1. The first occurrence of mass loss begins at approximately 430 K, accompanied by the escape of H2O, as in the following chemical reaction reported by Lee [14]:
This phenomenon is also consistent with the liquid-like behavior such as H2O flow at the surface above T P reported by Lee [25]. The onset of mass loss around 430 K was taken to indicate the beginning of thermal decomposition. This temperature indicating mass loss was commonly believed to indicate high-temperature phenomena caused by thermal decomposition. This change might to be related to the loss of H2O suggested by Lee [14].
Solid-state NMR was used to analyze the structure of the protons in NH4H2PO4 in a rotating frame. Figure 3 shows the in situ 1H MAS NMR spectrum of NH4H2PO4 as a function of temperature. The 1H MAS NMR spectrum consists of two peaks at chemical shifts of δ = 6.72 and 15.02 ppm at room temperature. The spinning sidebands are marked with asterisks. The signals at chemical shifts of 6.72 and 15.02 ppm are assigned to the ammonium and hydrogen-bonded protons, respectively. Two types of hydrogen bonds exist in NH4H2PO4, as shown in Fig. 1: the ammonium protons (hydrogen-bonded protons in N–H–O between NH4 and PO4) and the hydrogen-bonded protons (hydrogen-bonded protons in O–H–O between two PO4 groups). The strong and weak signals in the spectrum assigned to the ammonium and hydrogen-bonded protons are consistent with the fact that there are four protons in the NH4 groups and two hydrogen-bonded protons, respectively. The intensity for the hydrogen-bonded protons above 420 K is very weak, and a magnified view of this spectrum is shown in the inset of Fig. 3. The chemical shifts for the ammonium and hydrogen-bonded protons do not change; however, the relative intensities of two signals decrease with increasing temperature (Fig. 4). The relative intensity for two signals decreases somewhat continuously and does not change significantly near T P.
Structural analysis of the 31P in NH4H2PO4 was also performed using 31P MAS NMR. The in situ 31P MAS NMR spectrum for NH4H2PO4 is shown in Fig. 5 as a function of temperature. At room temperature, the 31P MAS NMR spectrum shows one signal in NH4H2PO4 at a chemical shift of δ = 1.09 ppm with respect to the reference TMS signal. The temperature dependence of the chemical shifts of the 31P NMR signal of NH4H2PO4 is shown in the inset in Fig. 5. The 31P chemical shift slowly and monotonically increases with increasing temperature. Therefore, the structural geometry of 31P ions in PO4 groups of NH4H2PO4 changed continuously. Overall, the chemical shift is sensitive to the electrical environment of the nucleus. The temperature dependence of the chemical shift is indicative of an electronic instability attributable to a deformation of the PO 3−4 tetrahedra.
The NMR spectra of 14N (I = 1) in NH4H2PO4 single crystals were obtained using static NMR at a Larmor frequency of ω 0/2π = 43.342 MHz. The in situ 14N NMR spectra of NH4H2PO4 single crystals as a function of temperature are plotted in Fig. 6a. Two resonance lines are obtained owing to the quadrupole interaction of the 14N nucleus. Furthermore, the resonance frequencies of the 14N signals are plotted in Fig. 6b. The resonance frequency increases until 260 K and decreases above 260 K with increasing temperature. Near T P, they are somewhat continuous, and the interval between the resonance frequencies is very narrow. Note that these temperature-dependent changes in the 14N resonance frequencies are generally attributed to changes in the structural geometry near T P = 430 K. Here, the electric field gradient (EFG) tensors at the N sites are varied, reflecting the changing atomic configurations around the 14N nuclei.
Conclusions
The structural behavior of NH4H2PO4 near the characteristic temperature T P was studied by examining the chemical shift and resonance frequency using MAS NMR and static NMR, respectively. At high temperature, the changes occurring in the chemical shift and resonance frequency were associated with changes in the atomic positions for two types of hydrogen bonds, phosphorus ions, and nitrogen ions. From these results, the symmetries of the environments of the N–H–O bonds and O–H–O bonds are varied and were related to the structural change of the tetrahedral PO4 groups and NH4 groups in the crystal structure.
The chemical shift and resonance frequency do not exhibit anomalous behavior near the characteristic temperature TP. In order to understand the high-temperature phenomena around T P in NH4H2PO4 crystal, the TG curve was obtained as a function of temperature. The high-temperature phase transformation around T P was replaced by the onset of partial polymerization at reaction sites on the surface; the mass of NH4H2PO4 decreases at 430 K (T d), which is interpreted as the onset of partial thermal decomposition. The mass loss in the sample observed in the TG curve suggests that TP is not related to physical changes such as structural phase transition, but is rather related to a chemical change through thermal dehydration, as suggested by Torijano et al. [23] and Park et al. [24, 26]. These discrepancies among different research groups are apparently caused by a difference in experimental humidity conditions and crystal growth conditions. The observed change of symmetry of the crystal suggests a phase-transition-like phenomenon called high-temperature phase transformation at the characteristic temperature T P = 430 K.
References
Eichele K, Wasylishen RE. 31P NMR study of powder and single-crystal samples of ammonium dihydrogen phosphate: effect of homonuclear dipolar coupling. J Phys Chem. 1994;98:3108–13.
Osterheld RK, Markowitz MM. Polymerization and depolymerization phenomena in phosphate-metaphosphate systems at higher temperatures. IV. Condensation reactions of alkali metal hydrogen phosphates. J Phys Chem. 1956;60:863–7.
Thilo E. Condensed phosphates and arsenates in Advances in inorganic chemistry and radiochemistry, vol 4, edited by Emeleus HJ, Sharpe AG (Academic Press, New York, 1962).
Haile SH, Boysen DA, Chisholm CRI, Merle RB. Solid acids as fuel cell electrolytes. Nature. 2001;410:910–3.
Uda T, Boysen DA, Haile SM. Thermodynamic, thermomechanical, and electrochemical evaluation of CsHSO4. Solid State Ion. 2005;176:127–33.
Castillo J, Materon EM, Castillo R, Vargas RA, Bueno PR, Varela JA. Electrical relaxation in proton conductor composites based on (NH4)H2PO4/TiO2. Ionics. 2009;15:329–36.
Lim AR, Lee K-S. High temperature behavior of NH4H2PO4 studied by single-crystal and MAS NMR. Solid State Sci. 2013;21:54–8.
Sun C, Xue D. Crystallization behaviors of KDP and ADP. Optical Mater. 2014;36:1966–9.
Zhou H, Wang F, Xu M, Liu B, Liu F, Zhang L, Xu X, Sun X, Wang Z. Raman spectral characterization of NH4H2PO4 single crystals: effect of pH on microstructure. J Cryst Growth. 2016;450:6–13.
Sangwal K, Mielniczek-Brzo E. Antisolvent crystallization of aqueous ammonium dihydrogen phosphate solutions by addition of acetone at different rates. Cryst Res Technol. 2016;51:475–90.
Ganesh V, Shkir M, Alfaify S, Yahia IS. Effect of Co2+ doping on solubility, crystal growth and properties of ADP crystals. J Cryst Growth. 2016;449:47–56.
Gorodylova N, Kosinova V, Dohnalova Z, Sulcova P, Belina P. Thermal stability and colour properties of CuZr4(PO4)6. J Therm Anal Calorim. 2016;126:121–8.
Lim AR, Kim SH. Structural and thermodynamic properties of Tutton salt K2Zn(SO4)2·6H2O. J Therm Anal Calorim. 2016;123:371–6.
Lee K-S. Hidden nature of the high-temperature phase transitions in crystals of KH2PO4-type: is it a physical change? J Phys Chem Solids. 1996;57:333–42.
Lines ME, Glass AM. Principles and Applications of Ferroelectrics and Related Materials. Oxford: Clarendon Press; 1977.
Lasave J, Koval SF, Migoni RL. Coexistence of ferroelectric and antiferroelectric microregions in the paraelectric phase of NH4H2PO4 (ADP). Phys B. 2009;404:2749–50.
Ishibashi Y, Ohya S, Takagi Y. A theory of the phase transition in ADP. J Phys Soc Japan. 1972;33:1545–50.
Tenzer L, Frazer BC, Pepinsky R. A neutron structure analysis of tetragonal NH4H2PO4. Acta Cryst. 1958;11:505–9.
Hewat AW. Location of hydrogen atoms in ADP by neutron powder profile refinement. Nature. 1973;246:90–1.
Keeling RO Jr, Pepinsky R. An X-ray diffraction study of the transition in NH4H2PO4 at 148 K. Z Kristallogr. 1955;106:236–65.
Matsushita E, Matsubara T. The role of hydrogen bonds in antiferroelectricity of NH4H2PO4. J Phys Soc Jpn. 1987;56:200–7.
Viswanath RS, Miller PJ. High temperature phase transition in NH4H2PO4. Solid State Commun. 1979;32:703–6.
Torijano E, Vargas RA, Diosa JE, Mellander BE. High temperature phase transitions of NH4H2PO4. Phys Stat Solidi (b). 2000;220:659–62.
Park JH, Lee K-S, Kim JB. Impedance relaxation of KH2PO4 at high temperatures. J Phys: Condens Matter. 1996;8:5491–9.
Lee K-S. Surface transformation of hydrogen-bonded crystals at high-temperatures and topochemical nature. Ferroelectrics. 2002;268:369–74.
Park JH, Lee K-S, Choi BC. High-temperature transformation in KH2PO4 and RbH2PO4 crystals. J Phys: Condens Matter. 2001;13:9411–9.
Acknowledgements
This research was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education Science and Technology (2016R1A6A1A03012069) and (2015R1A1A3A04001077).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, A.R., Chae, S.A. Phase-transition-like phenomenon of NH4H2PO4 observed using MAS NMR and static NMR near characteristic temperature. J Therm Anal Calorim 130, 885–889 (2017). https://doi.org/10.1007/s10973-017-6457-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6457-3