Abstract
This study reports the investigation of liposomal formulations of lidocaine in the form of a free base (LID). LID was encapsulated into large multilamellar vesicles composed of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC). Samples of a mass ratio of LID with respect to DMPC ranging from 1 to 10% were investigated. The effects of the increasing LID concentration on the bilayer membrane were determined in terms of size, polydispersity index, zeta potential, encapsulation efficiency (EE %) and partition coefficient. Furthermore, differential scanning calorimetry (DSC) studies were also carried out to analyze the effect of LID on the liposome phase transition temperature and to calculate the EE % with an unfrequented method. The EE % results obtained by different experimental procedures were quite ambiguous, but the DSC measurements confirmed the ultracentrifugation direct method. The calculated partition coefficients of these two methods were in good agreement, too. Our research revealed a less known application field of DSC, as a fast and reliable tool to determine EE%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays there is a great interest in new delivery systems for local anesthetics, especially for topical application because of its easy-to-use, painless and systemic side effect-free nature [1–4]. Liposomes are spherical vesicles (usually 0.05–5 µm in diameter) that are formed with energy input when certain phospholipids are hydrated in aqueous media [5]. The vesicles consist of one or more concentrically ordered phospholipid bilayers: the fatty acid chains are in the core of the bilayer, while the hydrophilic heads are oriented to the aqueous phase [6]. Liposomes improve drug bioavailability, reduce systemic toxicity and increase the half-lives of drugs in vivo [7–11]. Furthermore, these carriers enable a more intense localization of the active agent in the layers of the skin [12].
DMPC is a liposome-forming saturated neutral phospholipid with a smaller head group widely used as a model system of biomembranes since lecithins are a major component of most mammalian cell membranes [13, 14].
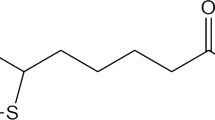
Local anesthetics (LA) comprise two major components: a lipophilic aromatic group and a polar region, connected by an intermediate carboxyl group in an amide bond [15]. Therefore, they can interact with the liposome membranes, usually by sitting in the lipid region, but a fraction of molecules is also retained in the aqueous phase [16]. Lidocaine (LID) is a commonly used local anesthetic with fast onset and intermediate duration of action (90–240 min) [17].
While in most studies and in the marketed formulations this local anesthetic is used in the hydrochloride form, we have chosen the base form because of its improved lipophilicity, thus better penetration properties through the lipophilic stratum corneum and the ability to form a depot in the hydrophilic dermis [18]. Furthermore, hydrophobicity is also crucial for drug partitioning into the nerve fibers, thus an appropriate amount of LA molecules remain within that membrane [17]. In addition, it was shown before that uncharged LID preferentially interacts with neutral membranes [19], such as DMPC.
In our studies, a series of samples was prepared showing a constant lipid concentration and increasing concentrations of the LID to examine the effects of lidocaine on the membrane properties.
The liposome preparations are always a mixture of entrapped and unentrapped drug fractions. To determine the encapsulation efficiency (EE %), the first step for the most common methods is the separation between the encapsulated drug (within the liposomes) and the free drug. This separation can be performed with mini-column centrifugation, dialysis membrane and ultracentrifugation [20]. After the separation, most of the published studies focus on measuring the unentrapped drug concentration in the supernatant and subtract this concentration from the total drug concentration (indirect method) [21–25]. The other technique is that when the supernatant is removed after the separation, the lipid bilayer (containing drug-loaded liposomes) is disrupted with organic solvent, and the released material is quantified (direct method). The above-mentioned procedures are obviously very laborious and time-consuming; furthermore, the attained results depend on the separation, which may not be complete [26].
On the other hand, differential scanning calorimetry (DSC) can serve as a powerful tool for the quality control of liposomes without needing to separate them. This method can give information about the drug–lipid interactions, size, partition coefficient and encapsulation efficiency with one measurement [27]. In our work, we focused on the comparison of the generally used ultracentrifugation method and DSC for the evaluation of encapsulation efficiency.
The principle of using DSC for this approach is based on the reduction in temperature of the main phase transition depending on the partitioning between LID and the lipid in the fluid or in the gel phase [28].
If the drug mixes ideally with the fluid phase of the membrane but is completely excluded from the gel, the difference between the actual phase transition temperature (T) and the temperature of the main phase transition of pure lipid (T 0) can be written as (∆T m = T 0–T)
where R is the ideal gas constant (1.9858775 cal K−1 mol−1), ΔH is the enthalpy of the main phase transition and \(X_{\text{d}}^{\text{b}}\) is the molar fraction of the drug bounded in the liposome [29]. So, the shift in the melting temperature is independent of the special properties of the drug (as long as the ∆H of melting is not strongly affected) and proportional to its mole fraction in the membrane [30].
Knowing the mass of the liposome measured, units can be converted from mole fraction to mass [31]:
where \(c_{\text{d}}^{\text{b}}\) is the concentration of the bounded drug and \(c_{\text{lipid}}\) is the lipid concentration. From these values, the encapsulation efficiency can be calculated:
To characterize a formulation, it is also useful to determine its membrane–water partitioning properties because lipophilicity plays an important role in biological activity. The direct calculation of solute partitioning into bilayers can also be monitored by DSC [31]. According to previous empirical evidence, the free drug concentration is proportional to the mole ratio of drug to lipid in the membrane (R), so the mole ratio partition coefficient (K R) [30, 32, 33] can be calculated:
where \(c_{\text{d}}^{\text{total}}\) is the total drug concentration, \(c_{\text{d}}^{\text{free}}\) is the unentrapped drug concentration and \(X_{\text{d}}^{\text{total}}\) is the maximum bounded drug fraction.
Experimental
Materials
Phospholipid (1,2-dimyristoyl-sn-glycero-3-phosphocholine, DMPC) was supplied by Avanti Polar Lipids. Chloroform, ethanol, N-(2-hydroxyethyl) piperazine-N’-(2-ethanesulfonic acid) (HEPES), sodium chloride and lidocaine were obtained from Sigma-Aldrich.
Preparation of liposomes
Liposomes were prepared with the dry film hydration method [6, 34]. Stock solutions were prepared with chloroform of DMPC and LID. Aliquots were added to individual vials to reach 5 mg mL−1 lipid and 1-2-3-4-10 w/w% LID. These solutions were dried under rotation (RVC 2-18 Speed Dry Rotational Vacuum Concentrator, Martin Christ Gefriertrocknungsanlagen GmbH, Germany, 30 °C, 1.5 h, 1500 rpm). The lipid film was then placed in a vacuum desiccator overnight, to ensure the complete removal of the solvent [35]. The hydration of the film was done with 1 mL HEPES buffer (20 mM, containing 154 mM NaCl, pH = 7.4) at room temperature, alternating with vortex agitation for 5 min. Liposomal formulations were stored at 4 °C and used within 1 week.
Particle size and zeta potential measurements
Measurements were taken with a Malvern Nano ZS based on dynamic light scattering. The system works according to the phase analysis light scattering (PALS) principle, and the data are automatically evaluated on the basis of the Smoluchowski equation (the particle size is much larger than the Debye length, ≈1 nm). The sample was thermostated to 25 °C with a built-in Peltier device. Measurements were taken in standard disposable cuvettes using Malvern’s dip cell. Each measurement was taken in triplicate. To ensure the validity of the data, a zeta standard was measured every 30 min. For these measurements, the samples were diluted with their aqueous phase in order to avoid multiscattering phenomena. The polydispersity index was also evaluated as a measurement of the homogeneity of the dispersion.
Ultracentrifugation
The encapsulation efficiency of the drug was determined for several preparations using the ultracentrifugation method combined with spectrophotometry. About 1 g of each preparation was placed into Beckman polycarbonate centrifuge tubes and diluted to 1.5 mL with HEPES buffer. The samples were centrifuged in a Beckman Coulter Optima XE-90 Ultracentrifuge for 3 h at 35,000 rpm at 4 °C. The supernatant was removed, leaving the pellet containing the liposomes at the bottom of the tubes. Then a washing step was performed: 1.5 mL of the HEPES buffer was added to the pellet and the centrifugation process was repeated. After that, the supernatant was removed again. Both the supernatant (“indirect method”) and the pellet (dissolved in 1 mL ethanol—“direct method”) were measured with UV spectrometer at 262 nm to determine the concentration of LID [36].
It is a crucial to carefully adjust the experimental parameters for the complete pelleting, but some studies reported micellization for LA-loaded liposomes [37, 38], which can cause difficulties in determining encapsulation efficiency in many preparations.
To prove that all the lipids are pelleted down, we performed Bartlett assay [39] before the centrifugation process and after redissolving the pellets; thus, we can be sure that the pellets contain all the lipids and there are no micelles or liposomes in the supernatant.
DSC measurements
DSC measurements were performed using a MicroCal VP-DSC device (MicroCal Inc., Northampton, USA). Before the calorimetric experiments, the solutions were degassed and then filled into the sample cell (0.4988 mL). A heating rate of 1 °C min−1 in the 5–80 °C range was applied. Phase transition temperatures and enthalpy (∆H) scales were calculated. As a reference, a 20 mM HEPES buffer solution was used. Three up and down scans were performed for each sample to prove the reproducibility. All curves shown in the figures originate from the first heating scan. The Origin 7.0 software was used to subtract the baselines from the curves and to convert the raw data into data of molar heat capacity. The thermodynamic parameters were obtained by using the non-two-state model provided by the software.
Statistical analysis
One-way ANOVA followed by the Bonferroni test was used to determine the statistical differences between the results by using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Differences were regarded as significant if p < 0.05*, p < 0.01** and p < 0.001***.
Results and discussion
Particle size and zeta potential measurements
The light scattering analysis showed that the sizes of liposomes were in the range of 1844 ± 562.9 nm and 4842 ± 275.57 nm. The results presented a decrease in vesicle size and a reduction in homogeneity with increasing the amount of the added LID, as indicated by the growth of the polydispersity index (Table 1). The polydispersity index (PDI) of the investigated vesicles showed values from 0.279 ± 0.253 to 1.000 ± 0.000, representing heterogenous populations (PDI > 0.3) of vesicles [40].
These findings could be explained by considering that the drug will be located within the liposomal bilayer and could alter the microstructure of the vesicular membrane, and reduce the liposomal membrane organization.
On the other hand, measurements of zeta potential values showed that it was barely influenced by the presence of the drug, so a contribution of the drug to the liposomal charge can be excluded (Table 1). Therefore, the nearly zero charge of the vesicles could be attributed to the properties of the DMPC molecules, which is a zwitterionic lipid that, at physiological pH, forms membranes with practically zero surface charge density [41]. The slight difference in the values could be explained by the measurement difficulties around the value zero.
DSC
The effect of LID on the thermotropic behavior of DMPC was investigated by DSC as a function of the anesthetic concentration.
Literature data report that pure DMPC liposomes show a strong and sharp main endothermic transition near 24 °C, with ∆H ≈ 6000 cal mol−1, because of the conversion of the gel phase to the lamellar liquid crystal phase [42]. These bilayer membranes also exhibit a weak pre-transition peak at about 13.5 °C, arising from the conversion of a lamellar gel phase to a rippled gel phase [43]. Figure 1 shows the heating curves of the DMPC multilamellar vesicles in the absence and presence of different LID amounts up to 10 w/w%.
Table 2 gives the values of T m (main transition temperature) and ∆H (enthalpy of transition) measured in all the formulations in the heating cycles.
The values measured for the T m, ∆H in pure DMPC liposomes (23.85 °C, 5293 cal mol−1, respectively) were in good agreement with the literature data [44].
The most outstanding feature of the series of curves is that the main phase transition for DMPC–represented by the peak in Cp—is progressively lowered and broadened with increasing concentrations of LID. The position of the main peak is reduced from T m = 23.85 °C for DMPC down to about 22.66 °C with 10 w/w% LID. This suggests a chain disordering effect in the lipid membrane (Fig. 2).
The analysis of ∆H, given by the peak area, can provide information about the impact (location) of the LID molecules in the phospholipid bilayers. In the investigated formulations, LID does not affect ∆H but causes a decrease in T m. This can be explained by a superficial interaction between the LID and the DMPC molecules and/or the intercalation of LID molecules between the chains of the lipids without reducing the stability of the membrane [45].
The pre-transition peak is broad and nearly symmetrical and, because of the formation of an intermediate metastable phase, slowly turns into the lamellar gel phase [43]. This peak became smaller by the addition of LID and disappeared at the concentration of 10 w/w%. The vanishing nature of the pre-transition is common upon the addition of membrane solutes [46, 47].
Encapsulation efficiency
The encapsulation efficiency values of the liposomal formulations obtained by different experimental methods are represented in Fig. 3 and Table 3.
The results are quite ambiguous. With the most widespread indirect method, we measured notably higher EE % values (9.4–32.2%) and the measurements also resulted in a high standard deviation, while the results of the direct method (3.5–10.1%) and the DSC measurements (5.4–13.0%) correlate nicely. These differences could be explained by some possible experimental errors in connection with the indirect method. First of all, indirect method is a derived result, and it is evident that each experimental step and each further calculation will introduce an experimental error and, moreover, may cause a loss of product (for example adsorption on the vial wall or pipette tips). The other probable explanation is the imperfect separation and the presence of liposomes in the supernatant as well.
According to Nernst’s partitioning law (1891) for dilute solutions, the ratio of concentrations in two separated phases is the constant partition coefficient (K R). The calculated average K R values are also similar for the direct and DSC methods (0.00903 ± 0.00455 mM−1 and 0.00895 ± 0.00468 mM−1) and really different for the indirect method (0.0375 ± 0.0306 mM−1).
Considering these outcomes, we can conclude that the direct method and DSC confirmed each other’s results, while the indirect method suffered from an unknown error source.
Conclusions
In conclusion, we successfully developed and characterized a liposomal lidocaine formulation. The effect of LID on the liposome structure is notable even in the presence of very small amounts of foreign substances added. The encapsulation efficiency of the local anesthetic on the lipid bilayer of DMPC multilamellar vesicles was investigated using DSC. The results were compared with those obtained with ultracentrifugation.
We conclude that the DSC method is more convenient compared to the techniques used generally for the determination of encapsulation efficiency in cases when phase transition measurements are taken with the aim of obtaining further information. These findings should be extended for higher lipid concentrations and other formulations.
Although this work is still preliminary, it provides an integrated approach to the study of encapsulation efficiency with a novel method. Moreover, it provides guiding lines for future investigations.
References
Mezei M, Gulasekharam V. Liposomes-a selective drug delivery system for the topical route of administration: gel dosage form. J Pharm Pharmacol. 1982;34(7):473–4.
Margalit R. Liposome-mediated drug targeting in topical and regional therapies. Crit Rev Ther Drug Carr Syst. 1995;12(2–3):233–61.
Fresta M, Puglisi G. Application of liposomes as potential cutaneous drug delivery systems. In vitro and in vivo investigation with radioactively labelled vesicles. J Drug Target. 1996;4(2):95–101.
Planas ME, Gonzalez P, Rodriguez L, Sanchez S, Cevc G. Noninvasive percutaneous induction of topical analgesia by a new type of drug carrier, and prolongation of local pain insensitivity by anesthetic liposomes. Anesth Analg. 1992;75(4):615–21.
Kamath MP, Shenoy BD, Tiwari SB, Karki R, Udupa N, Kotian M. Prolonged release biodegradable vesicular carriers for rifampicin—formulation and kinetics of release. Indian J Exp Biol. 2000;38(2):113–8.
de Araujo DR, Cereda CM, Brunetto GB, Vomero VU, Pierucci A, Neto HS, et al. Pharmacological and local toxicity studies of a liposomal formulation for the novel local anaesthetic ropivacaine. J Pharm Pharmacol. 2008;60(11):1449–57.
de Araujo DR, Cereda CM, Brunetto GB, Pinto LM, Santana MH, de Paula E. Encapsulation of mepivacaine prolongs the analgesia provided by sciatic nerve blockade in mice. Can J Anaesth. 2004;51(6):566–72.
Cereda CM, de Araujo DR, Brunetto GB, De Paula E. Liposomal prilocaine: preparation, characterization, and in vivo evaluation. J Pharm Pharm Sci. 2004;7(2):235–40.
Grant GJ, Bansinath M. Liposomal delivery systems for local anesthetics. Reg Anesth Pain Med. 2001;26(1):61–3.
Grant SA. The Holy Grail: long-acting local anaesthetics and liposomes. Best Pract Res Clin Anaesthesiol. 2002;16(2):345–52.
Grant GJ, Barenholz Y, Bolotin EM, Bansinath M, Turndorf H, Piskoun B, et al. A novel liposomal bupivacaine formulation to produce ultralong-acting analgesia. Anesthesiology. 2004;101(1):133–7.
Mura P, Maestrelli F, Gonzalez-Rodriguez ML, Michelacci I, Ghelardini C, Rabasco AM. Development, characterization and in vivo evaluation of benzocaine-loaded liposomes. Eur J Pharm Biopharm. 2007;67(1):86–95.
Bonora S, Trinchero A, Torreggiani A, Tamba M. A DSC and Raman study of the interaction between tricresyl phosphates (TCP) and phospholipid liposomes. Croat Chem Acta. 2007;80(1):81–9.
Anderson M, Omri A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004;11(1):33–9.
de Jong RH. Local anesthetics. 1st ed. St. Louis: Mosby-Year Book; 1994.
De Paula E, Schreier S. Use of a novel method for determination of partition-coefficients to compare the effect of local-anesthetics on membrane-structure. Biochem Biophys Acta. 1995;1240(1):25–33.
Collins VJ. Principles of anesthesiology: general and regional anesthesia. 3rd ed. Philadelphia: Lea & Febiger; 1993.
Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9.
Davio SR, Low PS. The effect of anesthetic charge on anesthetic-phospholipid interactions. Biochem Biophys Acta. 1981;644(2):157–64.
Laouini A. Preparation, characterization and applications of liposomes: state of the art. J Colloid Sci Biotechnol. 2012;1:147–68.
Cereda CM, Brunetto GB, de Araujo DR, de Paula E. Liposomal formulations of prilocaine, lidocaine and mepivacaine prolong analgesic duration. Can J Anaesth. 2006;53(11):1092–7.
Franz-Montan M, Baroni D, Brunetto G, Sobral VR, da Silva CM, Venancio P, et al. Liposomal lidocaine gel for topical use at the oral mucosa: characterization, in vitro assays and in vivo anesthetic efficacy in humans. J Liposome Res. 2015;25(1):11–9.
Glavas-Dodov M, Goracinova K, Mladenovska K, Fredro-Kumbaradzi E. Release profile of lidocaine HCl from topical liposomal gel formulation. Int J Pharm. 2002;242(1–2):381–4.
Mashimo T, Uchida I, Pak M, Shibata A, Nishimura S, Inagaki Y, et al. Prolongation of canine epidural anesthesia by liposome encapsulation of lidocaine. Anesth Analg. 1992;74(6):827–34.
Sun NH, Zhu YY, Yuan L, Lang B. Nano-liposomes of entrapment lidocaine hydrochloride on in vitro permeability of narcotic. Pak J Pharm Sci. 2015;28(1):325–8.
Bano M. Determination of partition coefficient by the change of main phase transition. Gen Physiol Biophys. 2000;19(3):279–93.
Biltonen RL, Lichtenberg D. The use of differential scanning calorimetry as a tool to characterize liposome preparations. Chem Phys Lipids. 1993;64(1–3):129–42.
Kaminoh Y, Tashiro C, Kamaya H, Ueda I. Depression of phase-transition temperature by anesthetics: nonzero solid membrane binding. Biochem Biophys Acta. 1988;946(2):215–20.
Inoue T, Miyakawa K, Shimozawa R. Interaction of surfactants with vesicle membrane of dipalmitoylphosphatidylcholine. Effect on gel-to-liquid-crystalline phase transition of lipid bilayer. Chem Phys Lipids. 1986;42(4):261–70.
Heerklotz H. Interactions of surfactants with lipid membranes. Q Rev Biophys. 2008;41(3–4):205–64.
Redmanfurey NL, Antinore MJ. Determination of partition-coefficients between dimyristoylphosphatidylcholine and water using differential scanning calorimetry. Anal Chim Acta. 1991;251(1–2):79–81.
Schurtenberger P, Lindman B. Coexistence of simple and mixed bile salt–lecithin micelles: an NMR self-diffusion study. Biochemistry. 1985;24(25):7161–5.
Almog S, Kushnir T, Nir S, Lichtenberg D. Kinetic and structural aspects of reconstitution of phosphatidylcholine vesicles by dilution of phosphatidylcholine–sodium cholate mixed micelles. Biochemistry. 1986;25(9):2597–605.
Cereda CMS, Tofoli GR, De Brito RB, De Jesus MB, Fraceto LF, Groppo FC, et al. Stability and local toxicity evaluation of a liposomal prilocaine formulation. J Liposome Res. 2008;18(4):329–39.
Puranik SB, Pai R, Pai PNS, Rao GK. Gas chromatographic determination of residual levels of methanol and chloroform from liposomal, microspheres and nanoparticles. Int J Chem Sci. 2008;6(2):693–704.
Pathak P, Nagarsenker M. Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech. 2009;10(3):985–92.
Foldvari M, Gesztes A, Mezei M, Cardinal L, Kowalczyk I, Behl M. Topical liposomal local anesthetics: design, optimization and evaluation of formulations. Drug Dev Ind Pharm. 1993;19(19):2499–517.
Fernandez MS. Formation of micelles and membrane action of the local anesthetic tetracaine hydrochloride. Biochem Biophys Acta. 1980;597(1):83–91.
Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104(1):10–4.
Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258(1–2):141–51.
Ionov M, Garaiova Z, Waczulikova I, Wrobel D, Pedziwiatr-Werbicka E, Gomez-Ramirez R, et al. siRNA carriers based on carbosilane dendrimers affect zeta potential and size of phospholipid vesicles. Biochem Biophys Acta. 2012;1818(9):2209–16.
Foggia MD. DSC and Raman study of DMPC liposomes in presence of Ibuprofen at different pH. J Therm Anal Calorim. 2016;127(2):1407–17.
Tenchov B. On the Reversibility of the phase-transitions in lipid–water systems. Chem Phys Lipids. 1991;57(2–3):165–77.
Marsh D. Handbook of lipids bilayers. Boca Rata: CRC Press; 1990.
Panico AM, Santagati A, Cardile V, Urso D, Gentile B, Ronsisvalle G. Calorimetric study on the interaction of thienopyrimidine derivatives with phosphatidylcholine membranes. Colloid Surf B. 2003;28(1):77–81.
Wang PY, Chen JW, Hwang F. Anisodamine causes acyl-chain interdigitation in phosphatidylglycerol. FEBS Lett. 1993;332(1–2):193–6.
Pedersen TB, Frokjaer S, Mouritsen OG, Jorgensen K. A calorimetric study of phosphocholine membranes mixed with desmopressin and its diacylated prodrug derivative (DPP). Int J Pharm. 2002;233(1–2):199–206.
Acknowledgements
The authors are thankful to the Erasmus+ Programme enabling the mobility to perform the experiments. The assistance of the workers of the Department of Pharmaceutical Technology and Biopharmacy (University of Freiburg) is highly appreciated.  Supported BY the ÚNKP-16-3 New National Excellence Program of the Ministry of Human Capacities.
Supported BY the ÚNKP-16-3 New National Excellence Program of the Ministry of Human Capacities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakonyi, M., Berkó, S., Budai-Szűcs, M. et al. DSC for evaluating the encapsulation efficiency of lidocaine-loaded liposomes compared to the ultracentrifugation method. J Therm Anal Calorim 130, 1619–1625 (2017). https://doi.org/10.1007/s10973-017-6394-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6394-1