Abstract
The pyrolysis kinetic analysis of the three pseudocomponents of biomass, namely cellulose, hemicellulose and lignin, were investigated using a thermogravimetric (TG) analyzer. The multi-peaks method was used to fit the Gaussian distribution model of DTG curves. The activation energies of three pseudocomponents pyrolysis were evaluated using sinusoidally modulated temperature method. The results showed that the multi-peaks methods can fit the DTG curves of cellulose, hemicellulose and lignin successfully. There was only one reaction stage for the pyrolysis of cellulose and hemicelluloses. There were two reaction stages for the pyrolysis of lignin. The average E was 112.6, 162.8 and 156.8 kJ mol−1 for cellulose, hemicellulose and lignin, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing anthropogenic CO2 emission and global warming have challenged the human to find new and better ways to meet the world’s increasing needs for energy while reducing greenhouse gases [1]. The world currently faces a systematic problem of increased CO2 emissions, decreased soil-carbon content and global warming. Generation of transportation fuels from biomass has gained significant interest in the recent years as it is a clean, sustainable and renewable energy source [2]. Pyrolysis of biomass is one of the thermochemical processes carried out in the absence of oxygen and bio-oil, and syngas and biochar are produced in the process [3]. Bio-oil is easy to transport, making biomass a dominant choice for the replacement of fossil fuels [4]. The content of the syngas is primarily CO, H2, CH4 and CO2. The literature [5] has reported that the gases can be used as the heat source in a recycling fluidized bed reactor. Biochar can be used as a soil amendment and has attracted attention due to its ability for long-term improvements in soil physical and chemical properties [6].
Kinetic investigations are one of the most important applications of thermal analysis for lignocellulosic biomass. There are a variety of models available for analyzing the kinetics of biomass thermal decomposition studies, including first-order [7], discrete activation energy distributions [8], isoconversional method [9–11] and sequential models [12]. It is well known that cellulose (40–50 mass%), hemicellulose (25–35 mass%) and lignin (16–33 mass%) are the building blocks of biomass material [2]. Several researchers [13–15] have investigated this kind of three parallel reaction model of biomass pyrolysis. The model assumes that lignocellulosic biomass contains three independently reacting pseudocomponents (hemicellulose, cellulose and lignin). Instead of the study of kinetics of specified biomass, a lot of literatures [13, 16] are intended to analyze the thermal decompositions of main constituents of biomass in the inert environment using a thermogravimetry (TG). Mamleev et al. [17] developed the modulated thermogravimetry (MTG) method to calculate the activation energy of the decomposition process. Previous kinetic research focuses on multiple heating rates [7–12]. In MTG, the perturbations caused by the sinusoidal temperature modulation are connected with derivatives of mass loss by simple scaling, where activation energy plays a role of a scaling parameter. The ratio of the experimentally measured perturbations to the experimental derivative is used for the model-free computation of activation energy. The latter variant is free from a number of assumptions and restrictions made in the isoconversional computations. In particular, it allows the use of a single decomposition curve and it remains in force even in the case of multistage decomposition with conjugated processes [18, 19].
In the previous research, the efforts focused on characterizing the pyrolysis oil [20, 21] and the kinetic of biomass pyrolysis [16, 22, 23]. To better understand the decomposition behaviors of cellulose, hemicellulose and lignin in inert atmosphere. The present work was to investigate the pyrolysis kinetic of three pseudocomponents using sinusoidally modulated temperature method. The multi-peaks method was used to fit the Gaussian distribution model of DTG curves of the three pseudocomponents. It was anticipated that the outcomes of this study would provide some new insights into the pyrolysis processes biomass.
Methods
Materials
In this experimental study, cellulose (CAS No.: 9004-34-6), hemicellulose (CAS No.: 9014-63-5) and lignin (CAS No.: 8068-05-1) were supplied from Sigma-Aldrich Co., Ltd (USA). Prior to experiment, the feedstock materials were put into an oven to outgas the water at the temperature of 378 K for 12 h.
TG analysis
A TG balance was used for all kinetic analysis tests. Non-isothermal TG was performed using a TA Instruments Q5000IR analyzer. The temperature range was from 313 to 1200 K, with heating rate (HR) 5 K min−1. Ultrahigh purity N2 was used at a constant flow rate of 100 mL min−1.
Determination of the E
The modulation of temperature is the perturbation of a temperature–time relationship using sinusoidal component [17–19]. According to the technique of TA Instruments, the modulated temperature is specified in Eq. (1):
where T 0 is the initial temperature, a is a heating rate, t is the time, ω is a frequency, L is the amplitude of the modulation. In this work, L = 0.002 K, 1/ω = 2 s.
The extent of dα/dt is calculated according to Eq. (2):
where a i is the partial degree of decomposition, w i is the mass contributions of each Gaussian model.
The perturbation in the derivative caused by the temperature modulation can be expressed as Eq. (3):
where a u and T u are the predicted data of the experimental functions of a m and T m, E is the activation energy.
By simple regrouping of the coefficients in Eq. (3), the following discrete function was introduced as Eq. (4):
where \(t_{\text{k}} = \left( {\frac{1}{2} + k} \right)/2\omega\); k = 0, 1, 2…
From here activation energy can be determined by Eq. (5):
Results and discussion
Pyrolysis behavior of cellulose, hemicellulose and lignin
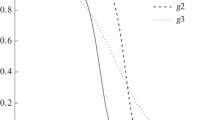
In this research, a typical heating rate of 5 K min−1 was used to investigate the pyrolysis behavior of cellulose, hemicellulose and lignin. The results of the TG and DTG curves of the three pseudocomponents are shown in Fig. 1. Figure 1a shows that the pyrolysis behavior was different for the cellulose, hemicellulose and lignin. There was no mass loss up to 520, 426 and 440 K for cellulose, hemicellulose and lignin, respectively. The mass remaining of the three pseudocomponents were also different. At the temperature of 700 K, almost all cellulose was decomposed with a very low solid residue (6.3%) left. However, high solid residue was left for hemicellulose (23.2%) and lignin (48.5%) at 1200 K. As shown in Fig. 1b, it was evident that cellulose exhibited a tall narrow peak and hemicellulose had a lower peak with a flat tailing, while lignin had two DTG peaks. Cellulose degradation happened between 514 and 670 K, and hemicellulose began to thermally decompose at 426–800 K. There were two degradation stages for the pyrolysis thermal events of lignin: The first degradation stage took place at 440–840 K and the second degradation stage took place at 840–1162 K [13].
Multi-peaks method to determine the Gaussian distribution model
Multi-peaks method was used to determine the Gaussian distribution model using the Levenberg–Marquardt algorithm. The DTG curves of cellulose, hemicellulose and lignin based on Gaussian distribution are shown in Fig. 2. Figure 2 shows that there were 1, 3 and 5 Gaussian peaks for the DTG curves of cellulose, hemicellulose and lignin, respectively. The Gaussian peak can indicate the partial reaction of decomposition of the three pseudocomponents. Cai et al. [24] also reported that single Gaussian model can fit the pyrolysis process of cellulose very well. The cellulose molecule used in this work is a very long chains of β(1,4) linked d-glucose units without any branches. It showed that there was only one chemical reaction in the pyrolysis process of cellulose. The main hemicellulose component is xylan, which is composed of 1,4-linked b-d-xylopyranose (b-d-Xylp) units that can be substituted at C-2 and/or C-3 by short and flexible side chains. The hemicelluloses had some branches structure. Figure 2b shows three chemical reactions can illustrate the pyrolysis process of hemicellulose. Lignin is a highly cross-linked polyphenolic aromatic polymer and is composed of p-hydroxyphenyl, guaiacyl and syringyl units. The thermal decomposition of lignin occurred in a broad temperature range, and there are two reaction stages in the DTG curve [13]. The first reaction stage can be illustrated using two chemical reactions and the later reaction stage can be divided into three chemical reactions.
Kinetic analysis of cellulose, hemicellulose and lignin
In this research, the modulated temperature is determined according Eq. (1), L = 0.002 K, 1/ω = 2 s. If a pyrolysis process under study is controlled by only one chemical reaction, the kinetic for the case of the modulated temperature can be described Eq. (3). For the partial chemical reaction Gaussian models in Fig. 2, the values of E α can be determined by Eq. (5). Values of E α at the different values of α of the partial chemical reaction model are shown in Fig. 3. The DTG curve of cellulose pyrolysis can be illustrated using single Gaussian curve. E α shown in Fig. 3a were the E α of cellulose pyrolysis. Values of E α at the different values of α of hemicelluloses and lignin can be calculated by the partial chemical reaction models of Fig. 3b–i.
Figure 4 shows the perturbations of derivatives of mass loss calculated as the difference between the modulated and unmodulated derivatives. One can see that the prediction resulting from the interpolation becomes bigger with increasing in DTG data. The real experiment contains restrictions both on amplitude and on period of the modulation. If these restrictions are exhausted, the remaining experimental parameter can govern accuracy. Mamleev et al. [25] investigated the effect of heating rate on the perturbations of derivatives of mass loss calculated as the difference between the modulated and unmodulated derivatives. These results showed that the prediction resulting from the interpolation becomes worse with increasing heating rate. It is better to do experiment under lower heating rate.
E is the equal to height of potential barrier on elementary step of chemical reaction. Values of E α at the different values of α of the three pseudocomponents are shown in Fig. 5. Figure 5 shows that the average E was 112.6, 162.8 and 156.8 kJ mol−1 for cellulose, hemicellulose and stage lignin, respectively. Cai et al. [26] reported the activation energy values for solid-state reactions are between 50 and 350 kJ mol−1. The E α of cellulose pyrolysis were ranged from 110 to 130 kJ mol−1 using Freidman method [23]. The E α of hemicellulose and lignin also fit the literatures very well [26, 27]. Thus, based on the above results from different researchers, the E α value in this work seemed to be reasonable. It was concluded that the activation energies were required to decompose different intermediate products. The decomposition of both hemicellulose and lignin occurred in a wide temperature range, and the activation energy was larger than cellulose. The results would provide some new insights into the pyrolysis processes biomass.
Conclusions
The kinetic analysis of the cellulose, hemicellulose and lignin were investigated using a TG analyzer. The multi-peaks method was used to fit the Gaussian distribution model of DTG curves of the three pseudocomponents. The E α of three pseudocomponents were evaluated using sinusoidally modulated temperature method. There were 1, 3 and 5 Gaussian peaks for the DTG curves of cellulose, hemicellulose and lignin, respectively. The Gaussian peak can indicate the partial reaction of decomposition of the three pseudocomponents. The activation energies were required to decompose different intermediate products. The decomposition of both hemicellulose and lignin occurred in a wide temperature range, and the activation energy was larger than cellulose. The average E was 112.6, 162.8 and 156.8 kJ mol−1 for cellulose, hemicellulose and lignin, respectively.
References
Lee JW, Hawkins B, Day DM, Reicosky DC. Sustainability: the capacity of smokeless biomass pyrolysis for energy production, global carbon capture and sequestration. Energy Environ Sci. 2010;3(11):1695–705.
Vinu R, Broadbelt LJ. A mechanistic model of fast pyrolysis of glucose-based carbohydrates to predict bio-oil composition. Energy Environ Sci. 2012;5(12):9808–26.
Chen T, Liu R, Scott NR. Characterization of energy carriers obtained from the pyrolysis of white ash, switchgrass and corn stover—biochar, syngas and bio-oil. Fuel Process Technol. 2016;142:124–34.
Mahinpey N, Murugan P, Mani T, Raina R. Analysis of bio-oil, biogas, and biochar from pressurized pyrolysis of wheat straw using a tubular reactor. Energy Fuels. 2009;23(5):2736–42.
Bridgwater AV. Principles and practice of biomass fast pyrolysis processes for liquids. J Anal Appl Pyrol. 1999;51(1–2):3–22.
Enders A, Hanley K, Whitman T, Joseph S, Lehmann J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour Technol. 2012;114:644–53.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand. 1956;57(4):217–21.
Burnham AK, Braun RL. Global kinetic analysis of complex materials. Energy Fuels. 1998;13(1):1–22.
Baroni ÉdG, Tannous K, Rueda-Ordóñez YJ, Tinoco-Navarro LK. The applicability of isoconversional models in estimating the kinetic parameters of biomass pyrolysis. J Therm Anal Calorim. 2015;123(2):909–17.
Wang J, Zhao H. Error evaluation on pyrolysis kinetics of sawdust using iso-conversional methods. J Therm Anal Calorim. 2016;124(3):1635–40.
Cruz G, Crnkovic PM. Investigation into the kinetic behavior of biomass combustion under N2/O2 and CO2/O2 atmospheres. J Therm Anal Calorim. 2015;123(2):1003–11.
Braun RL, Burnham AK. Analysis of chemical reaction kinetics using a distribution of activation energies and simpler models. Energy Fuels. 1987;1(2):153–61.
Zhou H, Long Y, Meng A, Li Q, Zhang Y. The pyrolysis simulation of five biomass species by hemi-cellulose, cellulose and lignin based on thermogravimetric curves. Thermochim Acta. 2013;566:36–43.
Cai J, Wu W, Liu R. Sensitivity analysis of three-parallel-DAEM-reaction model for describing rice straw pyrolysis. Bioresour Technol. 2013;132:423–6.
Li Z, Zhao W, Meng B, Liu C, Zhu Q, Zhao G. Kinetic study of corn straw pyrolysis: comparison of two different three-pseudocomponent models. Bioresour Technol. 2008;99(16):7616–22.
Zhang J, Chen T, Wu J, Wu J. Multi-Gaussian-DAEM-reaction model for thermal decompositions of cellulose, hemicellulose and lignin: comparison of N2 and CO2 atmosphere. Bioresour Technol. 2014;166:87–95.
Mamleev V, Bourbigot S, Bras ML, Lefevre J. Three model-free methods for calculation of activation energy in TG. J Therm Anal Calorim. 2004;78:1009–27.
Mamleev V, Bourbigot S. Modulated thermogravimetry in analysis of decomposition kinetics. Chem Eng Sci. 2005;60(3):747–66.
Mamleev V, Bourbigot S, Le Bras M, Yvon J, Lefebvre J. Model-free method for evaluation of activation energies in modulated thermogravimetry and analysis of cellulose decomposition. Chem Eng Sci. 2006;61(4):1276–92.
Chen T, Wu C, Liu R, Fei W, Liu S. Effect of hot vapor filtration on the characterization of bio-oil from rice husks with fast pyrolysis in a fluidized-bed reactor. Bioresour Technol. 2011;102(10):6178–85.
Chen T, Deng C, Liu R. Effect of selective condensation on the characterization of bio-oil from pine sawdust fast pyrolysis using a fluidized-bed reactor. Energy Fuels. 2010;24(12):6616–23.
Chen T, Zhang J, Wu J. Kinetic and energy production analysis of pyrolysis of lignocellulosic biomass using a three-parallel Gaussian reaction model. Bioresour Technol. 2016;211:502–8.
Chen T, Wu J, Zhang J, Wu J, Sun L. Gasification kinetic analysis of the three pseudocomponents of biomass-cellulose, semicellulose and lignin. Bioresour Technol. 2014;153:223–9.
Cai J, Wu W, Liu R, Huber GW. A distributed activation energy model for the pyrolysis of lignocellulosic biomass. Green Chem. 2013;15(5):1331–40.
Mamleev V, Bourbigot S. Calculation of activation energies using the sinusoidally modulated temperature. J Therm Anal Calorim. 2002;70:565–79.
Cai J, Han D, Chen C, Chen S. Application of the golden section search algorithm in the nonlinear isoconversional calculations to the determination of the activation energy from nonisothermal kinetic conversion data. Solid State Sci. 2010;12(5):829–33.
Ranzi E, Cuoci A, Faravelli T, Frassoldati A, Migliavacca G, Pierucci S, et al. Chemical kinetics of biomass pyrolysis. Energy Fuels. 2008;22(6):4292–300.
Acknowledgements
Financial support from the National Natural Science Foundation of China (Project No. 51406220) and Shanxi Province Coal-based Key Technology Research and Development Program (Project No. MD 2014-03) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, T., Li, L., Zhao, R. et al. Pyrolysis kinetic analysis of the three pseudocomponents of biomass–cellulose, hemicellulose and lignin. J Therm Anal Calorim 128, 1825–1832 (2017). https://doi.org/10.1007/s10973-016-6040-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6040-3