Abstract
Flame retardant thermoplastic polyurethane (TPU) composites with low smoke release have been prepared by melt-blending TPU with commercially available carbon black (CB) and ammonium polyphosphate (APP). The experimental data from SDT indicated that an appropriate amount of CB and APP can decrease the amount of smoke production in the test with or without flame. The CCT results showed that CB and APP greatly decrease smoke production rate, total smoke release, and smoke factor of flame retardant TPU composites compared with that of pure TPU. Indeed, CB is considered as an effective smoke suppression agent and a good synergism with APP in flame retardant TPU composites, which can greatly improve the structure of char residue realized by TG and SEM results. The TG and DTG results showed that CB can decrease the initial decomposition temperature and improve the thermal stability at high temperature for flame retardant TPU composites. More interest, the TG-IR study indicates that the volatilized products are CO2, ammonia compound, acid anhydride, water, alkane compounds, and aromatic compounds according to the temperature of onset formation. This is a very meaningful result in fire safety materials fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The flammability of polymers and the smoke produced when they burn still present a major hazard in the developed world [1]. It has been reported that most fire deaths are due to toxic gases, oxygen deprivation, and other effects that have been widely referred to smoke inhalation instead of burns in the USA [2]. The flammability of composites cannot be predicted from knowledge of properties of single components. In many cases, the visibility impairing and narcotic irritating effect of fire gases are regarded as the decisive factor preventing many fire victims from perceiving their possibilities of escape [3]. Recently, the additional fire and smoke hazards from communication cables routed through under floor and/or over-head spaces coupled with the rapidly rising demands for IT systems have added to the problem. So it can been found consistently that smoke, released by the materials discussed above, indeed represents a potential hazard to people’s lives; in fact, not only for transportation applications but also for furniture and accessories, smoke-related parameters are considered critical features for developing safety procedures and evacuation plans [4]. Within this scenario, the effects of various smoke suppressants on flammability of a flame retardant polymer have been become much important.
As an engineering plastic, TPU has been applied in many fields such as automotive parts, electrical and electronic industries, mechanical transmission parts, household and cosmetic parts, due to its excellent physical properties, chemical resistance, abrasion resistance, and good adhesion to chemicals and self-lubrication performance [5–8]. However, the production of much smoke and toxic gases (i.e., chlorine compounds, benzene, and other aromatic compounds) during burning of TPU and its high flammability have greatly limited its broad applications in various fields mentioned above. So, the improvement in the flame retardance and smoke suppression during combustion of TPU becomes increasingly vital to satisfy the increasing requirements in the practical use [9–11]. Many investigations demonstrated that halogen-containing flame retardants show effective flame retardant properties in TPU and a high price-performance. Compounds such as bromine and chlorine, because of their low bonding energy with carbon atoms, could be readily released and take part in the burning process, especially with the gas-phase mechanism by quenching the free-radicals [12, 13]. However, the utilization of such retardants has been limited due to generation of toxic gas, affecting human health and the environment. Therefore, it becomes a real request that new flame retardant systems instead of halogenated substances should be developed to meet the constantly changing demand of new regulations, standards, and test methods [14]. Intumescent flame retardants, as halogen-free system, developed quickly in the past few years [15]. It generally includes acid source, carbon source, and gas source in this intumescent flame retardant system. TPU can be used as carbon source. So, intumescent flame retardant TPU composites can be obtained by incorporating APP, used both as acid source and gas source. The intumescent flame retardant TPU composites show high flame retardant properties. However, there is still large smoke production in the combustion process. In order to reduce the smoke production, some smoke suppression agents are used into TPU/APP composites. In our previous work [2], ferrite yellow was used as smoke suppression agent into TPU/APP) composites. Results show that ferrite yellow can be used as efficient flame retardant synergism and smoke suppression agent.
Carbon black (CB) is used widely in polymer science, especially in rubber science as reinforcing material [16–18]. There is a strong interaction between CB and polymer molecular chain, which may slow the precipitation of pyrolysis volatiles in the combustion process, resulting in good flame retardant properties [19]. It has reported that CB nanoparticles can trap peroxy radicals at elevated temperature to form a gelled-ball cross-linked network, which act as a barrier to both heat and mass transfer [20]. To the best of our knowledge, no work has been reported the synergistic effects between CB and APP on achievement of smoke suppression and flame retardant properties in TPU composites.
In this paper, CB was used as flame retardant and smoke suppression synergism to improve the flame retardant efficiency and smoke suppression effect in TPU composites based on APP. The synergistic flame retardant and smoke suppression properties of CB with APP were intensively investigated using CCT, SDT, TG, and LOI. And, the synergistic flame retardant and smoke suppression mechanism were further studied using SEM and TG-IR.
Experimental
Materials
Thermoplastic polyurethane (TPU) was produced by Bayer, German. Ammonium polyphosphate (APP) with particle size of 2500 mesh was purchased from new thin Metal and Chemical Co., Ltd., Guangzhou, China. Carbon black (REGAL 250R, 99 % GR) with average particle size of 24 nm was purchased from Cabot Corporation, USA.
Sample preparation
TPU/APP/CB composites were prepared by melt-blending method. Before processing experiment, TPU was dried in an oven at 80 °C for 8 h; APP and CB were dried in an oven at 100 °C for 10 h. A certain amount of TPU was melted in the mixer at 175 °C. Then, a certain amount of APP and CB was added into the mixer, respectively. The blends were mixed for 10 min and hot pressed into sheets in the dimensions of 100 × 100 × 3 mm3 using the plate vulcanizing machine. The formulations of flame retardant TPU composites are listed in Table 1.
Measurements
A smoke density test machine (JQMY-2, Jianqiao Co, China) was used to measure the smoke characteristics according to ISO 5659-2 (2012). Each specimen with dimensions of 75 × 75 × 2.5 mm3 was wrapped in aluminum foil and exposed horizontally to an external heat flux of 25 kW m−2 with or without the application of a pilot flame.
The cone calorimeter (Stanton Redcroft, UK) tests were performed according to ISO 5660 standard procedures. Each specimen with dimensions of 100 × 100 × 3 mm3 was wrapped in aluminum foil and exposed horizontally to an external heat flux of 50 kW m−2.
Scanning electron microscopy (SEM) studies were performed using a Hitachi X650 scanning electron microscope.
LOI was measured according to ASTM D2863. The apparatus used was an HC-2 oxygen index meter (Jiangning Analysis Instrument Company, China). The specimens used for the test were of dimensions 10 × 6.5 × 3 mm3.
Thermogravimetric analysis (TG) of the sample was performed using a DT-50 (Setaram, France) instrument. About 10.0 mg of sample was put in an alumina crucible and heated from ambient temperature to 700 °C. The heating rates were set as 20 K min−1 (nitrogen atmosphere, flow rate of 60 mL min−1).
Thermogravimetric analysis/infrared spectrometry (TG-IR) of the cured sample was performed using a DT-50 (Shimadzu, Japan) instrument that was interfaced to a Varian 2000 FTIR spectrometer. About 10.0 mg of the TPU sample was put in an alumina crucible and heated from 260 to 700 °C. The heating rate was set as 20 K min−1 (nitrogen atmosphere, flow rate of 60 mL min−1).
Results and discussion
Smoke density test (SDT)
Polymer materials can release a large amount of toxic gases and smoke during the combustion process. The luminous flux curves of flame retardant TPU composites with and without flame in the smoke density test that gives detailed information about the smoke production are presented in Fig. 1a, b, respectively. As shown in Fig. 1a, it can be seen that even though the luminous flux, in the case of TPU-0, rapidly decreases in the first 200 s and gets the lowest luminous flux value (12.6 %) at 510 s, it slightly increases gradually after 510 s. The decrease in luminous flux mainly apparent in the first 450 s, and it attains the lowest value (48.9 %) at 600 s as APP is added into TPU in the smoke density test. This indicates that smoke produced by TPU-1 is significantly less than that of TPU-0. Indeed, the polyphosphoric acid compounds formed by the decomposition of APP at low temperature can catalyze the carbonization of TPU to generate condensed carbon shell on the surface of TPU-1, which results in the decrease of smoke production. In the case of TPU/APP/CB systems, the luminous flux of the samples increased rapidly. The luminous flux of TPU-2 is up to 92.1 % at the end of experiments, which is much higher than that of TPU-1 (56.0 %). It is concluded that CB has an apparently synergistic smoke suppression effect with APP in TPU composites.
There is a small decrease in the luminous flux of TPU-0 at the first 390 s as shown in Fig. 1b. During the period, a small amount of smoke produced from TPU-0. However, the luminous flux rapidly reduced after that and reaches 27.7 % at 1200 s. And, the luminous flux of TPU-1 containing only APP is much lower than that of the pure TPU (TPU-0) in the initial 720 s. This phenomenon resulted by two aspects: One is that APP can decompose at low temperature to form some smoke precursors and char residue shell, and the smoke precursors lead to low luminous flux before 720 s. Another is that the formation of char residue shell can decrease the generation rate of smoke precursors. It is interesting that the luminous flux is significantly improved when 0.50 mass% CB was incorporated into TPU/APP composite. As the content of CB is raised to 2.00 mass%, the maximum luminous flux value of TPU-3 reached to 62.4 % at 600 s, which is lower than that of TPU-2 (69.2 %). However, this behavior did not continue as the amount of CB was increased. From the result, it can be concluded that a moderate amount of CB is favorable for improving the smoke suppression property.
The difference of testing condition in the smoke density tests with and without flame is whether to ignite. The tests with flame are carried out using flame of methane gas to ignite the sample under the radiant heat flux of 25 kW m−2. And, the tests without flame are not ignited by any flame and just under the radiant heat flux of 25 kW m−2. Comparing Fig. 1b and Fig. 1a, it can be seen that there are many differences between the smoke density tests with flame and without flame. For example, the luminous flux of TPU-2 is 92.1 % in the test with flame, but it is only 56.1 % in the test without flame. This phenomenon illustrates that the smoke precursors formed in the thermal decomposition process and can be burnt out in the smoke density test with flame.
Cone calorimeter test (CCT)
Smoke production rate (SPR)
Smoke performance of material during combustion plays a vital factor concerning fire safety, heavy smoke can hinder escape, and toxic gases act as one killer during the fire hazard. The smoke production rate (SPR) curves of all samples are illustrated in Fig. 2. It is clearly seen that a significant decrease in the peak SPR value taken on as the flame retardants added. Compared with the peak SPR value of TPU-0, the peak SPR value of TPU-1 containing only APP is greatly decreased. The time to peak SPR of TPU-0, however, is delayed than that of other systems which may be attributed to the fact that APP decomposes at low temperature to form some smoke particulates. Moreover, it can be seen that the peak SPR value of TPU/APP/CB systems are decreased as the gradual increase in the amount of CB. From this result, it can be concluded that CB would change the structure of char residue layer; and the smoke production rate may decrease in the process of formation of carbon layer. Because of the protection of the carbon layer, combustible gases and smoke-forming materials reduce rapidly in the gas phase during combustion [21]. From the above results, it can be concluded that CB is an effective additive to suppress smoke production in the combustion process of TPU/APP composites.
Total smoke release (TSR)
The total smoke release (TSR) curves of TPU composites in the cone calorimeter test at a flux of 50 kW m−2 are shown in Fig. 3. It can be seen the TSR of TPU-0 is 628.7 m2 m−2, which is confirmed the fact that there is a large amount of smoke production during the combustion process of TPU. When APP is incorporated into TPU, the TSR value of TPU-1 is 354.8 m2 m−2 at the end of the test, which is 43.6 % lower than that of TPU-0. It also should be figured out the TSR of TPU-1 is larger than that of TPU-0 before 180 s. The above results are attributed to the fact that APP decomposes at low temperature to form polyphosphoric acid, which can react with TPU to generate some smoke particulates and char residue layer. The smoke particulates formed at low temperature result in high TSR value before 185 s. Then, the char residue layer formed at low temperature can effectively prevent the release of smoke particulates, resulting in low TSR value of TPU-1. When 0.50 mass% CB is incorporated into TPU/APP composite, the TSR value is reduced compared with TPU-1. Furthermore, the TSR value decreased with the increase in CB. When the loading of CB is raised to 2.00 mass%, the TSR value of TPU-4 is only 172.1 m2 m−2 at the end of the test, which is only 27.4 % of TPU-0 without any flame retardant additives. So, it can be concluded that CB has a perfect smoke suppression effect in flame retardant TPU based on APP. It has been reported that CB can be used as reinforcing agent and improves the viscosity of the system. So, it can be deduced that CB can improve the viscosity of molten carbon precursor from TPU/APP composites. When TPU is heated, the depolymerization reaction of TPU quickly occurred to form large amount of volatile organic compounds including polyol and isocyanate at about 220 °C, resulting in the rapid decrease in melt viscosity and high smoke particulates release. As mentioned before, APP can catalyze carbonization; CB can improve the viscosity of molten carbon precursor. So, the char residue layer can effectively reduce the release of smoke particulates. A low TSR value can be obtained by incorporating CB into TPU/APP composites.
Smoke factor (SF)
Figure 4 gives the smoke factor (SF) of all samples, and SF is the product of PHRR and TSR [22]. It is obvious that the SF value of TPU-0 is greatly decreased as APP was added. Furthermore, the samples containing both APP and CB show further decrease in SF values compared with TPU-1. It is apparent that the SF values for different samples decrease in turn as the amount of CB is increased. So it can be concluded that the addition of flame retardant significantly reduces the SF values of TPU composites. It has been reported that if a material has high smoke release and low heat release, there may be no combustion or extinguish to suppress smoke production; if combustion process has high heat release and low smoke production, it may result in fire hazard and a large amount of smoke production. Based on this theory, CB can not only decrease the heat release rare, but also reduce the smoke production rate. This means that CB can improve the fire safety level of TPU/APP/CB composites.
Heat release rate (HRR)
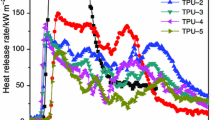
The heat release rate (HRR) curves for pure TPU and TPU blends with different amount of APP and CB are shown in Fig. 5. It can be seen that pure TPU burns quickly with a high PHRR value of 857 kW m−2 and the ignition time was relatively fast (70 s). In case of TPU/APP, however, the value of PHRR was dramatically reduced to 152.4 kW m−2, and two peaks can be identified in the HRR curve, which can be considered as characteristic feature for TPU/APP. The first peak followed immediately the ignition point, and the second peak corresponds to a huge increase in the HRR value over a short period of time. In this case, the first peak is identified as the development of the intumescent char, which could prevent oxygen from diffusing to the underlying substrate. After that, the HRR curves tended to a relatively steady case. The second peak is due to the degradation of the protective carbon layer gradually as the sample is continuously exposed to the heat, and the formation of a new protective char because of the flame retardant, which then gives rise to the third and then the fourth HRR peak [23, 24]. Moreover, when CB is incorporated into TPU/APP system, the PHRR value further decreases. The PHRR value of TPU-2 with 0.50 mass% CB is 88.5 kW m−2, which is much lower than that of TPU-1 (154.4 kW m−2). And the PHRR value of TPU-4 (90.2 kW m−2) is lower than that of TPU-1. The dramatic decrease in PHRR of the samples with the addition of CB can be interpreted that CB can change the structure of char residue, including compact and expansion degree, and mass of char residue in the cone calorimeter test. As shown in Fig. 5, it should be figured out the ignition time changed a lot for the samples with different content of CB. The reason is that when the samples containing CB are put into sample boxes under high heat flux in the cone calorimeter test, in which the samples become deformation with raised centers, resulting in very short ignition time.
Mass loss
The mass loss curves of TPU composites during the combustion test are shown in Fig. 6. By the addition of intumescent flame retardant agents in pure TPU under heat flow 50 kW m−2, the mass of TPU/APP and TPU/APP/CB systems decreases more slowly than that of the TPU system. There is about 65 % residue left after combustion for the TPU/APP system (TPU-1). In case of TPU/APP/CB, the mass loss is larger than that of TPU-1 before 150 s. However, the TSR of TPU/APP/CB is lower than that of TPU-1 (Fig. 3). This can be concluded that there are more volatile gases formed from the TPU/APP/CB samples than that from TPU-1. With the time prolonging, the mass loss of TPU-2 and TPU-3 became lower than that of TPU-1. These results indicate a moderate content of CB can decrease the decomposition rate of TPU/APP/CB composites in the cone calorimeter test, which is beneficial for the reduced smoke production. When the content of CB is raised to 2.00 mass%, the mass loss of TPU-4 is larger than that of TPU-1 during all the combustion process. In contrast, the TSR of TPU-4 is lower than that of TPU-1 during all test time (Fig. 3). Combining the HRR results as shown in Fig. 5, the ignition time and the time to PHRR of TPU-4 are the shortest among all samples. The above results mean there are a lot of flammable gases formed for TPU-4 at low temperature. But, the amount and release rate of flammable gases is not lager than that from TPU-1 because the PHRR of TPU-4 is lower than that of TPU-1. Also, some smoke particulate may be burnt out by the lot of flammable gases. The above analysis and discussion are based on the conservation law of matter. The char residue structure also can affect the smoke production, including the compact degree, expansion degree [25, 26]. An intumescent char layer creating a physical protective barrier for heat and mass transferring may emerge on the surface of the flame retardant sample during combustion. The char layer would prevent oxygen from diffusing to the underlying substrate or give a low volatilization rate. Then, the structure of char residue layer would be studied in the following.
Photographs and scanning electron microscopy (SEM) of char residue
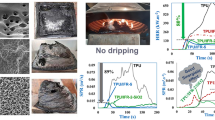
The digital photographs of the char residues for all the samples after the cone tests are shown in Fig. 7. It can be seen that the char residue layer has a concentric circle shape for the TPU-0 sample. This means that the viscosity of molten carbon layer cannot effectively bind the volatile substances during combustion process, resulting in large amount of smoke release and heat release. When APP is introduced into TPU, there is a continuous, compact char residue layer with high expansion degree formed on the surface of TPU-1 sample. However, there are some collapses in the char residue layer, which is generated after the volatile compounds released. This would make multi-peaks in the SPR and HRR curves. In a word, this char residue layer can act as barrier for the heat from flame zone into the underlying materials and can reduce the amount of volatile substances, especially smoke precursor. This result can illustrate the TSR result shown in Fig. 3. In case of TPU/APP/CB composites, it can be seen CB changes the structure of char residue layer. The compact and expansion degree of char residue layer from the samples with CB become better than that from TPU-1 without CB. Furthermore, there are almost no collapse in the char residue layer from the samples with CB, which mean this char residue structure has higher mechanical strength and long-term capability to protect the underlying materials from further pyrolysis and release of volatile compounds. This can illustrate the smooth SPR curves from TPU-2 to TPU-4 in Fig. 2.
The SEM of char residues for the TPU composites can further evaluate the influence of CB on the structure of intumescent char residue layer determines smoke suppression properties. For TPU-0S, there are many holes and crevices, and the carbon layer presents an irregular appearance. This cannot effectively bind volatile compound releasing, resulting in large SPR and TSR. And, a very smooth fracture surface can be observed as APP is added into TPU. Moreover, a compact appearance and smooth surface clearly as well as clear interface have been shown by the char residue of TPU-4S with 2.00 mass% CB. So it can be concluded that CB can change the structure of char residue layer, restraining the heat release and smoke generation.
Limiting oxygen index (LOI)
The LOI values of flame retardant TPU composites are presented in Fig. 8. It can be seen that the LOI value of the composite with APP is as high as 28.3 comparing with that of pure TPU with the LOI value of 21.3. And the values of the composites containing CB at the corresponding additive level are higher than that of TPU/APP composite. The highest LOI value of 30.1 is obtained by TPU-4 with 2.00 mass% CB. The above results imply that CB can improve the structure of char residue from TPU composites. Firstly, CB not only can increase the melt viscosity of the composites, resulting in high LOI value; secondly, CB can change the expansion degree and density of char residue, which play an important role in improving LOI value. Moderate expansion degree and density may correspond to high LOI value.
Thermogravimetric (TG)
Thermogravimetric (TG) is a high-precision method for the study of pyrolysis under well-defined conditions [27–30]. The TG also provides the identification of different surfactant environments and structure arrangements, and it can be also used for revealing thermal and structural stabilities [31–33]. Thermogravimetric (TG) curves of various flame retardant TPU composites under nitrogen atmosphere are shown in Fig. 9a. As shown in Fig. 9a, it can be seen that the curves can roughly be divided into three different regions; the first stage appears below 300 °C, which is due to the drying procedure and the release of some light volatiles. At the second stage (between 300 and 500 °C) is attributed to the great thermal decomposition of both TPU and APP, resulting in a significant drop in mass. At the third stage, the mass loss is not as significant as the previous period. To say it specially, the base TPU begins to decompose rapidly at about 333.9 °C, and this decomposition ends at about 600 °C with about 10 % char residue. The initial decomposition temperature of TPU-1 with only APP is 309.2 °C which is similar to TPU-0, but there are 20 % char residue at last. Compared with TPU-1, the char residue of TPU/APP/CB composites is large; the reason may be due to that APP can catalyze carbonization and CB can improve the viscosity of molten carbon precursor, so a rigid char residue layer can form and reduce mass loss.
The derivative TG (DTG) presents the mechanism of composites decomposition. And the DTG curves of various flame retardant TPU composites under nitrogen atmosphere are shown in Fig. 9b. For pure TPU, there is a two-phase morphology in which a soft phase containing either polyethers or polyesters is reinforced by condensation with a hard domain consisting of an aromatic diisocyanate extended with a short chain diol. And two peaks are shown in Fig. 9b, the first peak is the decomposition of hard domains comprising of urethane linkages at 370 °C to form alcohols and isocyanates, whereas the second stage is attributed to the further destruction of the C–C and C–O bonds on the main chain [34]. But for TPU-1, three peaks showed during decomposition. The first peak is attributed to the decomposition of APP to form ammonia gas and water vapors, etc., which can dilute the combustible fuel and reduce oxygen concentration. The reason for the second peak is APP can further degrade into polyphosphoric acid compounds which can catalyze the carbonization of TPU to generate some volatile compounds and condensed carbon shell on the surface of TPU-1. And, the third degradation process is the decomposition of the unstable carbon layer formed during the second stage. Because the protective layer on the polymer surface during the third degradation, the third maximum mass loss rate is lower than that of TPU-0. It also can be clearly seen that the addition of APP reduces the formation temperature of carbon layer, preventing TPU composites from further thermal degradation [15, 35]. For TPU/APP/CB composites, it can be seen that the initial decomposition temperature is lower than that of TPU-1. Which may due to that CB can as a kind of alkaline substance that catalyze APP decomposing. Compared with the TPU-1, the samples containing both APP and CB also have three peaks. And it is illustrated that the peaks of this composites are lower than TPU-1. This result means that the production rate of volatile compounds is reduced by CB. So it can be concluded that a good synergistic smoke suppression and flame retardant effects between CB and APP are shown in TPU composites.
It has been reported that the TG results in nitrogen atmosphere are correlated well with the mass results from cone calorimeter test. But, it can be found there is a contradiction comparing TG curve (Fig. 9a) with mass curve (Fig. 6). In the TG results, the char residue of TPU/APP/CB composites is much higher than that of TPU/APP sample in nitrogen atmosphere. However, the char residue of TPU/APP/CB composites is lower than that of TPU/APP sample in the cone calorimeter test. This can be illustrated in the following. In TG test, the test condition is under nitrogen atmosphere; there is no oxygen to oxidize the char residue degradation of the composites at higher temperature. In the CCT, the test condition is under air atmosphere. When the samples are put into sample box under high heat flux, there is a lot of pyrolysis gases formed. At this time, the solid phase is under inert atmosphere, and the char residues form the TPU/APP/CB samples are high than that of TPU/APP. After that a compact char residue shell formed on the surface of the sample, which can reduce the pyrolysis gases, resulting in low heat release rate. This leads the char residue shell can not effectively covered by the flame zone. So, part of the char residue with unstable structure is exposed to the high heat flux in air atmosphere. Smoldering is occurred on the surface of the sample, resulting in the further reduction of mass loss.
Thermogravimetric analysis/infrared spectrometry (TG-IR)
The TG-IR technique that directly gives identification of the volatilized products can significantly contribute to an understanding of thermal degradation mechanisms [14, 33, 36]. The volatilized products formed during the thermal degradation of the TPU composites were characterized by TG-IR technique, as shown in Fig. 10. The peaks at about 3327, 3000, 2989, 2335, 1750, and 900 cm−1 are attributed to H2O, aromatic compounds, hydrocarbon, CO2, and NH3, respectively [37]. NH3 is caused by the degradation of APP. Hydrocarbon and CO2 are caused by the degradation of TPU. For TPU-0, the intensity of characteristic CO2 peaks increases until it reaches the maximum at 460 °C, and then turns into a rapid decrease. But for TPU-1 and TPU-4, the intensity of this peak is lower than that of pure TPU when the products obtained at higher temperature, suggesting that during the thermal degradation, the further decomposition of carbon-containing compounds can lead to the elimination of CO2. More interesting is that, comparing with the pure TPU, the aromatic compounds peaks (3000 cm−1) for TPU-1 and TPU-4 are decreased, that may due to that APP degrade to polyphosphoric acid compounds which can catalyze the further decomposition of aromatic compounds to generate condensed carbon shell on the surface of TPU-1. This result also indicates that CB can reduce the production of aromatic compounds as smoke precursor, leading low smoke release.
Conclusions
When carbon black is combined with APP in TPU composites, there is obviously synergistic smoke suppression and flame retardant effect. Carbon black can greatly enhance the thermal stability at high temperature in TG test under nitrogen atmosphere and reduce the aromatic compounds as smoke precursor realized by TG-IR results. Carbon black represents dramatically excellent smoke suppression properties in flame retardant TPU composites based on APP by changing the structure of char and not decreasing the mass loss residue in SDT and CCT. Other carbon materials may be used as smoke suppression agents in fire safety materials.
References
Carty P, Creighton JR, White S. TG and flammability studies on polymer blends containing acrylonitrile-butadiene-styrene and chlorinated poly(vinyl Chloride). J Therm Anal Calorim. 2001;63(3):679–87.
Chen XL, Jiang YF, Jiao CM. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J Hazard Mater. 2014;66:114–21.
Chen MJ, Chen CR, Tan Y, Huang JQ, Wang XL, Chen L, Wang YZ. Inherently flame-retardant flexible polyurethane foam with low content of phosphorus-containing cross-linking agent. Ind Eng Chem Res. 2014;53(3):1160–71.
Formicola C, Fenzo AD, Zarrelli M, Giordano M, Antonucci V. Zinc-based compounds as smoke suppressant agents for an aerospace epoxy matrix. Polym Int. 2011;60(2):304–11.
Liu Y, Liu MF, Xie DY, Wang Q. Thermoplastic polyurethane-encapsulated melamine phosphate flame retardant polyoxymethylene. Polym-Plast Technol. 2008;47(3):330–4.
Harashina H, Tajima Y, Itoh T. Synergistic effect of red phosphorus, novolac and melamine ternary combination on flame retardancy of poly (oxymethylene). Polym Degrad Stabil. 2006;91(9):1996–2002.
Allcorn EK, Natali M, Koo JH. Ablation performance and characterization of thermoplastic polyurethane elastomer nanocomposites. Compos Part A Appl Sci Manuf. 2013;45:109–18.
Xu Y, Chen M, Ning X, Chen XL, Sun ZD, Ma YH, Yu J, Zhang ZB, Bo XJ. Influences of coupling agent on thermal properties, flammability and mechanical properties of polypropylene/thermoplastic polyurethanes composites filled with expanded graphite. J Therm Anal Calorim. 2014;15(1):689–95.
Jiang SH, Shi YQ, Qian XD, Zhou KQ, Xu HY, Lo SM, Gui Z, Hu Y. Synthesis of a novel phosphorus- and nitrogen-containing acrylate and its performance as an intumescent flame retardant for epoxy acrylate. Ind Eng Chem Res. 2013;52(49):17442–50.
Bourbigot S, Samyn F, Turf T, Duquesne S. Nanomorphology and reaction to fire of polyurethane and polyamide nanocomposites containing flame retardants. Polym Degrad Stabil. 2010;95(3):320–6.
Jiao CM, Zhao XL, Song WK, Chen XL. Synergistic flame retardant and smoke suppression effects of ferrous powder with ammonium polyphosphate in thermoplastic polyurethane composites. J Therm Anal Calorim. 2015;120(2):1173–81.
Laoutid F, Bonnaud L, Alxandre M, Lopez-Cuesta JM, Dubois P. New prospects in flame retardant polymer materials: from fundamentals to nanocomposites. Mater Sci Eng, R. 2009;63(3):100–5.
Wu JP, Zhang Y, Luo XJ, She YZ, Yu LH, Chen SJ, Mai BX. A review of polybrominated diphenyl ethers and alternative brominated flame retardants in wildlife from China: levels, trends, and bioaccumulation characteristics. J Environ Sci China. 2012;24(2):183–94.
Velencoso MM, Ramos MJ, Klein R, Lucas AD, Rodriguez JF. Thermal degradation and fire behaviour of novel polyurethanes based on phosphate polyols. Polym Degrad Stabil. 2014;101:40–51.
Subasinghe A, Bhattacharyya D. Performance of different intumescent ammonium polyphosphate flame retardants in PP/kenaf fibre composites. Compos Part A Appl Sci Manuf. 2014;65:91–9.
Janowska G, Rybiński P. Influence of carbon black on thermal properties and flammability of cross-linked elastomers. J Therm Anal Calorim. 2008;91(3):697–701.
Zhang QJ, Zhan J, Zhou KQ, Lu HD, Zeng WR, Stec AA. The influence of carbon nanotubes on the combustion toxicity of PP/intumescent flame retardant composites. Polym Degrad Stabil. 2015;115:38–44.
Omnès B, Thuillier S, Pilvin P, Grohens Y, Gillet S. Effective properties of carbon black filled natural rubber: experiments and modeling. Compos Part A Appl Sci Manuf. 2008;39(7):1141–9.
Wen X, Tian NN, Gong J, Chen Q, Qi YL, Liu Z, Liu J, Jiang ZW, Chen XC, Tang T. Effect of nanosized carbon black on thermal stability and flame retardancy of polypropylene/carbon nanotubes nanocomposites. Polym Adv Technol. 2013;24(11):971–7.
Chen XL, Jiao CM. Thermal degradation characteristics of a novel flame retardant coating using TG-IR technique. Polym Degrad Stabil. 2008;93(12):2222–5.
Dong YY, Gui Z, Hu Y, Wu Y, Jiang SY. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly(methyl methacrylate). J Hazard Mater. 2012;209:34–9.
Ricciardi MR, Antonucci V, Zarrelli M, Giordano M. Fire behavior and smoke emission of phosphate-based inorganic fire-retarded polyester resin. Fire Mater. 2012;36(3):203–15.
Tsai KC. Orientation effect on cone calorimeter test results to assess fire hazard of materials. J Hazard Mater. 2009;172(2):763–72.
Schartel B, Hull TR. Development of fire-retarded materials-Interpretation of cone calorimeter data. Fire Mater. 2007;31:327–54.
Zhang P, Song L, Lu HD, Hu Y, Xing WY, Ni JX, Wang J. Synergistic effect of nanoflaky manganese phosphate on thermal degradation and flame retardant properties of intumescent flame retardant polypropylene system. Polym Degrad Stabil. 2009;94(2):201–7.
Hu WZ, Zhan J, Wang X, Hong NN, Wang BB, Song L, Stec AA, Hull TR, Wang J, Hu Y. Effect of functionalized graphene oxide with hyper-branched flame retardant on flammability and thermal stability of cross-linked polyethylene. Ind Eng Chem Res. 2014;53(8):3073–83.
Paliesková J, Pajtášová M, Feriancová A, Ondrušová D, Holcová K, Vavro J. Thermal properties of fillers based on organoclays in the polymeric materials. J Therm Anal Calorim. 2015;119(2):939–43.
Li LL, Wang G, Wang SY, Qin S. Thermogravimetric and kinetic analysis of energy crop Jerusalem artichoke using the distributed activation energy model. J Therm Anal Calorim. 2013;114(3):1183–9.
Chen XL, Zhuo JL, Jiao CM. Thermal degradation characteristics of flame retardant polylactide using TG-IR. Polym Degrad Stabil. 2012;97(11):2143–7.
Chen XL, Jiao CM, Zhang J. Microencapsulation of ammonium polyphosphate with hydroxyl silicone oil and its flame retardance in thermoplastic polyurethane. J Therm Anal Calorim. 2011;104(3):1037–43.
Simkovic I. TG/DTG/DTA evaluation of flame retarded cotton fabrics and comparison to cone calorimeter data. Carbohyd Polym. 2012;90(2):976–81.
Jiao CM, Dong J, Chen XL, Li SX. Influence of T31 content on combustion and thermal degradation behaviors on flame-retardant epoxy composites. J Therm Anal Calorim. 2013;114(3):1201–6.
Kunze R, Schartel B, Bartholmai M, Neubert D, Schriever R. TG-MS and TG-FTIR applied for an unambiguous thermal analysis of intumescent coatings. J Therm Anal Calorim. 2002;70(3):897–909.
Li YT, Li B, Dai JF, Jia H, Gao SL. Synergistic effects of lanthanum oxide on a novel intumescent flame retardant polypropylene system. Polym Degrad Stabil. 2008;93(1):9–16.
Pan MZ, Mei CT, Du J, Li GC. Synergistic effect of nano silicon dioxide and ammonium polyphosphate on flame retardancy of wood fiber–polyethylene composites. Compos Part A Appl Sci Manuf. 2014;66:128–34.
Giron D. Applications of thermal analysis and coupled techniques in pharmaceutical industry. J Therm Anal Calorim. 2002;68(2):335–57.
Isitman NA, Kaynak C. Nanoclay and carbon nanotubes as potential synergists of an organophosphorus flame-retardant in poly(methyl methacrylate). Polym Degrad Stabil. 2010;95(9):1523–32.
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (Nos. 51106078, 51206084), the University Research and Development Projects from Shandong Province (J14LA13), and the Major Special Projects of Science and Technology from Shandong Province (2015ZDZX11011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Zhao, X., Ma, C. et al. Smoke suppression properties of carbon black on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J Therm Anal Calorim 126, 1821–1830 (2016). https://doi.org/10.1007/s10973-016-5815-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5815-x