Abstract
The influence of poly(ethylene glycol) (PEG) on thermal characteristics and thermal degradation of poly(vinyl chloride)/poly(ethylene oxide) (PVC/PEO) blends was investigated by differential scanning calorimetry and dynamic thermogravimetry. The thermal degradation characteristics of PEO remain unchanged upon PEG addition, while for all other investigated blends their values decreased, particularly at higher PVC content. Kinetic analysis of the non-isothermal degradation of investigated PVC/PEO/PEG blends was performed using isoconversional Friedman method in combination with the multivariate nonlinear regression method. The PEG addition lowers the values of the activation energies of PVC/PEO blends. The kinetic analysis indicated four-stage degradation mechanism for all investigated blends.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(vinyl chloride) (PVC) is one of the most important thermoplastics and has a wide applicability due to its good compatibility with a range of different additives. Because of today’s environmental requirements, it is necessary to reduce the amount of biologically non-degradable plastic waste. Hence, conventional additives for PVC, such as potentially harmful low molar mass compounds (phthalates) or polymeric modifiers (chlorinated polyethylene), can be replaced with non-toxic and biodegradable materials such as poly(ethylene glycol) (PEG) and poly(ethylene oxide) (PEO). PEG refers to an oligomer (molecular weight below 20,000), and PEO is polymer of ethylene oxide commercially available in the wide range of molecular weights (20,000–8,000,000) [1]. Besides the differences in molecular weight, PEG and PEO have different end groups; PEG has –OH and PEO has –CH3 end group. PEO is known as semicrystalline, biocompatible, biodegradable, non-ionic and water-soluble polymer of considerable industrial significance which finds applications in many different branches of industry [2]. Likewise, PEG is used as lubricant, intermediate, binder, solvent, carrier and coating in the cosmetic, pharmaceutical, paper, food, textile and chemical specialty fields [3]. Regardless PEG and PEO are used in different applications and have different physical properties (e.g., viscosity) due to chain length effect; their chemical properties are nearly identical.
Although the effect of PEG addition on the thermal properties of PVC blends has already been studied [4, 5], there is a lack of compliance. Pielichowski [4] added PEG to PVC blends, and through the increase of initial decomposition temperature and suppression of low molecular volatile products, emission concluded that PEG improves their thermal properties. However, Wu et al. [5] 10 years later utilized PEG for highly efficient dechlorination of PVC at atmospheric pressure. Latter authors confirmed the effects of PEG by comparing the thermal degradation behaviors of PVC and PVC/PEG blends, i.e., observing shift to lower values of the characteristic degradation temperatures of PVC (T 5%, T max).
Therefore, in this work, the influence of PEG on thermal properties of PVC/PEO blends was investigated by differential scanning calorimetry (DSC) and non-isothermal thermogravimetry (TG) in an inert atmosphere. The results of the non-isothermal thermogravimetry are often used for the determination of thermal stability of polymers and kinetic analysis [6]. Kinetic analysis aims to calculate kinetic parameters of the investigated process, i.e., the activation energy (E), the pre-exponential factor (A) and kinetic model (f(α)), the so-called kinetic triplet. Hence, additional goal of this study is to calculate the true kinetic triplets of the non-isothermal degradation of PVC/PEO/PEG blends. For this purpose, isoconversional Friedman (FR) method [7] in combination with the multivariate nonlinear regression method was used.
Experimental
Materials
The powders of PEG (M v = 3400), PEO (M v = 100,000) and PVC (M v = 86,000) were purchased from Sigma-Aldrich and Solvin, respectively. PVC/PEO blends of different compositions with the addition of 10 mass% PEG (100/0/10, 80/20/10, 60/40/10, 50/50/10, 40/60/10, 20/80/10 and 0/100/10) were prepared by mixing polymer powders in a laboratory extruder (Dynisco, Qualitest North America) at 160 °C and screw speed 180 rpm. The samples were hot-pressed after extrusion at 120 °C for 30 s. In order to prevent thermal degradation of PVC, during the sample preparation 2 mass% Ca/Zn stabilizer (Reapack B-NT/7060) was added. PVC/PEO/PEG blend 100/0/10 could not be prepared due to thermal degradation during extrusion.
Differential scanning calorimetry
The thermal characteristics of PVC/PEO/PEG blends were investigated by means of differential scanning calorimetry (Mettler Toledo DSC 823e) in the nitrogen atmosphere (50 cm3 min−1). The calibration was performed with metallic indium. For the determination of glass transition temperature (T g), the samples of approximately 20 mg were pressed in aluminum pans and heated at a rate of 20 °C min−1 from −90 to 120 °C, cooled at the same rate to −90 °C and reheated to 120 °C. The samples were kept at −90 and 120 °C for 10 min. The T g was determined from second heating cycle according to international standard ISO 11357-2 [8] as the extrapolated onset temperature (T eig), as midpoint temperature (T mg) and as the extrapolated end temperature (T efg). Corresponding change of the specific heat capacity (Δc p) was determined as well. The melting point of the samples of approximately 10 mg was determined from DSC curves obtained at the heating rate of 10 °C min−1 from 25 to 120 °C according to international standard ISO 11357-3 [9], as the extrapolated onset temperature (T eim), as peak temperature (T pm) and as the extrapolated end temperature (T efm). Corresponding heat of fusion (ΔH f) as the area under the peak to the baseline was determined as well.
Thermogravimetric analysis
Thermogravimetric measurements of the PVC/PEO/PEG blends were taken by using PerkinElmer Pyris-1 TGA thermobalance at the heating rates of 2.5, 5, 10 and 20 °C min−1 in a temperature range 50–650 °C under a steady flow of nitrogen (20 cm3 min−1). Samples weighing approximately 10 mg were used for the analysis. To evaluate the thermal stability of the investigated polymers, different criteria can be used. From thermogravimetric curves (TG) (mass versus degradation temperature) and corresponding derivative thermogravimetric curves (DTG) (mass loss rate versus temperature), the following characteristics were determined: the onset temperature (T onset), the temperature at 5 % mass loss (T 5%), the temperature at the maximum degradation rate (T max), the maximum degradation rate (R max), the conversion at the maximum degradation rate of (α max), the final mass (m f) and the mass loss (Δm) for the corresponding degradation steps.
Kinetic analysis
The non-isothermal TG data can be used for the kinetic analysis of the investigated process. Kinetic analysis of the solid-state reactions that are ruled by a single process is based on Eq. (1):
where α is the degree of conversion, β is the linear heating rate (°C min−1), T is the absolute temperature (K), R is the general gas constant (J mol−1 K−1), and t is the time (min). It is suggested that prior to any kinetic analysis, one should investigate the complexity of the process by determining the dependence of E on α by isoconversional methods [10–13]. Namely, this dependence is considered as reliable criterion of the process complexity and isoconversional methods are considered as the most reliable methods for the calculation of E and E versus α dependence of thermally activated reactions [10–13]. If E does not depend on α, the investigated process is simple (overall single stage) and can be described by unique kinetic triplet. Otherwise, the process is complex and the shape of the E versus α curve indicates the possible reaction mechanism [10–13]. Therefore, E values and E versus α dependence have been calculated by means of isoconversional method which can determine them without knowledge or assumption of kinetic model (model free). On the other hand, they do not give any information about A and f(α). Hence, in this study, the kinetic analysis was performed using model fitting multivariate nonlinear regression method. A series of reaction types is listed in Table 1. This list contains classic homogeneous reactions and typical solid reactions. In comparison with the known reaction types [12, 13], the list is extended with the combined autocatalytic types C1 and Cn [14]. The degradation kinetics parameters were evaluated by using the Netzsch Thermokinetics 3.1 software.
Isoconversional methods
Isoconversional methods enable determination of E directly from experimental α–T data (α = (m 0 − m)/(m 0 − m f), where m 0, m and m f refer to the initial, actual and residual mass of the sample obtained at several heating rates without the knowledge of f(α). Isoconversional Friedman (FR) method has been used. FR method is a linear differential method based on Eq. (2):
The plots ln[β(dα/dT)] versus 1/T obtained for α = const. from α–T curves recorded at several heating rates should be straight lines whose slopes allow calculation of E by means of FR method.
Results and discussion
Differential scanning calorimetry
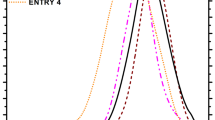
The thermal stability of each polymer in the blend can be significantly changed due to possible interaction of the polymer blend components and their degradation products. Differential scanning calorimetry is one of the techniques that can be utilized to study interaction in polymer blends by monitoring the shift of the characteristic transition temperatures. The results of the DSC investigation of PVC/PEO/PEG blends are shown as normalized DSC curves. The DSC heating curve of PVC/PEO/PEG blend 0/100/10 shows one glass transition temperature (T g) at −56 °C (T eig), −51 °C (T mg) and −45 °C (T efg) (Fig. 1) and one endotherm (T m) which represents the melting of the crystal phase of semicrystalline PEO at 60 °C (T eim), 68 °C (T pm) and 79 °C (T efm) (Fig. 2). The DSC curve characteristics of other investigated blends are shown in Table 2. The exception is PVC for which determination of T g in blends is not possible due to overlap with an latter endotherm. By increasing PVC content, T g of PEO remains unchanged, while Δc p decreases. Figure 2 shows the comparison of normalized DSC curve characteristics of PVC/PEO blends with and without PEG addition. Glass transition temperature of PEO expressed as T mg does not change by PEG addition, while for the blend 80/20/10 is increased by 4 °C toward T g of PVC (Fig. 2a). On the other hand, the melting temperatures of all investigated samples expressed as T eim and T pm remain unchanged, while T efm and ΔH m as well decrease by increasing PVC content (Table 2). By PEG addition, the melting temperatures of all investigated samples expressed as T eim decrease; therewith, the maximum reduction showed blends with a higher PVC content (Fig. 2b). The depression of temperatures of melting at higher PVC content (80 %) indicates that PVC/PEO/PEG system is only slightly miscible in the melt.
Thermogravimetric analysis
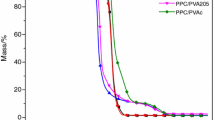
It was shown in our previous study [15] that the PVC and PVC/PEO blends degrade through two main stages, while the degradation of PEO occurs through one main degradation stage. The non-isothermal thermogravimetric curves and corresponding derivative thermogravimetric curves of PVC/PEO/PEG blends obtained at 2.5 °C min−1 are shown in Fig. 3a and b, respectively. Collected data from TG/DTG curves (Table 3) were used to assess the effect of PEG addition on the degradation pattern of PVC/PEO blends. PEO with PEG addition (0/100/10) exhibits only a single-stage degradation that occurs between 367 °C (T onset) and 450 °C. The peak temperature is observed at 388 °C, and the mass loss at the end of this stage is found to be 96.5 %. On the other hand, a two-stage degradation pattern is seen in the case of all PVC/PEO/PEG blends and occurs between 252 and 279 °C (T onset) in the first degradation stage and between 411 and 418 °C in the second degradation stage. As the PEO content is increased, the characteristic degradation temperatures of PVC/PEO/PEG blends (T 5%, T onset, T max) are increased, which is in accordance with our previous study [15]. The mass loss in the first degradation stage, Δm, increased linearly as the PEO content increased in the blend, while mass loss in the second degradation stage decreased. As expected, the final mass losses in the first and second stage decreased linearly as the PEO content increased.
Comparison of the thermal degradation characteristics of PVC/PEO blends with and without PEG addition at the heating rate of 2.5 °C min−1 (first degradation stage) is shown in Figs. 4 and 5. The characteristic degradation temperatures of PEO remain unchanged upon PEG addition, while for all other investigated blends the latter temperatures decreased, particularly at higher PVC content (Fig. 4). Other thermal degradation characteristics of PVC/PEO blends, i.e., R max, Δm and m f, upon PEG addition remain almost unchanged. Once again, exception of this pattern is sample with highest PVC content (80/20/10) which showed greater mass loss and smaller final mass than that of the PVC/PEO blend without PEG addition (Fig. 5). These results are consistent with the work of Wu et al. [5] where the addition of PEG shifted characteristic degradation temperatures of PVC to lower values and also resulted in higher mass loss which strongly proved a significant dechlorination degree of PVC. The fact that PVC sample with PEG addition (100/0/10) could not be prepared due to thermal degradation during extrusion confirms latter conclusions.
Comparison of the thermal degradation characteristics of PVC/PEO blends with and without PEG addition at the heating rate of 2.5 °C min−1 (first degradation stage): the temperature at 5 % mass loss (T 5%) (a), onset temperature (T onset) (b) and the temperature at the maximal rate of degradation (T max) (c)
The TG/DTG curves scanned at higher heating rates (5, 10 and 20 °C min−1) are similar to those at 2.5 °C min−1 and shifted to higher temperatures. At higher heating rates, polymer decomposition processes are more vigorously and consequently result to higher maximum rates of degradation.
Non-isothermal degradation kinetics analysis
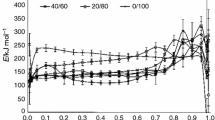
By using isoconversional FR method in combination with the multivariate nonlinear regression method, in our previous study [16] kinetic analysis of PVC/PEO blends indicated four-stage degradation mechanism, except for PVC sample which showed five-stage degradation mechanism. Collected data from non-isothermal thermogravimetric analysis of PVC/PEO/PEG blends were used to assess the effect of PEG addition on the degradation mechanism of investigated blends. Firstly, the dependence of E on α is established using FR method. For each selected α = const., the corresponding plots according to Eqs. (2) are obtained and from their slopes values of E are calculated. The dependence of E on α evaluated at whole conversion range by means of FR method is shown in Fig. 6. It can be concluded from Fig. 6 that E depends on α in a whole conversion range for all investigated samples, which may be result of complex mechanism of degradation. Since E depends on α, the process cannot be described adequately by a single reaction model and a single pair of Arrhenius parameters. It is advised to resort to a multistep kinetic analysis that would yield an individual reaction model and a pair of Arrhenius parameters for each of the reaction steps. Such an analysis can be accomplished by using the model fitting multivariate nonlinear regression method [12, 13]. The presence of two or more inflection points or maxima for all investigated samples (Fig. 6) indicates that degradation takes place at least in three main steps. Likewise, it can be concluded that the PEG addition lowers the values of the activation energies of PVC/PEO blends as shown for sample 80/20/10 in Fig. 7.
In our previous study [16], we have concluded that from the kinetic point of view PEO degradation can be adequately described by a four individual reaction models and pairs of Arrhenius parameters. Upon detailed examination of apparent activation energy of degradation of PEO with PEG addition calculated by Friedman method (Fig. 6), one can notice four areas with different E values. As expected, other investigated samples also showed four different areas of apparent activation energy (Fig. 6). To justify the occurrence of the four stages of the degradation, a deconvolution procedure described in details by Perejón et al. [17] has been performed. MagicPlot Pro computer program and different fitting functions (Gaussian and Lorentzian) have been used for the deconvolution process. The fitting results for the two selected samples (50/50/10 and 0/100/10) are given in Fig. 8. Figure 8 shows experimental DTG curves (circles) at 2.5 °C min−1, fitted (dotted) and cumulative (solid lines) curves. Cumulative curves are obtained by the summation of individual fitting curves. It is obvious from Fig. 8a that PEO (sample 0/100/10) DTG curve can be well fitted with four functions whose cumulative curve fits experimental one, supporting the above-mentioned conclusion that its thermal degradation can be adequately described by a four individual reaction steps. Similar results are obtained for other PVC/PEO/PEG blends (Fig. 8b) and support earlier indications obtained by Friedman method.
In order to find the most probable kinetic model of thermal degradation of PVC/PEO/PEG blends, the multivariate nonlinear regression as the method of analysis was employed. Analysis was based on the results of the activation energy from Friedman method as well as on the shape and slope of experimental points and isoconversional lines from Friedman plots. In case of multistep reactions, only few can be said about the reaction type of the individual reaction steps. For the start reaction, information can be gained by means of the Friedman analysis. If the experimental points at the start of the reaction (α = 0.02–0.10) show a lower slope than the isoconversion lines, this is a indication of the presence of a diffusion reaction. On the other hand, if the experimental points at the start of the reaction have a steeper slope than the isoconversional lines, this is a indication of the presence of an autocatalytic activated reaction (reaction type C) [18]. Applying the statistical criteria, F test and correlation coefficient, and especially similarity of obtained E with E calculated by Friedman method made it possible to find best fit of the f(α) function that describes kinetic model of degradation. Table 4 shows apparent activation energy values obtained by the FR method for each degradation stage. The results of calculations for the most probable kinetic models on the basis of F test and correlation coefficient are summarized in Table 5. Finally, Fig. 9 shows the comparison of experimental points and curves calculated from multivariate nonlinear regression for the PVC/PEO/PEG blends 80/20/10, 50/50/10 and 0/100/10.
The best fit between the experimental data and the assumed kinetic models for PEO sample (0/100/10) was obtained for the four-stage degradation mechanism with consecutive reactions A → B → C → D → E. Since the remaining mass of the samples does not depend on the heating rate (Fig. 9), multistep unbranched reactions are present [18], in this case with consecutive reactions as well as PEO without PEG addition [16]. For investigated PVC/PEO/PEG blends, the best fit between the experimental data and the assumed kinetic models was also obtained for the four-stage degradation mechanism (Fig. 9a, b), which was confirmed by the high correlation coefficient above 0.9998. The apparent activation energy of the first stage of thermal degradation of investigated PEO (Fig. 6) amounts in the range 0.0–146.0 kJ mol−1 and reveals diffusion character (D4). Likewise, the apparent activation energy of thermal degradation of investigated PVC/PEO/PEG blends amounts in the range 85.3–105.4 kJ mol−1 and reveals diffusion character (D4) with high probability (Table 4). Exception of this pattern is sample with highest PVC content (80/20/10) which may be described by the reaction of n-th order with autocatalysis (Cn). In our previous study [16], all investigated PVC/PEO blends in the first stage of thermal degradation revealed diffusion character (D4). In this case, PEG addition evidently altered probable degradation mechanism from D4 to Cn at this stage. This is consistent with the conclusions made by the differential scanning calorimetry and thermogravimetric analysis and strongly proves that PEG addition enhances dechlorination degree of PVC. Regarding the autocatalytic effect of the Cn model, in our previous study [16], we described the thermal degradation of PVC sample and PVC/PEO blends with higher PVC content by the reaction of n-th order with autocatalysis (Cn). These results are in accordance with the well-known fact that dehydrochlorination process (most important successive stage of the PVC degradation) is described as fast, autocatalytic reaction. In our opinion, as we concluded in our previous study [16], Cn kinetic model reasonably describes dehydrochlorination of PVC and PVC/PEO blends with higher PVC content as well, both mathematically and functionally. Similar conclusion can be made by this study in which it was demonstrated that PEG addition evidently enhanced dechlorination degree of PVC in the PVC/PEO blends (autocatalytic activated reaction). The apparent activation energies obtained by Friedman method indicated that the first stage of the degradation of all others samples might occur by diffusion mechanism. At the first stage, at low conversion degrees on Friedman plot, the experimental points showed a lower slope than the isoconversion lines, which is in accordance with results of nonlinear regression. At the beginning of degradation, there is a solid or high viscosity melt system and the mass transfer processes became rate determining. Hence, the decomposition products must diffuse to the surface to be evaporated and diffusion of the volatile products toward the surface is rate-controlling process for the first stage [19]. In case of sample with highest PVC content (80/20/10), autocatalytic dehydrochlorination process of PVC became rate-controlling process for the first stage.
The second stage of thermal degradation of PEO sample with PEG addition may be described by Avrami–Erofeev reaction type (An) with higher E value which amount in the range 194.0–205.6 kJ mol−1. These results are in accordance with the literature [16, 20] where the An is considered to be the best suited kinetic model for the description of thermal degradation of PEO. Furthermore, the second stage for the PVC/PEO/PEG blends with highest PEO content (40/60/10 and 20/80/10) may be described by the An reaction type which corresponds to the thermal degradation process of PEO, while samples with the highest PVC content (50/50/10, 60/40/10 and 80/20/10) may be described by reaction of n-th order with autocatalysis (Cn) which corresponds to the dehydrochlorination process of PVC. The apparent activation energies of this stage are slightly larger and amount in the range 95.4–143.4 kJ mol−1. Evidently, as the PEO content increased the probable mechanism changed from the reaction of n-th order with autocatalysis (Cn) to the Avrami–Erofeev reaction type (An). These results are consistent with our previous work [16], except for sample 40/60/10 where PEG addition changed probable mechanism from the Cn to the An reaction type (Table 4).
The apparent activation energy of the third stage of thermal degradation of investigated PEO sample with PEG addition amounts in the range 181.6–192.0 kJ mol−1 and can be described by the reaction of the Avrami–Erofeev reaction type (An). However, the third stage of thermal degradation of PVC/PEO/PEG blends occurs by the Avrami–Erofeev reaction type (An) and by the reaction of n-th order with autocatalysis (Cn). If compared to our previous study [16], PEG addition randomly changed probable mechanism of thermal degradation of PVC/PEO blends as follows: samples 50/50/10 and 40/60/10 changed from the An to the Cn reaction type, sample 20/80/10 changed from the Cn to the An reaction type, while probable mechanism of samples 80/20/10 and 60/40/10 remains unchanged (Table 4). The apparent activation energies of this stage are in the range of 117.3–165.9 kJ mol−1 and are higher if compared to the previous stage.
The final fourth stage of thermal degradation of PVC/PEO/PEG blends is described by the reaction of n-th order (Fn) with the apparent activation energies in the range 36.6–231.6 kJ mol−1 (Table 4). This is in accordance with our previous work [16]. However, the final stage of thermal degradation of PEO sample with PEG addition occurs by the Avrami–Erofeev reaction type (An) with the apparent activation energy in the range 0.0–205.0 kJ mol−1. The Avrami–Erofeev coefficient of thermal degradation of PEO sample at this stage amounts 0.5 (Table 4), and it is identical to the referent value (n = 0.50; A0.5) [21]. This fact would support the assumption that the PEG addition to the PEO sample altered the final stage of thermal degradation which now takes place through a mechanism of nucleation and growth of nuclei by a diffusion-controlled process. If compared to the previous stages, latter stage showed the highest apparent activation energy values. Higher values could be accounted by the higher collision frequencies at the higher degradation temperatures [22]. Finally, the comparison of apparent activation energy values obtained by isoconversional methods and by multivariate nonlinear regression method for all degradation stages confirmed the fit of most probable kinetic models (Tables 4 and 5).
Conclusions
The influence of PEG addition on thermal characteristics and thermal degradation of PVC/PEO blends was investigated by differential scanning calorimetry and non-isothermal thermogravimetry in an inert atmosphere. Characteristic transition temperatures, i.e., T g and T m, of PEO sample do not change by PEG addition, while for the PVC/PEO blends, T g values increased and T m values decreased with higher PVC content. PEO sample with PEG addition exhibits only single-stage degradation, while a two-stage degradation pattern is seen in the case of all PVC/PEO/PEG blends. The thermal degradation characteristics of PEO remain unchanged upon PEG addition, while for all other investigated blends, their values decreased, particularly at higher PVC content, which is in accordance with literature where the addition of PEG shifted characteristic degradation temperatures of PVC to lower values and also resulted in higher mass loss.
In order to find the most probable kinetic model of thermal degradation of PVC/PEO/PEG blends, isoconversional Friedman method in combination with the multivariate nonlinear regression as the method of analysis was employed. The kinetic analysis indicated four-stage degradation mechanism with consecutive reactions which was confirmed by the high correlation coefficient. The apparent activation energy of the first stage of thermal degradation of investigated samples revealed diffusion character (D4). Exception of this pattern is sample with highest PVC content (80/20/10) which may be described by the reaction of n-th order with autocatalysis (Cn). The second stage of thermal degradation of PEO sample with PEG addition and blends with highest PEO content (20/80/10 and 40/60/10) may be described by the An reaction type, while samples with the highest PVC content (50/50/10, 60/40/10 and 80/20/10) may be described by Cn reaction type. The third stage of thermal degradation of investigated blends occurs by the An and Cn reaction type. If compared to our previous study, PEG addition changed probable mechanism of thermal degradation of PVC/PEO blends from the Cn to the An reaction type and vice versa. Exceptions of this pattern are samples 80/20/10 and 60/40/10 whose probable mechanisms remain unchanged. The final fourth stage of thermal degradation of PVC/PEO/PEG blends is described by the reaction of n-th order (Fn), except for the PEO sample with PEG addition which occurs by the An reaction type. The final stage showed the highest apparent activation energies. These values are within the range of the results determined by employed isoconversional Friedman method.
References
Kahovec J, Fox RB, Hatada K. Nomenclature of regular single-strand organic polymers. Pure Appl Chem. 2002;74:1921–56.
Pielichowski K, Flejtuch K. Non-oxidative thermal degradation of poly(ethylene oxide): kinetic and thermoanalytical study. J Anal Appl Pyrol. 2005;73:131–8.
Sheth M, Kumar RA, Dave V, Gross RA, McCarthy SP. Biodegradable polymer blends of poly(lactic acid) and poly(ethylene glycol). J Appl Polym Sci. 1997;66:1495–505.
Pielichowski K. Thermal energy storage systems based on poly(vinyl chloride) blends. Eur Polym J. 1999;35:27–34.
Wu Y-H, Zhou Q, Zhao T, Deng M-L, Zhang J, Wang Y-Z. Poly(ethylene glycol) enhanced dehydrochlorination of poly(vinyl chloride). J Hazard Mater. 2009;163:1408–11.
Patrutescu C, Vlase G, Turcus V, Ardelean D, Vlase T, Albu P. TG/DTG/DTA data used for determining the kinetic parameters of the thermal degradation process of an immunosuppressive agent: mycophenolate mofetil. J Therm Anal Calorim. 2015;121:983–8.
Friedman HL. Kinetic of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic resin. J Polym Sci C. 1963;36:183–95.
ISO 11357-2: 2009 plastics—differential scanning calorimetry (DSC)-Part 2: determination of glass transition temperature.
ISO 11357-3: 2009 plastics—differential scanning calorimetry (DSC)—part 3: determination of temperature and enthalpy of melting and crystallization.
Vyazovkin S, Lesnikovich AI. An approach to the solution of the inverse kinetic problem in the case of complex processes: part 1. Methods employing a series of thermoanalytical curves. Thermochim Acta. 1990;165:273–80.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Sunol JJ. ICTAC Kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Opfermann J. Kinetic analysis using multivariate non-linear regression: I. Basic concepts. J Therm Anal Calorim. 2000;60:641–58.
Jakić M, Vrandečić NS, Klarić I. Thermal degradation of poly(vinyl chloride)/poly(ethylene oxide) blends: thermogravimetric analysis. Polym Degrad Stab. 2013;98:1738–43.
Jakić M, Vrandečić NS, Erceg M. Kinetic analysis of the non-isothermal degradation of poly(vinyl chloride)/poly(ethylene oxide) blends. J Therm Anal Calorim. 2016;123:1513–22.
Perejón A, Sánchez-Jiménez PE, Criado JM, Pérez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2011;115:1780–91.
NETZSCH Thermokinetics 3.1, Netzsch-Geraetebau GmbH, Software Manual.
Byczynski L. Thermal degradation studies of poly(urethane-siloxane) thermosets based on co-poly(dimethyl)(methyl, 3-glycidoxypropyl)siloxane and epoxy-terminated urethane oligomer. Thermochim Acta. 2014;592:58–66.
Vrandečić NS, Erceg M, Jakić M, Klarić I. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochimic Acta. 2010;498:71–80.
Sestak J. Thermophysical properties of solids: their measurements and theoretical thermal analysis. Amsterdam: Elsevier; 1984.
Radhakrishnan Nair MN, Thomas GV, Gopinathan Nair MR. Thermogravimetric analysis of PVC/ELNR blends. Polym Degrad Stab. 2007;92:189–96.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jakić, M., Vrandečić, N.S. & Erceg, M. The influence of poly(ethylene glycol) on thermal properties of poly(vinyl chloride)/poly(ethylene oxide) blends. J Therm Anal Calorim 127, 663–674 (2017). https://doi.org/10.1007/s10973-016-5768-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5768-0