Abstract
The characterization of radiation stability of polyurethane nanocomposites by chemiluminescence and optical spectroscopies (FTIR and UV–VIS) is presented. Samples consisting of polyurethane and various percentages of polyhedral oligomeric silsesquioxane (POSS), namely 0, 2, 4, 6, 8 and 10 mass% loading, were aged by γ(137Cs)-irradiation. The polyurethane composites with 2 and 4 mass% POSS have presented slower rate of degradation in comparison with unmodified polymer. The consequences of the increase POSS loading are analyzed starting from the structural configuration of POSS which allows the scavenging of free radicals formed in polymer phase. The received moderate doses (10 and 20 kGy) like a sterilization procedure at a dose rate of 0.4 kGy h−1 brought significant changes in the thermal behavior of our hybrid composites, splitting the formulations into two groups: one group is characterized by the increasing thermal stability, while the second group presents an advancing oxidative degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On the last period considerable attention has been paid to the synthesis [1–5] and characterization [6–10] of polyurethanes because of the multitude of synthesis routes and the expending operation ranges. The application areas of polyurethanes (flexible or rigid foams, coatings, sealants, adhesives or thermoplastic items—in particular automotive, aircraft and biomedical products) for the manufacture of long-term products have been continuously enlarged due to their satisfactory stability and stress resistance [11–19]. Polyurethanes are characterized by –NHCO–O– units, which generate various decomposition products previously reported [20]. The mechanism of their formation follows a three stage process (Fig. 1). The scission of macromolecules is caused not only by the energetic depositing, but also by the attack of free radicals on the methylene group in the neighborhood of urethane moieties [21]. Spectral investigations on the photodegradation of poly(ether urethanes) [22] confirm the presented approach of the degradation progress in polyurethanes. The long-term service of medical wear subjected to several degrading factors requires the improvement in the stability of polyurethanes. The modification of polyurethane matrix with polyhedral oligomeric silsesquioxane (POSS) offers numerous utile alternatives for the enlargement of various application areas such as scaffolds, catheters, blood vessels and drug delivery membranes [23].
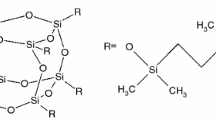
The general formula of POSS, [R(SiO)3/2]n, is associated with a large molecule size (about 25 Å on axis direction) in comparison with average dimension of polyurethane hard domain [24]. The structural configuration of POSS allows to be penetrated by free radicals acting as efficient scavenging cage. A detailed study on the physical and electrical characteristics of PU/POSS nanocomposites was already published [25] emphasizing the modifications induced by POSS nanophase by the interaction with basic polyurethane.
Several other polymer materials modified with POSS were qualified for various applications, because this nanofiller ameliorates their functional features. Different types of polymers modified with POSS were studied: high density polyethylene [26], polypropylene [27, 28], polystyrene [29], polyethylene terephthalate [30], polycarbonate [31] and many others. The similar effect in the increasing the usage temperature, stability to oxidation, mechanical properties of host material is based on the sorption activity of nanofiller [32].
The radiation effects on POSS composites were scarcely investigated. Choi et al. [33] managed the grafting of POSS particles on polypropylene by γ-exposure and the decomposition temperature decreased with about 10 %, while de-elasticity of composed samples increased sharply. Gorna and Gogolewski [34] have demonstrated that radiation treatment applied to polyurethane/POSS hybrid composites generates materials properly used for cardiovascular implants, artificial skin, pericardial patched and scaffolds for repair of articular cartilage because of their adequate compliance, fatigue resistance and sufficient tear and burst strengths. In the circumstances of firing or ignition, when the oxidative degradation of polyurethanes is accelerated by the reaching local high temperatures, an efficient stability can be obtained by the modification of basic material with nanoclay [35, 36]. For the delaying of degradation induced by light stressing activity, the addition of antioxidant is also a proper solution [37].
The thermal and radiation stabilization of polymers can be easy obtained by the presence of antioxidant additives [38, 39] by scavenging effect. The resistance of polyurethanes to the oxidation by ignition can be efficiently improved by the addition of flame retardants like ammonium polyphosphate [40] and these types of intumescent polyurethane foam systems may be used where potential fire events would be imminent.
This paper presents the radiation stability of polyurethane/polyhedral oligomeric silsesquioxane hybrid composites starting from previous studies on radiochemistry of polyurethanes [41] and thermal stability of these nanosystems [42]. Complementary determinations by optical spectroscopies and chemiluminescence prove the stabilization potential of POSS during accelerated degradation promoted by high-energy radiation.
Materials and methods
The basic polyurethane matrix was prepared starting from 4,4′-methylenebis(phenylisocyanate) and poly(tetramethyleneglycol) as is has been previously reported [43]. Nanocomposites were obtained by the substitution of chain extender with 1,2-propanediol-heptaisobutyl-POSS. The loading polyhedral oligomeric silsesquioxanate of each sample is listed in Table 1.
The radiochemical aging was performed in an irradiation machinery (Gammator M-38) provided with 137Cs source by exposure in air at room temperature. The dose rate was 0.4 kGy h−1. Because the γ-irradiated metals provide energetic electrons (δ-electrons) as the result of interaction of incidental radiation with material sheet, during γ-irradiation our composite samples were covered with aluminum thin foil for more efficient energetic transfer. In this manner the energy received by polymer samples is maximized; otherwise, the samples would absorb only a small part of dissipated energy from electromagnetic rays. The total doses are placed on the medium dose range up to 100 kGy, but the most relevant effect of stabilization was observed at 10 and 20 kGy). The measurements were carried out immediately after the end of irradiation. For avoiding the errors brought about by sample preparation, the irradiation procedure by accumulation was applied.
Chemiluminescence (CL) determinations were carried out with LUMIPOL 3 (Slovakia) equipment. The measurement temperatures for isothermal regime were carefully selected ensuring convenient degradation rate. The applied chemiluminescence procedure for isothermal measurements was previously reported [44]; the nonisothermal experiments were carried out according with the method described by Rychlý et al. [45]. This equipment maintains constant temperature with an error of ±0.2 °C for the accomplishment of isothermal measurements, while the rate of heating for nonisothermal assays was established as optimal value of 2 °C min−1. The activation energies, Ea, were calculated by Arrhenius method at three temperatures around central values. The first set of Ea was evaluated in the range of 98, 100 and 102 °C, while the second and the third sets used the oxidation rates measurements were done at 138, 140 and 142 °C. Although the temperatures are closed to each other, the rates were enough different to allow the accurate calculation of activation energies for oxidation of polyurethane samples (supplementary documents).
Optical spectroscopies (FT-IR and UV–VIS) were performed on JASCO 4200 and JASCO V570 spectrophotometers, respectively. For FT-IR investigation, the vibration band between 3350 and 3650 cm−1, which corresponds to bond hydroxyls, while UV–VIS records were performed on the spectral region from 400 to 300 nm, which is ascribed to a n − π* transition in ketone structures. The successive records for increasing dose were done on the same point of each sample avoiding the involvement of any thickness deviation.
Results and discussion
The polyurethane-based POSS nanocomposites are pendant on the relative proportion of components. The evidence on microphase morphology in POSS phase [24] brings about the problem of interaction degree between sample constituents, which determines the cage effect on small molecule products generated during degradation. The main advantage of the filler presence consists of the control on diffusion process, either for molecular oxygen that penetrates material or for the scavenging of the intermediates of degradation. It must be taken into account the energetic effect of phenyl rings that act as energy-depositing structural unit where excitation is retained for a long time [46]. Apart from the structural stability conferred by geometrical configuration, the phenyl content is an additional factor that increases the radiation stability of POSS.
Optical spectroscopy
Even though composite films were thick enough for transmission measurement, UV–VIS and FT-IR records have revealed modifications in the accumulation of degradation products occurred during radiolysis because two degradation agents (high-energy radiation and oxygen) induce combined effects.
Figure 2 illustrates the increase in the hydroxyl content due to the simultaneous actions of γ-radiation (scission of bonds) and diffused oxygen (oxidation reactions involving free radicals) according with the mechanism of oxidative degradation. The evolution of the ratios of transmission at each dose divided by initial transmission value for aged PU/POSS samples which were subjected to the hard action of γ-rays is shown in Fig. 3. This bonded-hydroxyl group accumulation which seems to follow quasi-linear dependency on irradiation dose depicts the formation of this type of function directly from peroxyl intermediates. The increase in the absorbance values of neat polyurethane at 320 nm (Fig. 4) proves the accumulation of carbonyl-containing degradation products, which determines the variation of absorbances in this spectral region for polyurethane/POSS hybrids (Fig. 5). The continuous augmentation of relative absorbance figures as destructive energy is transferred on polymer samples is the consequence of the scission occurred in polyurethane molecules. The differences in the oxidation level indicate the contribution of dispersed POSS nanoparticles to the propagation of oxidation during irradiation.
Absorption ratios (A D/A 0) for PU/POSS composites recorded at 320 nm. The meaning of coloring is the same as in Fig. 3
According to ESR study, the formation of primary radical >Ċ(NCO) initiates the addition of molecular oxygen to form peroxyl moieties [9]. Their amounts depends on the irradiation dose because of the increased scission number at increasing dose and on the POSS loading because it acts as the adsorbent of intermediates.
Chemiluminescence
The thermal degradation of neat polyurethane advances differently, if the applied temperature is increasing (Fig. 6). At medium temperature (100 °C), oxidation takes place smoothly that does not present a well-defined oxidation induction time, because the oxidation process progresses at low rate. At higher temperature (140 °C) one large peak appears which describe the scissions of polyurethane segments. According with the mechanism presented in Fig. 1 and with the photodegradation mechanism of polyurethanes and with the photodegradation mechanism of polyurethanes [47], the radicals can appeared either by the breaking of urethane units or by the scission of vicinal methylene groups. At 140 °C, when the energetic transfer is more intensive, the second peak appears demonstrating the formation of double bonds by releasing carbon dioxide. By enhancing degradation temperature, the main peak becomes more conspicuous and shifted toward shorter appearance times.
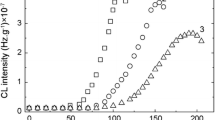
The scission rate that characterizes the organic component is modified by the presence of POSS nanoparticles. At 100 °C the filler nanoparticles act as scavengers for degradation initiators (Fig. 7a). The continuous descendant CL intensity is the proof for the subtraction of oxidizing intermediates, which are prevented to react with oxygen being locked inside POSS geometry. The increase in temperature at 120 °C, the differences between the developments of oxidation in the six PU/POSS composites appears (Fig. 7b). The samples containing 2 and 4 mass% POSS present higher thermal stability relative to the neat polyurethane. The other three POSS loadings induce faster oxidation than pristine material. It can be supposed that an equilibrium adsorption/desorption of radicals is turned onto the expelling the covalent inclusions. If the samples with low POSS content are slightly oxidized over 120 min, the samples incorporating higher amounts of POSS present an accelerated degradation on the first 40 min followed by a pseudo-steady-state progress of oxidation. The sample with 10 % POSS has a similar behavior as neat PU so that it may be suppose that on the surface of filler nanoparticles the uptake and release rates are similar.
The contribution of nanocomponent to the stability of PU/POSS hybrids is illustrated by the variation of CL intensity emitted on the first minute of isothermal investigation. This value, which depicts the initial concentration of radicals, increase from neat polyurethane to the sample containing 6 mass% POSS, followed by the significant diminution (Fig. 8). Even different irradiation doses (0, 10 and 20 kGy) are applied, the degradation level is intensified, and the shape of variation curves remains the same. In all the cases, the most instable system is the POSS nanocomposite in which the filler concentration is 6 mass%.
The accelerated degradation in organic substrate from PU/POSS nanocomposites during γ-irradiation proceeds with different rates depending on filler content (Fig. 9). If at 10 kGy the decrease in the CL intensities happens similarly starting from corresponding to initial higher emissions (Fig. 8), a constant degradation rate is attained after 10 min from the start of heating. The increase in dose at 20 kGy modifies the behavior of sample containing 6 mass% POSS. It is considerably degraded during γ-exposure, when it receives a large amount of energy becoming quite susceptible to oxidation. The order of stability established for unirradiated studied systems is maintained. The oxidative degradation is initiated in polyurethane and POSS nanofiller can modified the rate of process by modification of reacting entities as the result of surface interaction. The evolution of oxidation state in PU/POSS samples is also related on the modification in the length of polyurethane molecules according with the proposed mechanism and previously reported data [37].
Isothermal CL spectra measured at 100 °C on hybrid samples irradiated at a 10 kGy and b 20 kGy. The meaning of signs is the same as in Fig. 7
The same sequence of thermal stability is obtained by nonisothermal chemiluminescence determinations. The oxidation starts in polyurethane/POSS composites at the temperature around 130 °C, except in sample with 6 % filler, where oxidative degradation begins before attaining 100 °C (Fig. 10). The CL emission intensities at elevated temperatures are moderate, which suggests the relative thermal stability at these temperatures.
Nonisothermal CL spectra of PU/POSS samples. The meaning of signs is the same as in Fig. 7a
The accelerated degradation by γ-irradiation of polyurethane/POSS hybrids is the result of the influence of inorganic particles of hard and soft blocks of elastomer. The influence of filler content is revealed by the restriction of oxidation at low POSS content and the acceleration of degradation for the concentration exceeding 6 mass%. Actually, the chemiluminescence measurements can characterize the interaction of the two components in order to evaluate the influence of filler concentration on the material stability (Table 2).
The scavenging action of POSS structure is well understood by the activation energy figures which vary in the direct connection with the free interstitial volume and the allowed amount of intermediates temporary captured in the octahedral configuration (Table 3). According with the oxidative degradation mechanism, the nanoparticles of POSS retain free organic radicals in a certain proportion, which attains saturation at about 4 %. The exceeding POSS loading diminishes the thermal stability of studied hybrid systems because the accumulation of hosted entities does not allow the undefined amount. The increase in the degradation temperature involves higher concentrations of absorbing entities determining a competition for space occupation and, indeed, higher activation energies (Table 3). When the oxidation is on the terminal step, the degradation products are highly accumulated and the hindering each other controls the oxidation level. The POSS nanoparticles play the role of absorbent, which acts slower and slower when the amount of intermediates exceeds a certain quantitative limit.
Conclusions
The PU/POSS nanocomposites with different compositions present selective stabilities in respect with filler loading. Although the thermal stability differentiates studied systems, the slow oxidation rates in low POSS content formulations is obtained in comparison with pristine polymer. The sequence of stability levels obtained by either isothermal or nonisothermal chemiluminescence places nest polyurethane between improved materials (PU modified with 2 and 4 mass% POSS) and less stable composites containing 6, 8 and 10 mass% POSS. Even though γ-irradiation modifies the molecular length of elastomer, the behavior of irradiated these nanocomposites reflects the contribution of filler to the initiation and progress of oxidative degradation in polymer component. The relevant stability of low POSS content in irradiated materials recommends them for the preparation of various high-performance items, which are subjected to accelerated degradation.
Due to the absorbent feature of POSS nanoparticles, these systems can be preferentially applied in the medical areas, manufacture of scaffolds, carriers of drugs, covering layers for injuries and many other purposes, including pharmaceutics. The assessment of oxidation strength by the application of γ-irradiation for accelerated degradation allows the correct qualification of products. The present results recommend the extension of utilization purposes as absorbent of radioactive contamination in the radiation chemistry laboratories or nuclear power plants (outer “clean” areas).
References
Jia QM, Zheng M, Chen HX, Shen RJ. Synthesis and characterization of polyurethane/epoxy intercalating network nanocomposites with organoclays. Polym Bull. 2005;54:65–73. doi:10.1007/s00289-005-0372-7.
Paciorek-Sadowska J, Czupryński B. New compounds for production of polyurethane foams. J Appl Polym Sci. 2006;102:5918–26. doi:10.1002/app.25093.
Pereira IHL, Ayres E, Patrío PS, Góes AM, Gomide VS, Eduardo P Jr, Oréfice RL. Photopolymerization and injectable polyurethanes for medical applications. Synthesis and biocompatibility. Acta Biomater. 2010;6:3056–66. doi:10.1016/j.actbio.2010.02.036.
Zou XW, Qin TF, Wang Y, Huang LH, Han YM, Li Y. Synthesis and properties of polyurethanes foams prepared from heavy oil modified by polyols with 4,4′-methylene-diphenylene isocyanate. Bioresour Technol. 2012;114:654–7. doi:10.1016/j.biortech.2012.03.030.
Madbouly SA, Otaigbe JU. Recent development in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films. Prog Polym Sci. 2009;34:1283–332. doi:10.1016/j.progpolymsci.2009.08.002.
Yildiz B, Özgür-Seydibeyoglu M, Seniha-Güner F. Polyurethane-zinc borate composites with high-oxidation stability and flame retardancy. Polym Degrad Stab. 2009;94:1072–5. doi:10.1016/j.polymdegradstab.2009.04.006.
Fernández CE, Bermúdez M, Versteegen RM, Meiji EW, Vancso GJ, Munoz-Guerra S. An overview of 12-polyurethane: Synthesis, structure and crystallization. Eur Polym J. 2010;46:2089–98. doi:10.1016/j.eurpolymj.2010.09.018.
Chou CW, Hsu SH, Chang H, Tseng SM, Lin HR. Enhanced thermal and mechanical properties and biostability of polyurethane containing silver nanoparticles. Polym Degrad Stab. 2006;91:1017–24. doi:10.1016/j.polymdegradstab.2005.08.001.
Przybytniak G, Kornacka E, Ryszkowaska, Bil M, Rafalski A, Woźniak P, Lewandowska-Szumiel M. Influence of radiation sterilization on poly(ester urethanes) designed for medical applications. Nukleonika. 2006;51(Suppl 1):S121–8.
Prisecariu C, Scortanu E, Agapie B. Synthesis and characterization of dibenzyl based polyurethane blends obtained via the one shot synthesis route. Proc Eng. 2011;10:984–9. doi:10.1016/j.proeng.2011.04.162.
Yang XF, Vang C, Tallman DE, Bierwagen GP, Croll SG, Rohlik S. Weathering degradation of a polyurethane coating. Polym Degrad Stab. 2001;74:341–51. doi:10.1016/j.polymdegradstab.2010.08.016.
Grad S, Kupscik L, Gorna K, Gogolewski S, Alini M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24:5163–71. doi:10.1016/S0142-9612(03)00462-9.
Park CH, Chang H, Minn KW, Park CY. Development of thermoplastic polyurethanes vascular prosthesis. J Appl Polym Sci. 2008;110:3267–74. doi:10.1002/app.28800.
Rosu D, Varganici C-D, Rosu L, Mocanu (Păduraru) M. Thermal degradation of thermosetting blends. In: Visakh PM, Arao Y, editors. Thermal degradation of polymer blends, composites and nanocomposites. Engineering materials. Switzerland: Springer Int. Pub; 2015. pp. 17–49. doi:10.1007/987-3-319-03464-5_2.
Kang JM, Erdodi G, Brendel CM, Ely D, Kennedy JP. Polyisobutylene-based polyurethanes. V. Oxidative-hydrolytic stability and biocompatibility. J Polym Sci Part A Polym Chem. 2010;48:2194–203. doi:10.1002/pola.23989.
Malikova M, Rychlý J, Matisová-Rychlá L, Csomorová K, Janiková I, Wilde H-W. Assessing the progress of degradation in polyurethanes by chemiluminescence. I. Unstabilised polymer films. Polym Degrad Stab. 2010;95:2367–75. doi:10.1016/j.polymdegradstab.2010.08.016.
Pârvu R, Podină C, Zaharescu T, Jipa S. Stability evaluation of polyurethane coatings by gamma irradiation. Optoelectr Adv Mater Rapid Commun. 2010;4:1815–8.
Rychlý J, Latuatti-Derieux A, Lavédrine B, Matisová-Rychlá L, Csomorová K, Malikova M, Csomorová K, Janiková I. Assessing the progress of degradation in polyurethanes by chemiluminescence. II. Flexible polyether- and polyester-type polyurethane foams. Polym Degrad Stab. 2011;96:462–9. doi:10.1016/j.polymdegradstab.2011.01.012.
Mondal S, Martin D. Hydrolytic degradation of segmented polyurethane copolymer for biomedical applications. Polym Degrad Stab. 2012;97:1553–61. doi:10.1016/j.polymdegradstab.2012.04.008.
Zhang YH, Xia ZB, Huang H, Chen HQ. Thermal decomposition of polyurethanes based on IPDI. J Anal Appl Pyrol. 2009;84:89–94. doi:10.1016/j.jaap.2008.11.008.
Fratričová M, Šimon P, Schwarzer P, Wilde H-W. Residual stability of polyurethane automotive coatings measured by chemiluminescence and equivalence of Xenotest and Solisi ageing tests. Polym Degrad Stab. 2006;91:94–100. doi:10.1016/j.polymdegradstab.2005.04.025.
Mendikute G, Irusta L, Fernandez-Berridi MJ. Infrared study of the photochemical behaviour of aromatic poly(ether urethanes): effect of various stabilizers. e-Polymer. 2009;. doi:10.1515/epoly.2009.9.1.1489.
Pielichowski K, Njuguna J, Janowski B, Pielichowski J. Polyhedral oligomeric silsesquioxane (POSS)—containing nanohybrid polymers. Adv Polym Sci. 2006;201:225–96. doi:10.1007/12_077.
Fu BX, Hsiao BS, White H, Rafailovich M, Mather PT, Jeon HG, Phillips S, Lichtenhan J, Schwab J. Nanoscale reinforcement of polyhedral oligomeric silsesquioxane (POSS) in polyurethane elastomer. Polym Int. 2000;49:437–40. doi:10.1002/(SICI)1097-0126(200005)49:5<437:AID-PI239>3.0.CO;2-1.
Raftopoulos RN, Pandis Ch, Apekis L, Pissis P, Janowski B, Pielichowski K, Jaczewska J. Polyurethane-POSS hybrids: molecular dynamics studies. Polymer. 2010;51:709–18. doi:10.1016/j.polymer.2009.11.067.
Joshi M, Butola BS. Studies on nonisothermal crystallization of HDPE/POSS nanocomposites. Polymer. 2004;45:4953–68. doi:10.1016/j.polymer.2004.04.057.
Rios-Dominguez H, Ruiz-Treviño FA, Contreras-Reyes R, Gonzáles-Montiel A. Synthesis and evaluation of gas transport properties in polystyrene-POSS membranes. J Membr Sci. 2006;271:94–100. doi:10.1016/j.memsci.2005.07.014.
Fina A, Tabuani D, Frache A, Camino G. Polypropylene-polyhedral oligomeric silsesquioxanes (POSS) nanocomposites. Polymer. 2005;46:7855–66. doi:10.1016/j.polymer.2005.06.121.
Zhou ZY, Cui LM, Zhang Y, Zhang YX, Yin NW. Preparation and properties of POSS grafted polypropylene by reactive blending. Eur Polym J. 2008;44:3057–66. doi:10.1016/j.eurpolymj.2008.05.036.
Kim H-U, Bang YH, Choi SM, Yoon KH. Morphology and mechanical properties of PET by incorporation of amine-polyhedral oligomeric silsesquioxane. Compos Sci Technol. 2008;68:2739–47. doi:10.1016/j.compscitech.2008.05.020.
Zhang W, Li XM, Guo XY, Yang RJ. Mechanical and thermal properties and flame retardancy of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS)/polycarbonate composites. Polym Degrad Stab. 2010;95:2541–6. doi:10.1016/j.polymdegradstab.2010.07.036.
Fina A, Tabuani D, Carniato F, Frache A, Boccaleri E, Camiono G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim Acta. 2006;440:36–42. doi:10.1016/j.tca.2005.10.006.
Choi JH, Jung CH, Kim DK, Ganesan R. Radiation-induced grafting of inorganic particles onto polymer backbone: a new method to design polymer-based nanocomposite. Nucl Instrum Methods Phys Res B. 2008;266:203–6. doi:10.1016/j.nimb.2007.11.012.
Gorna K, Gogolewski S. The effect of gamma radiation on the molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polym Degrad Stab. 2003;79:465–74. doi:10.1016/j.polymdegradstab.2010.01.032.
Piszczyk Ł, Danowská M, Mietlarek-Kropidłowská A, Szyszká M, Strankowski M. Synthesis and thermal studies of flexible polyurethane nanocomposites foams obtained using nanoclay modified with flame retardant compound. J Therm Anal Calorim. 2014;118:901–9. doi:10.1007/s10973-014-3878-0.
Paliesková J, Pajášová M, Feriancová A, Ondrušová D, Holcová K, Vavro J Jr, Mojumdar SC. Thermal properties of fillers based on organoclays in the polymer materials. J Therm Anal Calorim. 2015;119:939–43. doi:10.1007/s10973-014-4109-4.
Atiqullah M, Cibulková Z, Černá A, Šimon P, Hussain I, Al-Harthi MA, Anantawaraskul S. Effects of supported metallocene catalyst active center multiplicity on antioxidant-stabilized ethylene homo- and copolymers. J Therm Anal Calorim. 2015;119:581–95. doi:10.1007/s10973-014-4167-0.
Zaharescu T, Ilieş D-C, Roşu T. Thermal and spectroscopic analysis of stabilization effect of copper complexes in EPDM. J Therm Anal Calorim. 2016. doi:10.1007/s10973-015-4893-5.
Chao M, Li W, Wang X. Thermal decomposition kinetics and anti-oxidation performances of commercial antioxidants. J Therm Anal Calorim. 2015;120:1921–8. doi:10.1007/s10973-015-4525-0.
Luo F, Wu K, Lu M, Li X, Guan X. Thermal degradation and flame retardancy of microencapsulated ammonium polyphosphate in rigid polyurethane foam. J Therm Anal Calorim. 2015;120:1327–35. doi:10.1007/s10973-015-4425-3.
Ravat B, Grivet M, Chambaudet A. Evaluation of the degradation and oxidation of polyurethanes vs the electron irradiation parameters: fluence, flux and temperature. Nucl Instrum Methods Phys Res B. 2001;179:243–8. doi:10.1016/S0168-583X(01)00571-7.
Janowski B, Pielichovski K. Thermo(oxidative) stability of novel polyurethane/POSS nanohybrid elastomers. Thermochim Acta. 2008;478:51–3. doi:10.1016/j.tca.2008.08.015.
Lewicki JP, Pielichovski K, de la Croix PT, Janowski B, Todd D, Liggat JJ. Thermal degradation studies of polyurethane/POSS nanohybrid elastomers. Polym Degrad Stab. 2010;95:829–32. doi:10.1016/j.polymdegradstab.2010.01.032.
Setnescu R, Jipa S, Zaharescu T, Setnescu T, Gorghiu LM, Băncuţă I, Chelărescu ED. Copper diffusion in cable-insulating materials by chemiluminescence and DSC techniques. J Therm Anal Calorim. 2015;2015(122):251–9. doi:10.1007/s10973-015-4668-z.
Matisova-Rychla L, Rychlý J. Thermal oxidation of nonstabilized and stabilized polymers and chemiluminescence. J Polym Sci Part A Polym Chem. 2004;42:648–60. doi:10.1002/pola.10847.
Chapiro A (ed). Radiation chemistry of polymeric systems. New York: Interscience Publisher; 1962. pp. 91–93.
Rosu D, Rosu L, Mustaţă L, Varganici C-D. Effect of UV radiation on some semi-interpenetrating polymer networks based on polyurethane and epoxy resins. Polym Degrad Stab. 2012;97:1261–9. doi:10.1016/j.polymdegradstab.2012.05.035.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zaharescu, T., Pielichowski, K. Stabilization effects of POSS nanoparticles on gamma-irradiated polyurethane. J Therm Anal Calorim 124, 767–774 (2016). https://doi.org/10.1007/s10973-015-5191-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5191-y