Abstract
A heptanuclear trigonal prismatic polyhedron, [EuCu6(μ-OH)3(Gly)6Im6](ClO4)6·3H2O, was synthesized and characterized by elemental analysis and X-ray single-crystal diffraction. The complex crystallize is the rhombohedral R3 space group (a = b = 15.8673 Å, c = 23.4038 Å, V = 5103.0(6) Å3, Z = 3). In the clusters, Eu3+ ions are situated in the center of a prism formed by six copper atoms and coordinate to nine oxygen atoms with a tricapped trigonal prismatic coordination polyhedron. Six glycinato ligands and six imidazole terminal ligands (each coordinates to one Cu2+) were used in the construction of the cluster. The low-temperature molar heat capacity, C p,m, and thermodynamic properties of the complex were studied with an adiabatic calorimeter from 80 to 370 K. The thermodynamic functions [H T–H 298.15] and [S T–S 298.15] were derived in the temperature range from 80 to 370 K with a temperature interval of 5 K. The thermal properties of the complex from 300 to 800 K were studied by DSC and TG in nitrogen atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As biological trace elements, rare earth elements and transition metal elements (such as Cu) play a certain role in the human body. Amino acids are the basic unit of the human beings. And the amino acid has two or more types of coordinated atoms and can act as various kinds of bridging ligands by using the carboxylate [1–7]. Imidazole is also involved in the important biological processes [8, 9]. As the two nitrogen atoms in one imidazole molecule are a good resource to coordinate with metal ions, some complexes based on rare earth or transition metal ions and imidazole have been reported [10–12]. Here, europium ions, copper ions, glycine, and imidazole were chosen to prepare the complex, which may have special biological properties. For the structure of Cu2+ ions coordinating to the nitrogen of the imidazole rings have been found presenting in the biomolecules such as histidine [13–17]. The Cu2+ ions bind to the imidazole rings of several histidine residues, form an important functional group in proteins, and play a key role in the mechanism of several metalloenzymes [18–20].
The synthesization, crystal structures of rare earth elements, and transition metal elements’ complex were reported. The thermal stability of the complex was investigated. The basic and important thermodynamic data molar heat capacity, C p,m, and thermodynamic properties of the complex were studied, from which many other thermodynamic properties can be calculated [21, 22].
Experimental
Synthesis of the complex
The starting materials were the analytical reagent from the Beijing Chemical Reagent Co. The solutions of Eu3+ and Cu2+ were synthesized by dissolving excess Eu2O3 and CuO in a certain amount of perchloric acid (1:1). The concentrations of the above two solutions were determined by EDTA titration analysis. The glycine was added to the solutions of Eu3+ and Cu2+ for further 2 h. After the pH value of the reaction mixture was carefully adjusted to about 6.0 by the slow addition of 1.0 M NaOH solution, imidazole was added in molar ratio of Eu3+:Cu2+:Gly:Im = 1:6:6:6. The solution was filtered to remove the precipitate after stirring for a further 4 h and placed in a desiccator filled with the silica gel. Blue crystals were generated about one month later.
An elemental analysis apparatus (Model PE-2400 II, USA) was used to measure the C, H, N of the complex. The Cl amount in ClO −4 of the complex was determined by the ion chromatography (Waters Alliance® 2695 System with the Waters Conductivity Detector and Waters Micromass® Quattro micro™ Mass Spectrometer). The mass fraction of Eu and Cu was determined by the determination of ClO −4 of the complex. Found: Eu (7.389 %), Cu (19.136 %), C (17.794 %), N (11.409 %), H (3.009 %) and Cl (15.665 %), which is close to the theoretical value, Eu (7.282 %), Cu (18.296 %) C (17.265 %), N (12.081 %), H (2.753 %), and Cl (15.195 %). The sample formula was determined to be C30H57Cl6Cu6EuN18O42.
X-ray structure determinations
Suitable single-crystal structure was obtained as described below by the X-ray single-diffraction study. Data collections were performed on a CCD area-detector diffractometer with graphite monochromated Mo Kα radiation (λ = 0.71073 Å at 298 K) in the ω-scan mode. Unit-cell parameters were obtained by least squares methods in the θ ranges of 2.556°–28.183° for the complex. The structures were solved by direct methods with SHELXS-97 [23] and refined by full-matrix least squares methods on F 2 with SHELXL-97 [24]. Anisotropic displacement parameters were refined for all non-hydrogen atoms. Hydrogen atoms were generated geometrically, assigned appropriated isotropic thermal parameters, and included in structure factor calculations. CCDC 1025592 contains the supplementary crystallographic data.
Adiabatic calorimetry and DSC analysis of the complex
Adiabatic calorimetry is the most accurate approach to achieve the heat capacity. In the present study, heat capacity measurements were taken in a high-precision automatic adiabatic calorimeter over the temperature range from 80 to 370 K. The instrument was established in Thermochemistry Laboratory of Dalian Institute of Chemical Physics, Chinese Academy of Sciences. The structure and principle of the adiabatic calorimeter have been reported in detail elsewhere [25, 26]. A precision miniature platinum resistance thermometer (produced by Shanghai Institute of Industrial Automatic Meters, 16 mm long, 1.6 mm in diameter) measured the temperature of the sample cell. The thermometer was calibrated in terms of ITS-90 by Station of Low-Temperature Metrology and Measurements, Chinese Academy of Sciences, R 0 = 100.1384 Ω.
A differential scanning calorimeter (DSC-141, SETARAM, France) was utilized to perform the thermal analysis of the complexes from (300 to 700 K) at the heating rate of 10 K min−1 under nitrogen. The calibrations for the temperature and heat flux of the calorimeter were performed prior to the experiment. The temperature scale was calibrated by measuring the melting points of Hg, In, Sn, Pb, and Zn, at different heating rates, and the heat flux was calibrated by the Joule effect. Measurement of the melting temperature and the enthalpy of fusion of benzoic acid (NIST, Standard Reference Material 39i) were made in our laboratory to check the accuracy of the instrument.
The TG measurement of the sample was carried out by a thermogravimetric analyzer (Model: DT -20B, Shimadzu, Japan) at the heating rate of 10 K min−1 under a purity nitrogen with flow rate of 30 mL min−1. The reference crucible was filled with α-Al2O3. The TG-DTG equipment was calibrated by the SRM in the thermal analysis, CaC2O4·H2O(s).
Results and discussion
Structure of the complex
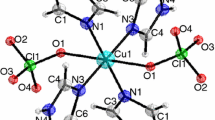
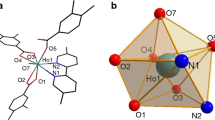
A summary of crystal data and structure refinement of the complex are provided in Table 1. The structure diagram was shown in Fig. 1. It can be seen from Fig. 1 that six Cu2+ ions exist around an Eu3+ ion in the center. Every identical two Cu2+ ions are connected by a chelating glycine ligand. Eu3+ ions have a nine-coordinated O9 donor set (site), and its coordination polyhedron may be best described as a tricapped trigonal prism. Six apexes are formed by the six carboxylate oxygen atoms from six glycine, and three caps are formed by the three oxygen atoms from three μ3-OH− groups. Each cation consists of three μ3-OH− groups, which are situated in the same place with Eu3+ ions, forming two equilateral triangles. Glycinato ligand adopts a π-coordination mode, chelating to two Cu2+ and one Eu3+ ions. Each imidazole ligand coordinates with one Cu2+ ion as a terminal ligand. The two corresponding imidazole ligands in the two layers are nearly parallel. The huge network of intra- or inter-molecular hydrogen bonds helps to stabilize the crystal. Three of the perchlorates show disorder site in the crystal, which were due to the rotation of the perchlorate groups.
Molar heat capacities and thermodynamic functions
The experimental molar heat capacities (C p,m) of [EuCu6(μ-OH)3(Gly)6Im6](ClO4)6·3H2O from 80 to 370 K are presented in Table 2, and the molar heat capacity values are plotted as a function of temperature in Fig. 2. The above figure shows that there is no phase change in the temperature range from 80 to 370 K.
A polynomial equation was obtained by the least square fitting by using the experimental molar heat capacities (C p,m) and the experimental temperatures (T).
From 80 to 370 K (solid phase):
where x is the reduced temperature, \( x = [{{(T/{\text{K}}) - 2 25]} \mathord{\left/ {\vphantom {{(T/{\text{K}}) - 2 25]} {1 45}}} \right. \kern-0pt} {1 45}} \), T is the experimental temperature, 225 is obtained from polynomial \( {{(T_{\hbox{max} } + T_{\hbox{min} } )} \mathord{\left/ {\vphantom {{(T_{\hbox{max} } + T_{\hbox{min} } )} 2}} \right. \kern-0pt} 2} \), 145 is obtained from polynomial \( {{(T_{\hbox{max} } - T_{\hbox{min} } )} \mathord{\left/ {\vphantom {{(T_{\hbox{max} } - T_{\hbox{min} } )} 2}} \right. \kern-0pt} 2} \), T max is the upper limit (370 K) of the above temperature region, T min is the lower limit (80 K) of the above temperature region. Correlation coefficient R 2 of least square fitting is 0.9999.
The thermodynamic functions [H T–H 298.15] and [S T–S 298.15] of the compound were calculated and are listed in Table 3, which is in the temperature range from 80 to 370 K with a temperature interval of 5 K in terms of the polynomials of heat capacity and the thermodynamic relationships as Eqs. (2–5).
DSC and TG analysis
DSC analysis of the complex from the room temperature to 900 K is plotted in Fig. 3. It showed that there were two peaks in the curve. The first one was a sharp endothermic peak at 515 K, which showed the fastest rate of the decomposition. For the coexistent of the ClO −4 ions and imidazole, the rate of the decomposition was so fast. The second peak was exothermic course, which was attributed to the formation of the oxides of elements Eu and Cu.
The TG–DTG curves plotted in Fig. 4 showed that mass loss of [EuCu6(μ-OH)3(Gly)6Im6](ClO4)6·3H2O begin at about 325 K and end at about 800 K. The whole process was divided into two stages. The mechanism was deduced as follows:
Conclusions
In summary, [EuCu6(μ-OH)3(Gly)6Im6](ClO4)6·3H2O is the rhombohedral R3 space group (a = b = 15.8673 Å, c = 23.4038 Å, V = 5103.0(6) Å3, Z = 3). In the cluster, Eu3+ ions are situated in the center of a prism formed by six copper atoms and coordinate with the nine oxygen atoms with a tricapped trigonal prismatic coordination polyhedron. Six glycinato ligands and six imidazole terminal ligands coordinated to six Cu2+. The molar heat capacities of the complex were collected from 80 to 370 K, and no phase change was found in this temperature range. Moreover, the molar enthalpy and the entropy of fusion and the thermodynamic functions, [H T–H 298.15] and [S T–S 298.15], were derived from the measured heat capacity data with a temperature interval of 5 K. The DSC test found that an endothermal process happens at about 515 K, which is due to the decomposition of the ClO −4 ions and imidazole molecules.
Supplemental data
CCDC 1025592 contains the supplementary crystallographic data for the complex.
These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/ or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK [Email: deposit@ccdc.com.ac.uk].
References
Yukawa Y, Inomata Y, Takeuchi T. The structure of dichloro(4-hydroxy-l-proline)cadmium(II). Bull Chem Soc Jpn. 1982;55:3135–7.
Inomata Y, Takeuchi T, Yukawa Y. Structure and properties of dichloro(l-proline)cadmium(II) Hydrate. Bull Chem Soc Jpn. 1983;56:2125–8.
Inomata Y, Yukawa Y, Yasukawa N. The structure of dichlorobis(l-proline)zinc(II). Bull Chem Soc Jpn. 1985;58:1591–2.
Itoh S, Inomata Y, Yukawa Y. Structure of aquachloro(4-hydroxy-L-prolinato)copper(II). Bull Chem Soc Jpn. 1987;60:899–902.
Nagase M, Yukawa Y, Inomata Y, Takeuchi T. Synthetic spectroscopic and X-ray crystallographic studies of bivalent metal complexes with amino acids having a thiazolidine Ring. I. Properties of bivalent metal complexes with (S)-4-thiazolidinecarboxylic acid and the structure of chloro((S)-4-thiazolidinecarboxylato)((S)-thiazolidinium-4-carboxylate)zinc(II). Bull Chem Soc Jpn. 1988;61:775–81.
Inomata Y, Nakagome S, Takeuchi T. Structure and properties of chloro(N, N-diethylglycinato)copper(II). Bull Chem Soc Jpn. 1989;62:1057–60.
Yukawa Y. Structures and properties of (L-prolinato) copper (II) complexes prepared from different solvents. J Chem Soc Dalton Trans. 1992;22:3217–21.
Allen FH. The Cambridge structural database: a quarter of a million crystal structures and rising. Acta Crystallogr Sect B. 2002;58:380–8.
Mura P, Casini A, Marcon G, Messori L. Synthesis, molecular structure and solution chemistry of the iridium(III) complex imidazolium [trans(bisimidazole)tetrachloro iridate(III)] (IRIM). Inorg Chim Acta. 2001;312:74–80.
Masciocchi N, Ardizzoia GA, Brenna S, Castelli F, Galli S, Maspero A, Sironi A. Synthesis and ab initio XRPD structure of group 12 imidazolato polymers. Chem Commun. 2003;16:2018–9.
Lambert F, Renault JP, Policar C, Badarau IM, Cesario M. A polymeric, layered bimetallic Mn(II)Fe(III) imidazolate network; crystal structure and magnetic properties. Chem Commun. 2000;1:35–6.
Huang XC, Zhang JP, Lin YY, Yu XL, Chen XM. Two mixed-valence copper(I, II) imidazolate coordination polymers: metal-valence tuning approach for new topological structures. Chem Commun. 2004;9:1100–1.
Choi YA, Keem JO, Kim CY, Yoon HR, Heo WD, Chung BH, Jung YW. A novel copper-chelating strategy for fluorescent proteins to image dynamic copper fluctuations on live cell surfaces. Chem Sci. 2015;6:1301–6.
Dede B, Karipcin F, Cengiz M. Novel homo-and hetero-nuclear copper (II) complexes of tetradentate Schiff bases: synthesis, characterization, solvent extraction and catalase—like activity studies. J Hazard Mater. 2009;163:1148–56.
William MA, Sarah CL. DFT calculations of the EPR parameters for Cu(II) DETA imidazole complexes. Phys Chem Chem Phys. 2009;11:8266–74.
Lakatos A, B′ela G, N′ora VN, Zita C, W′eber E, L′ıvia F, Tam′as K. Histidine-rich branched peptides as Cu(II) and Zn(II) chelators with potential therapeutic application in Alzheimer’s disease. Dalton Trans. 2012;41:1713–26.
Remelli M, Valensin D, Bacco D, Gralka E, Guerrini R, Migliorini C, Kozlowskic H. The complex-formation behaviour of His residues in the fifth Cu2+ binding site of human prion protein: a close look. New J Chem. 2009;33:2300–10.
Jorge AT, Luis RS, Mariona S. Computational calculations of pK a values of imidazole in Cu(II) complexes of biological relevance. Phys Chem Chem Phys. 2011;13:7852–61.
Potocki S, Valensinb D, Kozlowski H. The specificity of interaction of Zn2+, Ni2+ and Cu2+ ions with the histidine-rich domain of theTjZNT1 ZIP family transporter. Dalton Trans. 2014;43:10215–23.
Gralka E, Valensin D, Porciatti E, Gajda C, Gaggelli E, Valensin G, Kamysz W, Nadolny R, Guerrini R, Bacco D, Remelli M, Kozlowski H. CuII binding sites located at His-96 and His-111 of the human prion protein: thermodynamic and spectroscopic studies on model peptides. Dalton Trans. 2008;38:5207–19.
Novikov VV, Avdashchenko DV, Matovnikov AV, Mitroshenkov NV, Bud’ko SL. Heat capacity and thermal expansion of icosahedral lutetium boride LuB66. J Thermal Anal Calorim. 2014;116:765–9.
Galkin V, Gartvich Y. Thermal expansion and evaluation of almandine heat capacity. J Therm Anal Calorim. 2015;1–6. doi:10.1007/s10973-015-4768-9.
Sheldrick GM. A short history of SHELX. Acta Crystallogr Sect A. 2008;64:112–22.
Weng NS. Some strategies for the refinement of disordered structures with the SHELXL-97 refinement program. Chin J Struct Chem. 2005;24:1425–39.
Zhong WW, Di YY, Dou JM. Synthesis, structural characterization, and thermochemistry of complexes of pyridine 2,6-dicarboxylate with metals (Mg and Sr). J Thermal Anal Calorim. 2014;115:2527–33.
Wang Z, Li B, Zhang YH, Wang M, Kong YX, Di YY, Dou JM. Molar heat capacities and solution thermochemistry of n-undecylammonium bromide monohydrate C11H28BrNO(s). J Thermal Anal Calorim. 2015;120:1645–55.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (21103078, 21003069).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, L., Gao, XH., Lv, XC. et al. Low-temperature molar heat capacity and thermodynamic properties of rare earth complex. J Therm Anal Calorim 124, 429–435 (2016). https://doi.org/10.1007/s10973-015-5133-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5133-8