Abstract
In the framework of a systematic investigation of intermetallic systems, constituted by aluminium and silicon with a rare earth metal, the isothermal section at 500 °C of the Pr–Al–Si system has been experimentally investigated. The experimental techniques used were scanning electron microscopy, electron microprobe analysis and X-ray powder diffraction, and some samples have been analysed by differential thermal analysis. The existence of six ternary compounds has been confirmed, one of them showing a composition homogeneity range: τ1 PrAl2Si2 (hP5-Ce2SO2 type), τ2 Pr3Al4Si6 (hP13-Ce3Al4Si6 type), τ3 PrAlSi2 (hP8-CeAlSi2 type), τ4 Pr2Al3Si (hP3-AlB2 type), τ5 PrAl(1−x)Si(1+x) (tI12-αThSi2Si type) and τ6 Pr2AlSi (oS8-CrB type). A few compounds pertaining to the binary boundary systems Pr–Al and Pr–Si dissolve the third element.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multicomponent Al–Si-based alloys are important and widely used in industries; the addition of a rare earth metal to Al–Si alloys can result in an increase in their extremely useful thermal, mechanical and corrosion resistance properties; this implies the need for the development in alloys’ composition design. The addition of a rare earth element (R) can improve the technological properties and the stability at high temperatures of these alloys, through the formation of ternary phases [1–5]. Moreover, the addition of rare earth metals to casting Al–Si alloys modifies their microstructures [6] inducing a lowering of the eutectic temperature; for this reason, a measure of the “depression” in the eutectic temperature value has been proposed as a method to control the modification level in the alloy microstructure by means of thermal analysis measurements [7, 8].
To improve the understanding of the rare earths’ influence on the Al–Si alloy properties, the knowledge of the phase relationships and the constitutional properties of the ternary R–Al–Si systems can constitute an important support. For this purpose, the investigation of the different R–Al–Si systems (R = rare earth) is part of an ongoing research project by our research group with the aim to clarify the constitutional properties of these ternary systems ([9–11] and references therein). In this work, the Pr–Al–Si isothermal section at 500 °C, in the full concentration range, has been experimentally investigated by using scanning electron microscopy (SEM), electron microprobe analysis (EDXS), X-ray powder diffraction (XRPD) and differential thermal analysis (DTA). Some samples have been analysed by DTA.
Literature data
Boundary binary systems
The Al–Si phase diagram, based mainly on the work by Murray et al. [12], consists of a simple eutectic system with the eutectic reaction at 12.2 at.% Si and 577 °C. The Pr–Si system was assessed by Okamoto [13], and in this system, five intermetallic compounds have been recognized: Pr5Si3 (tI32-Cr5B3 type), Pr5Si4 (tP36-Zr5Si4 type), PrSi (oP8-FeB type), PrSi1.33 (oC20-Ho3Si4 type), PrSi2 (tI12-Th2Si type, T > 653 K and oI12-GdSi2 type T < 653 K). The Pr–Al phase diagram has been recently assessed by Jin et al. [14] confirming the following intermediate phases: Pr3Al (hP8-Ni3Sn type T < 330°, cP4-AuCu3 type T > 330 °C), Pr2Al (oP12-Co2Si type), PrAl (oP16-AlEr type T < 700 °C K, oC16-AlCe type T > 700 °C), PrAl2 (cF24-MgCu2 type), PrAl3 (hP8-Ni3Sn type) and Pr3Al11 (oI28-La3Al11 type T < 651 °C, tI10-Al3Ba type T > 651 °C).
Pr–Al–Si ternary system
Nakonechna et al. [15] investigated the Al richest corner of the Pr–Al–Si system at 400 °C. In this system, the following phases are known: PrAl2Si2 (hP5-CaAl2Si2 type) [15], Pr3Al4Si6 (hP13-Ce3Al4Si6 type) [15], PrAlSi2 (hP8-CeaAlSi2 type) [16] Pr2Al3Si (hP3-AlB2 type) [15], PrAl(1−x)Si(1+x) (tI12-αThSi2 type) [17], Pr2AlSi (tI12-αThSi2 type) [18].
Experimental
Ingots of high-purity metal Pr (99.9 mass% nominal purity), Si (99.999 mass% nominal purity) and Al (99.99 mass% nominal purity) were used to synthesize the alloys (1–2 g each). All the metals were provided by Newmet Koch, Waltham Abbey, England. The samples were prepared by arc melting, under argon atmosphere, stoichiometric amounts of the elements. The mass losses of the alloys during melting were less than 0.2 mass%. The samples, placed in alumina crucibles sealed under vacuum in quartz tubes, were then annealed at 500 °C for 3 weeks and subsequently quenched in cold water. A number of alloys were subjected to DTA by using the Netzsch model 404S apparatus (Selb, Germany), and the measurements were taken, both on heating and on cooling, at different rates (5–10 K min−1). The thermocouples were calibrated by using high-purity elements such as Al, Ag, Au. More details on the operating conditions are reported in [9].
Light optical microscopy (LOM), scanning electron microscopy and electron probe microanalysis based on energy-dispersive X-ray spectroscopy (EDXS) were used to characterize the samples, investigating the microstructure and determining the phase composition. For microscopic observation and metallographic analysis, the specimens were prepared by using SiC papers and diamond pastes down to 3 μm grain size. For the quantitative analysis, an acceleration voltage of 20 kV was applied for 50 s, and a cobalt standard was used for calibration. The software packaging Inca Energy (Oxford Instruments, Analytical Ltd, Bucks, UK) was employed to process X-ray spectra. In order to determine crystal structures and calculate lattice parameters, X-ray diffraction (XRPD) analysis was performed on pulverized samples by using a vertical diffractometer X’Pert MPD (Philips, Amelo, the Netherlands). The indexing of the obtained diffraction data was performed by comparison with the literature or calculated data (the program Powder Cell [19]), and the lattice parameters of the phases were calculated using the program LATCON [20].
Results and discussion
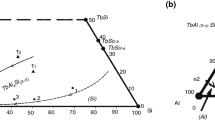
The data obtained in the investigation by SEM/EDXS and X-ray powder diffraction of the different alloys are reported in Table 1. Basing on the experimental results achieved, the Pr–Al–Si isothermal section at 500 °C is drawn and reported in Fig. 1a. Figure 1b shows the compositions of the alloys investigated. Photomicrographs of selected samples are shown in Fig. 2a–d.
Pr–Al–Si system. a The isothermal section at 500 °C of the Pr–Al–Si system. b Gross composition of the analysed alloys. The results of the characterization of the coded samples are shown in Table 1
Back-scattered electron (BSE) images of selected samples annealed at 500 °C (see Table 1). a Sample n. 3: τ2 (white), τ3 (light grey) and (Si) (black). b Sample n. 15: two-phase sample PrAlxSi1−x (white crystals) and βPr(AlxSi1−x)2 (black). c Sample n. 10: τ2 (white), τ3 (grey) and τ5 (black). d Sample n. 27: τ6 (white), Pr5(AlxSi1−x)3 (grey) and Pr(AlxSi1−x) (black)
Isothermal section
The following binary intermetallic compounds pertaining to the Pr–Al–Si system were confirmed at 500 °C: Pr5Si3 (tI32-Cr5B3), Pr5Si4 (tP36-Zr5Si4), PrSi (oP8-FeB), PrSi2 (tI12-Th2Si), Pr3Al (cP4-AuCu3), Pr2Al (oP12-Co2Si), PrAl (oP16-AlEr), PrAl2 (cF24), PrAl3 (hP8-Ni3Sn) and Pr3Al11 (oI28-La3Al11). Five ternary intermetallic compounds, already reported in literature, were identified at 500 °C: τ1-PrAl2Si2 (hP5-CaAl2Si2 type), τ2-Pr3Al4Si6 (hP13-Ce3Al4Si6 type), τ3-PrAlSi2 (hP8-CeaAlSi2 type), τ4-Pr2Al3Si (hP3-AlB2 type), τ5-PrAl(1−x)Si(1+x) (tI12-αThSi2 type) and τ6-Pr2AlSi (oS8-CrB).
The τ5 compound shows an homogeneity range, at a constant Pr content (33.3 at.%), extending towards both the Al richest and Si richest compositions with respect to the stoichiometric formula PrAlSi. The substitution with aluminium for silicon in the τ5 compound was suggested by Bobev et al. [17], but at 500 °C also the substitution with silicon for aluminium occurs. The compound exists in a range from 28 to 38 at.% aluminium. Figure 3 shows the trend of the τ5 phase cell volume versus the Al content.
On the basis of the similar atomic radii of the two Al and Si atoms, some solid solutions extend from the binary edges into the ternary field. From the binary Pr–Al system, only one intermetallic compound originates a ternary solid solution: Pr(Al1–xSix)3 (0 ≤ x ≤ 0.05, hP16-Ni3Ti). From the Pr–Si system, five ternary solid solutions form: Pr(AlxSi1−x)2 (0 ≤ x ≤ 0.35, tI2-ThSi2), Pr3(AlxSi1−x)5 (0 ≤ x ≤ 0.15, hP3-AlB2), Pr(AlxSi1−x) (0 ≤ x ≤ 0.2, oP8-FeB), Pr5(AlxSi4−x) (0 ≤ x ≤ 0.07, oP36-Pr5Ge4) and Pr5(AlxSi3−x) (0 ≤ x ≤ 0.1, hP16-Mn5Si3). The homogeneity ranges were determined by SEM/EDXS analysis, and the Al/Si mutual substitutions were confirmed by the variation of the lattice parameters of the phases, by moving from the binary into the ternary system. Solid solutions based on binary phases extending less that 2 % in the ternary field were not taken into account.
By comparing the four isothermal sections of the systems Nd–Al–Si [9], Pr–Al–Si, Dy–Al–Si [10] and Sm–Al–Si [11] at 500 °C, moving from the lighter to the heavier lanthanide, it can be observed that there is a regular trend of the number and stoichiometry of the phases. A few Pr–Al–Si samples were subjected to DTA. Figure 4 reveals the heating and cooling curves of a sample at composition Pr 1 at.%, Al 91 at.%; the thermal effect at 566 °C corresponds to the ternary eutectic L ⇄ (αAl) + (βSi) + τ1. The lowering of the eutectic temperature, due to the addition of the rare earth, has been confirmed.
Conclusions
The phase relationships in the Pr–Al–Si system at 500 °C has been drawn, by comparing and analysing the experimental results obtained for 31 samples; 23 three-phase fields and 9 two-phase fields have been determined. The regular trend in the chemical and physical properties through the lanthanides series leads the interaction of Pr with Al and Si at 500 °C to be similar with the other R–Al–Si (R = light rare earth) systems, especially in the Al–Si-rich side of the system. The stoichiometric ternary intermetallic compounds PrAl2Si2 (τ1) hP5-CaAl2Si2, Pr3Al4Si6(τ2) hP13-Ce3Al4Si6, PrAlSi2 (τ3) hP8-CeAlSi2, Pr2Al3Si (τ4) hP3-AlB2, PrAl(1−x)Si(1+x) (τ5) tI12-αTh2Si and Pr2AlSi (τ6) oS8-CrB have been confirmed, and the ternary compound PrAl(1−x)Si(1+x) (τ5) extends in a composition range from 28 to 38 at.% aluminium, at a constant praseodymium content. The phase cell volume increases regularly with the increase in aluminium content. The isothermal sections studied can be fruitfully compared, concerning the alloying behaviour of the rare earths with Al and Si: the elements Nd, Pr and Sm as examples of the behaviour of the light rare earths, while Dy as the heavy lanthanides. All the systems are characterized by intermediate phases with an R content up to 60 at.% rare earth. The number of phases decreases on going from the light to the heavy rare earths. Only the RAl2Si2 compound forms along the whole lanthanide series. With respect to the other R–Al–Si ternary systems, although the literature data are generally lacking, information is available concerning the Al–Si-rich portion of the different systems, likely due to the technological relevance of the alloys lying near the Al–Si eutectic composition. All the known systems show the tie triangle (Al), (Si) and RAl2Si2 (hP5-CaAl2Si2 type), in which is located the ternary eutectic equilibrium L ⇄ (αAl) + (βSi) + RAl2Si2 (τ1).
References
Miller WS, Zhuang L, Bottema J, Wittebrood AJ, De Pret P, Haszler A, Vieregge A. Recent development in aluminium alloys for the automotive industry. Mater Sci Eng, A. 2000;280:37–49.
Chen CL, Richter A, Thomson RC. Mechanical properties of intermetallic phases in multi-component Al–Si alloys using nanoindentation. Intermetallics. 2009;17:634–41.
Ye HJ. An overview of the development of Al–Si-alloy based material for engine applications. J Mater Eng Perform. 2003;12:288–97.
Zhu M, Jian Z, Yao L, Liu C, Yang G, Zhou Y. Effect of mischmetal modification treatment on the microstructure, tensile properties, and fracture behavior of Al–7.0%Si–0.3%Mg foundry aluminum alloys. J Mater Sci. 2011;46:2685–94.
Qiu H, Yan H, Hu Z. Effect of samarium (Pr) addition on the microstructures and mechanical properties of Al–7Si–0.7 Mg alloys. J Alloys Compd. 2013;567:77–81.
Mazahery A, Shabani MO. Modification mechanism and microstructural characteristics of eutectic Si in casting Al–Si alloys: a review on experimental and numerical studies. J Miner Met Mater Soc. 2014;166(5):726–38.
Heusler L, Schneider W. Influence of alloying elements on the thermal analysis results of Al–Si cast alloys. J Light Met. 2002;2:17–26.
Nogita K, Yasuda H, Yoshiya M, McDonald SD, Uesugi K, Tacheuchi A, Suzuki Y. The role of trace element segregation in the eutectic modification of hypoeutectic Al–Si alloys. J Alloys Compd. 2010;489:415–20.
Cardinale AM, Macciò D, Delfino S, Saccone A. Experimental investigation of the Nd–Al–Si system. J Therm Anal Calorim. 2011;103:103–9.
Cardinale AM, Macciò D, Delfino S, Saccone A. Phase equilibria of the Dy–Al–Si system at 500°C. J Therm Anal Calorim. 2012;108:817–23.
Cardinale AM, Macciò D, Delfino S, Saccone A. Phase equilibria in the Sm–Al–Si system at 500°C. J Therm Anal Calorim. 2014;116:61–7.
Murray JL, McAlister AJ. The Al–Si (aluminum–silicon) system. Bull Alloy Phase Diagr. 1984;5:74–84.
Okamoto H. Desk handbook: phase diagrams for binary alloys. Materials Park: ASM Internatinal; 2000.
Jin LL, Kang YB, Chartrand P, Fuerst CD. Thermodynamic evaluation and optimization of Al–La, Al–Ce, Al–Pr, Al–Nd and Al–Pr systems using the modified quasichemical model for liquids. CALPHAD Comput Coupling Phase Diagr Thermochem. 2011;35:30–41.
Nakonechna N, Lyaskovska N, Romaniv O, Starodub P, Gladyshevskii E. Pr–Al–Si phase diagram (0–0.33 at.fract. Pr) and crystal structure of the compounds. Visn Lviv Univ Ser Khim. 2001;40:61–7.
Muts N, Gladyshevskii RE, Gladyshevskii EI. Crystal structures of the compounds PrAl2Si2, Pr3Al4Si6 and PrAlSi2. J Alloys Compd. 2005;402:66–9.
Bobev S, Tobash PH, Fritsch V, Thompson JD, Hundley MF, Sarrao JL, Fisk Z. Ternary rare-earth alumo-silicides—single-crystal growth from Al flux, structural and physical properties. J Solid State Chem. 2005;178:2091–103.
Lyaskovska N, Romaniv O, Semus’o N, Gladyshevskii E. Crystal structures of the compounds RAl0.5−xSi0.5+x (R = La, Ce, Pr, Nd, Pr, Gd), R3Al4Si6 (R = La, Pr), and RAlSi2 (R = Pr, Nd). J Alloys Compd. 2004;367:180–4.
Kraus W, Nolze G. Powder cell for windows. Berlin: Federal Institute for Materials Research and Testing; 1999.
King G, Schwarzenbach D. Latcon. In: Hall SR, Du Boulay DJ, Olthof-Hazekamp R, editors. Xtal3.7 system. Perth: University of Western Australia; 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardinale, A.M., Macciò, D. & Saccone, A. Phase relationships of the R–Al–Si systems. J Therm Anal Calorim 121, 1151–1157 (2015). https://doi.org/10.1007/s10973-015-4906-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4906-4