Abstract
Osteoporosis is a systemic skeletal disease characterized by a disorder between bone reabsorption and formation with increase in bone fragility. The aim of this study was to evaluate the bone mass loss using solid-state techniques and biochemical assays to characterized bone samples of ovariectomized rats (OVX) and control group (CTR). The in vivo assays were carried out using biochemical analyses and bone mineral density (BMD) measurements. After the animals were euthanized, the femurs were examined using simultaneous differential thermal analysis (DTA), thermogravimetry/derivative thermogravimetry (TG/DTG) and atomic absorption spectrometric. The microstructure was examined using scanning electron microscopy (SEM). OVX had statistically higher averages (p < 0.05) for alkaline phosphatase activity in the serum. Therefore, our results demonstrated that the comparison of the OVX with the CTR showed no evidence of significant differences in the serum calcium and phosphorus levels (p > 0.05); however, OVX showed a significant weight gain (p < 0.0001) with a body mass increase of 30.23 ± 6.45 %. BMD results presented significant differences (p = 0.01985) between CTR and OVX. The total mass loss (TG) was 39.12 ± 0.45 % in OVX, while in CTR groups, it was the 32.45 ± 1.01 % (p = 0.0038). The results of the calcium amount in the bones of OVX (199.86 ± 8.08) were lower than the CTR (229.62 ± 10.02). SEM micrographs showed that OVX presented bone porosity, while CTR showed denser trabecular structure; this result corroborated with bone mass loss in OVX. The ovariectomy-induced bone loss in the rodent model can be evaluated using physical–chemical techniques based on thermal analysis, atomic absorption spectrometry and scanning electronic microscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The bone is a complex composite material consisting of approximately 10 % water, 30 % organic phase (mainly collagen fibrils) and 60 % inorganic material (predominantly carbonated hydroxyapatite) [1]. Bone is one of the most important biological structures in the field of biomineralization. There is a clear relationship between the weight fraction of the mineral phase in bone and its mechanical properties [2].
Osteoporosis is a progressive systemic disease that is associated with a diminution of bone mass and the deterioration of the microarchitecture, causing bone fragility and increasing the risk of fractures [3]. This disorder is characterized by an imbalance in bone remodeling in which the rate of bone reabsorption exceeds that of bone formation [4, 5].
Osteoporosis is a common metabolic disease that affects postmenopausal women as a result of a decrease in estrogen levels secondary to the loss of ovarian function. A deficiency in estrogen increases bone turnover rates such that bone reabsorption exceeds bone formation, which compromises trabecular bone [6]. Postmenopausal osteoporosis typically affects more than 80 % of the female population above 50 years [7] and is characterized by a rapid loss of mineralized bone tissue, disruption of the trabecular architecture of the bone and changes in the crystalline properties of mineral deposits, which result in the structural failure (fracture) of sites rich in cancellous bone [8]. Most fractures in postmenopausal women are associated with a mild-to-moderate trauma superimposed on a low bone mass [9], on changes in bone architecture and collagen fiber orientation or, as recently suggested, on changes in the chemical composition of collagenic and non-collagenic bone matrix [10].
The ovariectomization of the female rat is the most common experimental model used in research on postmenopausal osteoporosis. It has been reported that ovariectomy-induced bone loss is observed in the proximal tibial metaphysis after approximately 2 weeks, in the femoral neck after 4 weeks and in the lumbar vertebral body after 8 weeks [11].
Bone densitometry has been widely used in preclinical research to assess bone mass and mineral content in animal models of osteoporosis [11]. However, it has been reported that the analysis of bone densitometry can be challenging in growing animals because in these cases, apparent changes in bone mass may be a reflection of growth and size and not of changes in true mineral density [12, 13].
In the last few decades, physicochemical characterization techniques, e.g., atomic absorption spectrometric, scanning electronic microscopy and thermal analysis, have been used to assess mineral content in different samples, such as food, water and soil analysis, as well as in material engineering [1, 14–16]. Despite the sensitivity of these methods, their use has not been explored to evaluate bone loss in bioassay-guided experimental osteoporosis.
Thus, the goal of this study was to characterize the bone samples of ovariectomized female rats using solid-state physicochemical techniques. Furthermore, changes in the serum levels of alkaline phosphatase, phosphorus and calcium were assessed and were used as biochemical parameters of bone loss.
Materials and methods
Animals
Thirteen-week-old female Wistar rats (150–200 g) were obtained from the Biotherium of Tiradentes University (Sergipe, Brazil). This study was approved by the Institutional Animal Care of the Tiradentes University (CEUA/UNIT#110310R), and all procedures were carried out in accordance with Animal Care. The rats were randomly assigned into two groups: control group, with adult female rats (CTR; n = 6); ovariectomized group, with adult female rat that have undergone bilateral ovariectomy by lumbar access under general anesthesia (OVX; n = 6). The rats from both groups were weighed at the beginning of the study and after 60 days.
Biochemical analyses
Sixty days after surgery (ovariectomy), blood samples (1 mL) were collected by cardiac puncture from all of the animals. Serum was obtained after centrifugation at 10,000 rpm for 15 min. The alkaline phosphatase, phosphorus and calcium levels of the CTR and OVX animals were assayed using Architect C8000 (Abbott, USA) automated equipment.
Bone mineral density (BMD)
Sixty days after surgery, bone mass was determined using bone mineral density (BMD) and expressed in g cm−2. The measurements were taken with the bone mass of total body weight using dual X-ray absorptiometry (GE Healthcare densitometer, USA).
Bone sample
Subsequent to the collection of blood samples and assessment of the BMD measurements, the animals were euthanized in a CO2 chamber. The bone samples (femurs) were removed for the following treatments. Bones were dissected, boiled in distilled water to remove fat and subsequently dried in the oven at 80 °C. [1].
Thermal analysis
The bone samples were ground, and the powder was used for TG/DTA analysis. Simultaneous thermogravimetry (TG/DTG) and differential thermal analysis (DTA) were performed on a thermobalance model TGA 60 (Shimadzu, Kyoto Japan) using platinum crucibles. The bone samples were approximately 5 mg and were heated at a rate of β = 20 °C min−1 in air atmosphere (flow = 100 mL min−1) in the temperature range of 20–1100 °C, according to the method proposed by Jankovíc and collaborators (2009) [17]. The isothermal thermogravimetric test of animal bones was performed by 1 h a temperature of 1100 °C.
Atomic absorption spectrometry
The following methods were performed according to the Official Method 968.08 for determination of minerals in animal feed and pet food and Official Method 965.09 for atomic absorption spectrometric method (AOAC), with slight modifications. Bone digestion was performed by adding 5.0 mL HNO3 to each 0.1 g of the sample. Subsequently, the samples were transferred to volumetric flasks, and Milli-Q water was added to achieve a final volume of 25 mL. Calcium assessment using atomic absorption spectrometry was performed with air-C2H2 burners. Ionic interference was eliminated by adding La salt stock solution to the standard and sample solutions so that final dilutions contained 1 % La. A Perkin-Elmer (Analyst 300, USA) flame atomic absorption spectrometer equipped with hollow cathode lamps was used for the analysis. The instrumental parameters were adjusted according to the manufacturer’s recommendations. A Ca hallow cathode lamp operating at 422.7 nm was used as the radiation source. The lamp current was set at 10 mA. The flame composition was acetylene (gas pressure 2.94 × 104 Pa) and air (gas pressure 1.28 × 105 Pa).
Scanning electron microscopy (SEM)
The dry samples were coated with 10 nm of gold by sputtering with a Sputter Coater BAL-TEC MED 20 (Oxford, England). Femur neck microstructures were examined by scanning electron microscopy (SEM, JEOL JMS-6360-Lv, Japan; 20 kV) with energy-dispersive spectrometry (EDS, Oxford, England). An ISI DS-130 LaB6 SEM operated at 9–15 kV was employed to investigate the trabecular architecture (mesostructural level) of the femur necks. Decalcification was not necessary for analyzing these structures under SEM, and thus, they all remained calcified. SEM images were digitally photographed to best capture the trabecular structure.
Statistical analysis
Student’s t test (GraphPad Prism 5.01 computer program) was employed for statistical analysis of the results. All values were expressed as the mean ± SD. Differences below the probability level of 0.05 were considered statistically significant.
Results and discussion
The ovariectomized rat is the most common animal model for studying events associated with postmenopausal osteoporosis [17]. As shown in Table 1, the OVX presented higher values of alkaline phosphatase serum levels compared with CTR (p = 0.0025). Although increased levels of alkaline phosphatase has been considered to be a marker of bone formation, it has also been demonstrated that this enzyme is over stimulated as a compensatory result of decreased serum levels of estrogen, which is what occurred in OVX [18]. Surprisingly, no significant difference was observed in the serum calcium and phosphorus levels between experimental (OVX) and control groups (CTR; p > 0.05).
Plasmatic levels of phosphorus, calcium and alkaline phosphatase were measured to assess osseous metabolism. The ovariectomized group showed an increase in alkaline phosphatase levels and regular calcium and phosphorus levels. According to Serakides et al. [19], hypogonadism interferes with the plasmatic characteristics of osseous metabolism, particularly increasing the levels of alkaline phosphatase. In contrast, hypogonadism has little effect on the level of phosphorus. The ovariectomized group showed that the bone is in the remodeling biochemical process, represented by high levels of alkaline phosphatase. In addition, the calcium level was not inversely related to phosphorus, which may suggest a typical response to the action of parathyroid hormone (PTH) on bone, demonstrating the important role of bone in maintaining calcium levels [20, 28].
The ovariectomized group showed a significant weight gain (p < 0.0001), with a body mass increase of 30.23 ± 6.45 % after 2 months when compared to the rats before surgery (Fig. 1). The monthly weight gain of the rats that did not undergo surgery was 10.10 ± 3.2 %. It has been reported that estrogen deficiency is associated with atrophy of the uterus and weight gain. Thus, the success of ovariectomy in reducing estrogen levels can also be inferred by changes in the body weight of the ovariectomized animals [21, 22].
The combustion of animal bones under isothermal conditions is a complicated process because many different molecules are present in the bone samples. Figure 2 shows the TG curve of CTR and OVX. The TG curves for both bone samples showed three events with different intensities that correspond to the considered reaction steps (steps I, II and III). Water is an abundant component of bone, accounting for approximately 8.46 ± 1.19 % by mass loss in OVX and 9.73 ± 1.4 % in the control group. This mass loss was associated with loss of water (stage I). In the interval from 268 to 518 °C, a gradual mass loss (stage II), 19.91 ± 0.81 % in CTR and 25.85 ± 1.13 in OVX, was observed. A higher mass loss was observed in 268–518 °C. In this range, mass loss can be associated with thermal decomposition of the organic phase (stage II). It can be noted that in this stage, the loss of bulk water can be followed by deeper dehydration, with the loss of the ordered hydration shell that has been proposed to mediate the interactions between the mineral and collagen components of bone. Additionally, it can be noted that the samples with a high organic content usually lost carbon dioxide in two stages. The mass loss in the temperature range from 518 to 1100 °C was 2.8 ± 0.33 % in CTR and 4.81 ± 0.19 % in OVX. At this step, carbon dioxide is released from carbonated apatite [14]. The mass loss in this step can be attributed to adsorbed water and partial dehydration of hydroxyapatite [15]. The total mass loss was 39.12 ± 0.45 % in OVX, whereas in CTR groups, it was 32.45 ± 1.01 %, which shows significant difference (p = 0.0038) between the groups.
DTA curves (Fig. 3) for the bone sample show an endothermic peak in the temperature range of approximately 35–164 °C and exothermic events between 190 and 625 °C. The formation of bone requires hydroxyapatite (inorganic component), collagen (organic component) and water. These data confirm the results obtained by TG/DTG showing the first event of water loss. The second stage is related to the decomposition of the organic phase from 190 to 640 °C. The last exothermic event is related to the elimination of carbonaceous material and the dissociation of calcium carbonate (CaCO3). At this step, the organic material was separated from the mineral component [15].
The data analysis of bone mineral density (BMD) presented significant difference (p = 0.01985) between CTR (0.413 ± 0.021 g cm−2) and OVX groups (0.347 ± 0.009 g cm−2). These data show that after ovariectomy, a significant bone mineral loss occurred as expected. The ovariectomy osteoporosis model mimics the bone loss that occurs with estrogen deficiency in postmenopausal women. The OVX rat model is widely used as a preclinical model to evaluate bony changes in postmenopausal osteoporosis [23, 24].
The calcium bone content was assessed in CTR and OVX groups, and the comparisons between the two groups demonstrated significant differences in bone calcium levels (p = 0.016). The atomic absorption spectrometry results from the calcium amount in the bones of ovariectomized rats (199.86 ± 8.08 calcium/μg g−1) were lower than those observed in the CTR group (229.62 ± 10.02 calcium/μg g−1). These findings demonstrate the effectiveness of the experimental model for osteoporosis, as previously reported by Netto et al. (2006).
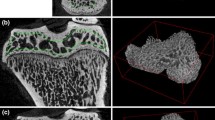
SEM micrographs revealed a structural difference in the trabecular architecture of the OVX and CTR femur (Fig. 4). OVX presented bone porosity, whereas CTR showed denser trabecular structure. The relevance of this finding lies in the fact that the dense bone structures of CTR increased bone strength and fracture toughness. Osteoporosis causes a loosened bone structure with poor mechanical performance in ovariectomized animals [25]. According to Changa et al. (2011), once bone mineral loss occurs, the features and arrangements of collagen fibers and hydroxyapatite crystals are also expected to change [26, 27]. The porosity observed in OVX may be a result of shorter and randomly distributed collagen fibers, as well as loosely packed hydroxyapatite crystals, in comparison with CTR. Furthermore, the SEM results appear to confirm our data from the TG/DTA analysis, bone mineral density (BMD) assay, atomic absorption spectrometry and biochemical assessment of alkaline phosphatase serum levels.
Conclusions
We provide the first evidence that ovariectomy-induced bone loss in a rodent model can be successfully assessed using physical–chemical techniques based on thermal analysis, atomic absorption spectrometry and scanning electronic microscopy. Therefore, these methods can be useful in analyzing the loss/gain of bone mass in further experimental studies on postmenopausal osteoporosis in a rodent model.
Abbreviations
- OVX:
-
Ovariectomized rats
- CTR:
-
Control group
- BMD:
-
Bone mineral density
- DTA:
-
Differential thermal analysis
- TG/DTG:
-
Thermogravimetry/derivative thermogravimetry
- SEM:
-
Scanning electron microscopy
- PTH:
-
Parathyroid hormone
References
Jankovic B, Kolar-Anic L, Smiciklas I, Dimovic S, Arandelovic D. The non-isothermal thermogravimetric tests of animal bones combustion. Part. I. Kinetic analysis. Thermochim Acta. 2009;495:129–38.
Giavaresi G, Fini M, Gnudi S, Aldini NN, Rocca M, Carpi A, Giardino R. Comparison of calcitonin, alendronate and fluorophosphate effects on ovariectomized rat bone. Biomed Pharmacother. 2001;55:397–403.
Cruz L, Assumpção E, Andrade SF, Conrado DJ, Kulkamp IC, Guterresa SS, Pohlmanna AR. Gastroresistant microparticles containing sodium alendronate prevent the bone loss in ovariectomized rats. Eur J Pharm Sci. 2010;40:441–7.
Pérez-Lopez FR. Postmenopausal osteoporosis and alendronate. Maturitas. 2004;48:179–92.
Stepan JJ, Alenfeld F, Boivin G, Feyen JHM, Lakatos P. Mechanisms of action of antiresorptive therapies of postmenopausal osteoporosis. Endocr Regul. 2003;37:225–38.
Netto CC, Franco M, Cunha MSCA, Miyasaka CK. Efeitos da ovariectomia experimental no metabolismo ósseo deratas wistar adultas: um modelo para estudo da osteoporose. Rev Ci Méd Biol. 2006;5(3):231–8.
Agrawal T, Verma AK. Cross sectional study of osteoporosis among women. Med J Armed Forces. 2013;69(2):168–71.
Okazaki N, Chiba K, Taguchi K, Nango N, Kubota S, Ito M, Osaki M. Trabecular microfractures in the femoral head with osteoporosis: analysis of microcallus formations by synchrotron radiation micro CT. Bone. 2014;2:113–6.
Coughlan T, Dockery F. Osteoporosis and fracture risk in older people. Clin Med. 2014;14(2):187–91.
Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–7.
Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58(5):424–30.
Turner RT, Maran A, Lotinun S, Hefferan T, Evans GL, Zhang M, Sibonga JD. Animal models for osteoporosis. Rev Endocr Metab Disord. 2001;2(1):117–27.
Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409.
Peters F, Schwarz K, Epple M. The structure of bone studied with synchrotron X-ray diffraction, X-ray absorption spectroscopy and thermal analysis. Thermochim Acta. 2000;361:131–8.
Kusrini E, Sontang M. Characterization of x-raydiffraction and electronspin resonance: effects of sintering time and temperature on bovine hydroxyapatite. Radiat Phys Chem. 2012;81:118–25.
Chen JR, Lazarenko OP, Zhang J, Blackburn ML, Ronis MJJ, Badger TM. Diet-derived phenolic acids regulate osteoblast and adipocyte lineage commitment and differentiation in young mice. J Bone Miner Res. 2014;29(5):1043–53.
Jee WSS, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskel Neuron Interact. 2001;1:193–207.
Funck-Brentano T, Biver E, Chopin F, Bouvard B, Coiffier G, Souberbielle JC, Garnero P, Roux C. Clinical utility of serum bone turnover markers in postmenopausal osteoporosis therapy monitoring. A Systematic Review Semin Arthritis Rheu. 2011;41(2):157–69.
Serakides R, Nunes VA, Nascimento EF, Silva CM, Ribeiro AFC. Relação tireóide-gônadas e níveis plasmáticos de fósforo, cálcio e fosfatase alcalina em ratas. Arq Bras Med Vet Zootec. 2000;52(6):579–85.
Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;45:1353–8.
Sato T, Fukazawa Y, Kojima H, Ohta Y, Iguchi T. Multiple mechanisms are involved in apoptotic cell death in the mouse uterus and vagina after ovariectomy. Reprod Toxicol. 2003;17:289–97.
Wegorzewska IN, Walters K, Weiser MJ, Cruthirds DF, Ewell E, Larco DO, Handa RJ, Wu TJ. Post ovariectomy weight gain in female rats is reversed by estrogen receptor alpha agonist, propylpyrazoletriol. Am J Obstet Gynecol. 2008;199:67.e1–5.
Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15(3):175–91.
Jee JH, Lee W, Lee BD. The influence of alendronate on the healing of extraction sockets of ovariectomized rats assessed by in vivo micro-computed tomography. Oral Surg Oral Med O. 2010;110(2):e47–53.
Bagi CM, Wilkie D, Georgelos K, Williams D, Bertolini D. Morphological and structural characteristics of the proximal femur in human and rat. Bone. 1997;21(3):261–7.
Changa YT, Chenb CM, Tub MY, Chend HL, Changa SY, Tsaib TC, Wanga YT, Hsiaoa HL. Effects of osteoporosis and nutrition supplements on structures and nanomechanical properties of bone tissue. J Mech Behav Biomed. 2011;4:1412–20.
Olszta MJ, Cheng SS, Jee R, Kumar YY, Kim MJ, Kaufman EP, Douglas LB, Gower M. Bone structure and formation: A new perspective. Sci Eng Rep. 2007;58:77–116.
Redlich K, Pietschmann P, Štulc T, Peterlik M. Comparative study on the effect of calcium channel blockers on basal and parathyroid hormone-induced bone resorption in vitro. Basic Clin Pharmacol Tocicol. 1997;80:262–5.
Acknowledgements
This work was supported by Grants from National Council of Technological and Scientific Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq/Brazil) and the Research Supporting Foundation of State of Sergipe (Fundação de Amparo à Pesquisa do Estado de Sergipe/FAPITEC/SE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lima, C.M., Serafini, M.R., Santos, G.P. et al. Use of bone physicochemical characterization and biochemical analyses in an experimental model. J Therm Anal Calorim 123, 2179–2184 (2016). https://doi.org/10.1007/s10973-015-4887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4887-3