Abstract
Polyesters were obtained from phthalic anhydride as acidic components and an alcoholic mixture of monoethylene glycol and n-propanol (in ratio 1:0.15). Molar ratio of the acid component versus alcohol component was 1:2, 1:1.8, 1:1.25 and 1:0.7. Obtained polyesters were characterized by: TG/DTG/Heat Flow (in synthetic air, respectively, in nitrogen atmosphere), EGA (evolved gas analysis), elemental analysis and FT-IR/UATR spectroscopy of polyesters and residues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyesters are widely used as technical polymers obtained by polycondensation of a large group of carboxylic components and di- and/or polyols. Aromatic carboxylic compounds (isophthalic and terephthalic acids) together with aliphatic carboxylic compounds (adipic acid and sebacic acid) as well as the phthalic and maleic anhydride are used as dicarboxylic components in the polymerization process. The most used diol, in the process, is the ethylene glycol. Also 1,2-propanediol and 1,3-butanediol and polyhydroxylic compound as trimethylol propane and pentaerythritol are often used during the synthesis process. Depending on the nature of the macromolecular chain (linear or branched as well as saturated or unsaturated), there are large variety of using fields: fibres and threads, paints and varnishes, polyurethanes, glass silk-polyester composites, etc. [1–7].
Only the polyesterification with di- and/or polyfunctional reaction partners conducted to very high molecular mass, with high viscosity, i.e. with manufacturing difficulties. In order to avoid these unpleasant aspects and to obtain a controlled polycondensation degree (mean value), monohydroxylic alcohols were used as chain breakers [8], especially n-propanol and n-butanol.
The aim of this paper is the study of the thermal behaviour of a linear phthalic anhydride—ethylene glycol polyester in respect of the anhydride–glycol–chain breaker molar ratio, n-propanol being the chain breaker.
The experimental protocol was similar with that presented in our previous papers [9–16] that regarded the thermal behaviour of different polymeric materials by means of a TG-DTG-FTIR-EGA hyphenated analytical technique.
Materials and methods
Synthesis
O-phthalic anhydride (AF) used in this study was purchased from Merk (mp. 130–131 °C), and p-toluene sulphonic acid (APTS) and monoethylene glycol (MEG) were purchased from Sigma-Aldrich (MEG; bp. = 196–197 °C; n20 = 1.430, d = 1.12 g cm−3). n-propanol (Pr-ol) was purchased from Fluka (bp. 96–97 °C; n20 = 1.385; d = 0.80 g cm−3).
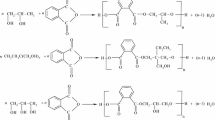
The synthesis takes place according to Scheme 1, catalyst being the p-toluene sulphonic acid.
Classical esterification equipment was used; a Dean-Stark separator was introduced between the condenser and the three-necked reaction vessel, for the water—alcohol separation and the alcohol recirculation, taking in to account that reaction water was removed as water—propanol azeotrope.
The reaction temperature in the range 170–230 °C is controlled especially by the azeotrope distillation. The reaction time (10–15 h) is determined by reaching a constant acidity index.
The data on the different molar ratio of the samples are systematized in Table 1.
Elemental analysis was performed with Costech Instruments-Elemental Combustion System 4010. Results are presented in Table 2.
FTIR spectra
The FTIR spectra were drawn up with a PerkinElmer Spectrum 100 device, using the UATR techniques for the solid phases (compounds before and after the thermal treatment) for identification of functional groups [17, 18] and an IR gas cell for the EGA technique. Evolved gases were identified using the Gas Vapor Library (Sadtler Spectral Databases).
Thermal analysis
The thermoanalytical curves TG, DTG and Heat Flow were obtained on a PerkinElmer Diamond device, using Al crucibles. The experiments were performed in dynamic atmosphere (100 cm3 min−1) of nitrogen, respectively, synthetic air, at heating rates of 10 °C min−1, respectively, 20 °C min−1, in synthetic air for the EGA study. The evolved gas analysis (EGA) was carried out by a coupled TG/FT-IR technique, using a PerkinElmer SPECTRUM 100 devices with an IR gas chamber connected to the exit of the DIAMOND furnace.
Results and discussions
Results of elemental analysis are presented in Table 2.
The FTIR analysis (see Fig. 1) of synthesized polyesters revealed a decreasing absorption of intensities of bands in the 1760–1670 cm−1, characteristic for ν C=O stretching vibrations of phthalic anhydride, indicating its consumption and confirming that polyester formation took place.
FTIR spectra of the four polymeric samples are similar. FTIR bands were observed at 2950–2800 cm−1, corresponding to the ν C–H (aliphatic –CH2– and aromatic –CH groups) vibrations. The shifting of the stretching vibration ν C=O from 1760–1670 to 1723–1720 cm−1 confirms the formation of polyester. The intense peaks that appear at 1270–1255 cm−1 are assigned to the stretching vibration ν C–O–C , the bands at 1120–1109 cm−1 to stretching vibration ν C–O–C and the ones at 1070–1042 cm−1 to the stretching vibration ν C–C-O of polyesters, respectively.
Peaks appearing around 742–709 cm−1 are assigned to the out-of-plane deformation vibration δ C–H , characteristic for 1,2-disubstituted benzene moiety.
Thermal analysis
The results of thermal analysis in dynamic synthetic air atmosphere are presented in Fig. 2.
Thermal stability until 330 °C decreases in the order: 1:2 > 1:1.25 > 1:1.8 > 1:0.7 (see Fig. 3). Over this temperature, the most stable is polyesters with molar ratio 1:1.25
The results of thermal analysis in dynamic nitrogen atmosphere are presented in Fig. 4.
Thermal stability decreases in the order: 1:2 > 1:1.25 > 1:1.8 > 1:0.7 (see Fig. 5) until 330 °C; over this temperature, the most reduced mass loss belongs to the polyester with molar a ratio of 1:1.25.
Comparative FT-IR/UATR spectroscopy of polyesters and residues was performed using a PerkinElmer Spectrum 100 FT-IR Spectrometer with UATR, by comparison of the recorded spectra of the polyesters with the ones of the residues after being heated, in air, at 280 and at 380 °C (all the studied samples are similar, as one example shown in Fig. 6).
The comparison of the UATR-FTIR spectra of the initial sample with one of the residues remaining after heated in air provides some valuable supplementary information:
-
the peak at 1255 cm−1 is shifted to higher values (until 1280 cm−1) with increasing of the thermodegradation degree/temperature;
-
at 1719–1720 cm−1, it is a sharp peak by both fresh and thermally treated samples, corresponding to acyclic ketones;
-
the bands in range of 1118–1112 cm−1 are assigned to a 1:2 disubstituted aromatic ring, but also to secondary alcohols;
-
the peaks of 742–744 cm−1 are for an aliphatic –CH2– skeletal vibration and for an out-of-plane C–H vibration of an 1:2 disubstituted aromatic ring;
-
the 910 cm−1 peak corresponding to low molecular phthalates is absent.
Evolved gases spectra
The FTIR spectra of the evolved gases by all the studied samples are similar (see an example in Figs. 7 and 8). The most significant peaks are at:
-
1863 cm−1; C=O stretching, CHR1=CH2 overtone of C–H stretching.
-
1806 cm−1; benzene ring substitution patterns, CH=CH2 overtone of C–H stretching.
-
1255 cm−1; aryl esters and/or unsaturated esters.
-
1103 cm−1; 1,2 disubstituted benzene ring.
-
910 cm−1; phthalates.
The IR spectra of the EG indicate a partial thermooxidative destruction of the polymeric chain. The heavy compound in the EG corresponds to low ortho-disubstituted phthalic compounds. Noticeable is the absence of the patterns for carbon oxides and water vapour, a clear sign for a “cutting” of the macromolecules into lower chain, some of them being “volatile” at the temperature/flow conditions. Another part of the initial macromolecular chain remains as “non-volatile” char, but essentially with the same functional groups.
Conclusions
This study presents the synthesis of polyesters obtained from phthalic anhydride as carboxylic components and a mixture of monoethylene glycol and n-propanol. Molar ratio of the acid component and alcohol component was 1:2, 1:1.8, 1:1.25 and 1:0.7. Physico-chemical characterization was performed by means of elemental analysis, FT-IR/UATR spectra of prepared samples and char remaining after thermal treatment at different temperatures, and TG/DTG/Heat Flow in air and in nitrogen, respectively.
Thermal stability is same in air as in nitrogen atmosphere, respectively. Thermal stability (until 300–320 °C) decreases in the order: 1:2 > 1:1.25 > 1:1.8 > 1:0.7.
According to the TG and EGA data, the following remarks are possible:
-
by the 1:2 molar ratio, a rather homogeneous product was obtained, i.e. the variation of polymerization degree is in a small range;
-
in comparison with this, by 1:1.25 molar ratio, two range of decomposition appear, one (30 mass%) is less thermostable, whereas 70 % is stable over 330 °C; this suggests the existence of two range of molecular mass distribution;
-
by 1:0.7 and 1:1.8, it clearly appear two well-separated decomposition steps, but the lower molecular part is higher (≈65 mass%, respectively, 55 %)
The above observations are useful for practical recommendation. So the used hyphenated techniques seem to be useful for establishing both manufacturing and working conditions of polyester materials.
References
Edlund U, Albertsson AC. Polyesters based on diacid monomers. Adv Drug Deliv Rev. 2003;55:585–609.
Guimarães DH, Brioude MM, Fiúza RP, Prado LASA, Boaventura JS, José NM. Synthesis and characterization of polyesters derived from glycerol and phthalic acid. Mater Res. 2007;10(3):257–60.
Cherian B, Thachil ET. Synthesis of unsaturated polyester resin—effect of choice of reactants and their relative proportions. Int J Polym Mater. 2004;53:829–45.
Tang J, Zhang Z, Song Z, Chen L, Hou X, Yao K. Synthesis and characterization of elastic aliphatic polyesters from sebacic acid, glycol and glycerol. Eur Polym J. 2006;42:3360–6.
Kadkin O, Osajda K, Kaszynski P, Barber TA. Polyester polyols: synthesis and characterization of diethylene glycol terephthalate oligomers. J Polym Sci Pol Chem. 2003;41:1114–23.
Soulis SD, Triantou D, Weidner S, Falkenhage J, Simitzis J. Structural analysis of biodegradable low-molecular mass copolyesters based on glycolic acid, adipic acid and 1,4 butanediol and correlation with their hydrolytic degradation. Polym Degrad Stab. 2013;97:2091–103.
Dogan E, Acar AE. The use of anhydride linkages to increase the glass transition temperatures of polymers containing carboxyl end groups: a perspective in powder coatings. Prog Org Coat. 2013;76:513–8.
Patel BR, Patel KS, Patel RN, Patel KD. Thermal and mechanical properties of modified polyester resin and jute composite, Pelagia Research Library. Der Chemica Sinica. 2014;5(1):47–54.
Vlase T, Vlase G, Doca N, Ilia G, Fuliaş A. Coupled thermogravimetric-IR techniques and kinetic analysis by non-isothermal decomposition of Cd2+ and Co2+ vinyl-phosphonates. J Therm Anal Calorim. 2009;97:467–72.
Doca N, Vlase G, Vlase T, Ilia G. Thermal behavior of Cd2+ and Co2+ phenyl-vinyl-phosphonates under non-isothermal condition. J Therm Anal Calorim. 2008;94(2):441–5.
Vlase T, Bolcu C, Vlase G, Mogos A, Doca N. Thermooxidative stabilization of a MDI Polyol polyisocyanate. J Therm Anal Calorim. 2010;99:973–9.
Albu P, Bolcu C, Vlase G, Doca N, Vlase T. Kinetics of degradation under non-isothermal conditions of a thermooxidative stabilized polyurethane. J Therm Anal Calorim. 2011;105(2):685–9.
Vlase T, Vlase G, Doca N, Iliescu S, Ilia G. Thermo-oxidative degradation of polymers containing phosphorus in the main chain. High Perform Polym. 2010;22(7):863–75.
Vlase T, Doca N, Vlase G, Bolcu C, Borcan F. Kinetics of non-isothermal decomposition of three IRGANOX-type antioxidants. J Therm Anal Calorim. 2008;92:15–8.
Bolcu C, Modra D, Vlase G, Doca N, Mihali C, Vlase T. Synthesis and thermal behaviour of some diisocyanate-silane compounds. J Therm Anal Calorim. 2014;115(1):489–94.
Bolcu C, Vlase G, Vlase T, Albu P, Doca N, Şisu E. Thermal behavior of some polyurethanes reticulated by aminated maltose. J Therm Anal Calorim. 2013;113(3):1409–14.
Silverstein RM, Clayton Basster G, Morrell T. Spectrometric identification of the compounds. 5th ed. New York: Wiley; 1995.
Cross AD. An introduction to practical infra-red spectroscopy. London: Butterworths; 1960.
Acknowledgements
This work was supported by POSCCE Grant No. 12PO102418/5124/22.05.2014, SMIS 50328: “New energetic efficient technology for synthesis of polyester copolymers.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albu, P., Vlase, G., Modra, D. et al. Thermal behaviour of the polyesters obtained with different molar ratios of carboxyl hydroxyl components. J Therm Anal Calorim 121, 1031–1037 (2015). https://doi.org/10.1007/s10973-015-4783-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4783-x