Abstract
The NaYF4:Yb3+,R3+ (R: none, Pr, Nd, Sm, Eu, Tb or Dy) materials were prepared with selected dopant concentrations, using co-precipitation synthesis, to study the effect of the dopant and its concentration on the structure of these materials. The thermal behaviour of the as-prepared materials was studied with differential scanning calorimetry (DSC) and thermogravimetry. The structures prior to and after annealing were identified with X-ray powder diffraction. The materials were mainly hexagonal with occasional slight cubic impurity. The DSC curves revealed the cubic-to-hexagonal phase transition at 400–450 °C which temperature changes irregularly with the R3+ dopant and its concentration. The specific enthalpy of this transition varies also in a complicated way but may be correlated with the completeness of the transition. The hexagonal-to-cubic transition temperature (ca. 670 °C) is rather constant regardless of the R3+ dopant or its concentration. The temperatures and specific enthalpies of the phase transitions are useful when choosing the optimum dopant concentrations. It is also possible to estimate the structure prior to annealing with significant savings in use of resources. Only with Sm3+ and Dy3+ doping, no visible up-conversion luminescence was observed—in addition to the Er3+ and Tm3+ impurity emission. Eventually, it was found that the hexagonal form gives much stronger up-conversion luminescence and changes in the rare earth concentration allows the formation of this form.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In up-conversion luminescence, absorption of two or more low-energy photons is followed by emission of a high-energy photon [1]. This phenomenon allows the conversion of NIR radiation usually to visible but nowadays increasingly also to NIR or UV radiation. Up-conversion luminescence can be useful in applications such as enhancement of photosynthesis [2], solar cells [3] and medical imaging [4]. In the two former applications, up-conversion luminescence materials can be used to convert otherwise unusable radiation to visible and thus to enhance the overall efficiency. In medical imaging, the up-converting materials enable NIR excitation, which is not absorbed by blood or tissues. Interference from autoluminescence and tissue damage are then generally avoided. A key aspect of up-conversion luminescence materials in all applications is obviously strong luminescence, and also nanoscale size is required in, e.g. medical imaging. Unfortunately, up-conversion luminescence is still rather weak for materials in nanoscale. It is thus important to enhance the performance of the materials to achieve as strong up-conversion luminescence as possible.
NaYF4 has been recognized as one of the most feasible hosts for efficient up-conversion luminescence [5–7]. The NaRF4 (R: e.g. Y, Yb and Er) materials have three different structures [8, 9]: low-temperature (LT) cubic (α-NaRF4; Fm\( \bar{3} \)m (#225), Z: 2 [10]), hexagonal (β-NaRF4; P63/m (#176), Z: 1.5 [5, 10]) and high-temperature (HT) cubic, whose structure lacks detailed characterization. The hexagonal β form is the best for up-conversion luminescence [5–7]; therefore, the materials should be obtained in this form. This is achieved by heating the cubic α form at a temperature between the cubic-to-hexagonal (α- to β-NaRF4) and hexagonal-to-HT cubic phase transitions, which occur around 400 and 700 °C, respectively. Both phase transitions are irreversible [11], and after cooling, all three structures can be quenched to room temperature.
Up-conversion luminescence has been observed from several rare earths (R: Pr [12], Nd [13], Sm [14], Eu [15], Tb [16], Dy [17], Ho [18], Er [5–7, 19, 20] and Tm [5, 20]). With the NaYF4 host, the hexagonal structure yields the strongest luminescence [5–7]. The goal is thus to have as small nanomaterials with as strong luminescence as possible. Naturally, the annealing temperatures should then be kept as low as possible to achieve this trade-off. In this work, NaYF4:Yb3+,R3+ (R: none, Pr, Nd, Sm, Eu, Tb or Dy) materials were studied to determine the optimum annealing temperature as a function of dopant and its concentration. Finally, a relationship between the thermal behaviour of the material and the structure was established. The structure and phase purity of the as-prepared and post-annealed materials were analysed with X-ray powder diffraction (XPD). The thermal behaviour of the as-prepared materials was studied with differential scanning calorimetry (DSC) and thermogravimetry (TG). The ambient up-conversion luminescence of the post-annealed materials was routinely verified with NIR laser excitation at 976 nm at room temperature.
Materials and methods
Materials preparation
The NaYF4:Yb3+,R3+ (R: none, Pr, Nd, Sm, Eu, Tb or Dy) materials were prepared with selected dopant concentrations (x Yb: 0.01–0.20 and x R: 0.001–0.04) using co-precipitation [19]. This involved the dissolution of solid NaF to distilled water, which was further mixed with aqueous solution of mixed YCl3, YbCl3 and RCl3 to obtain the stoichiometry desired. A Na/R ratio of 12.5 was used to force the formation of the NaRF4 phase instead of the RF3 one. After stirring for 1 h at room temperature, the precipitate thus formed was centrifuged (3766g) and washed thrice with water and once with ethanol. Each washing was followed by centrifuging. The precipitate was then dried in a vacuum desiccator at room temperature. Based on the DSC results, β form was obtained with annealing at 500 °C for 5 h in a static N2 + 10 % H2 gas sphere (the annealing temperature of 500 °C was considered sufficient to obtain the β-NaRF4). After annealing, the materials were cooled down freely to the room temperature under the same gas sphere. More N2 + 10 % H2 gas was added to the quartz reactor during the cooling to prevent replacement air to enter.
Characterization
The structure and phase purity of the materials were analysed with the XPD measurements. The patterns were collected at room temperature with a Huber G670 image plate Guinier camera (2θ range 4–100°, data interval 0.005°) with monochromatic copper Kα1 radiation (λ: 1.54056 Å). The asymmetry of the reflections at low angles is due to the apparatus used. Even for Rietveld structure refinements, this effect can still be corrected with success, however.
The thermal behaviour of the as-prepared materials was studied with a TA Instruments SDT Q600 simultaneous TGA-DSC apparatus between 25 and 720 °C in flowing N2 (flow rate: 100 cm3 min−1). A heating rate of 5 °C min−1 and sample mass of ca. 10 mg were used. The sample pan was made of aluminium oxide, and a similar but empty pan was used as the reference material. The specific enthalpies of the phase transitions were calculated by integration of the corresponding DSC signal area.
The up-conversion luminescence spectra of the materials inside a rotating capillary tube were measured at room temperature with an Ocean Optics PC2000-CCD spectrometer. The NIR excitation (λ exc: 976 nm) source was a Hamamatsu L9418-04 NIR laser diode. Optical long- and short-pass filters were used before and after the sample, respectively. The emission was guided to the spectrometer with an optical fibre.
Results and discussion
Structure and phase purity
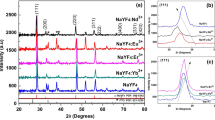
The as-prepared as well as post-annealed (for 5 h @ 500 °C) NaYF4:Yb3+,R3+ (R: Pr, Nd, Sm, Eu, Tb, Dy or none) have cubic and hexagonal structures, respectively, as revealed by the XPD measurements. Very slight amounts of the cubic form (Fig. 1) were also observed with the following annealed R3+ doped materials: Yb3+ only: x Yb: 0.10 or 0.15, Yb3+,Sm3+: x Yb: 0.20 and x Sm: 0.02, Yb3+,Nd3+: x Nd: 0.002 or 0.02, Yb3+,Eu3+ : x Eu: 0.002 or 0.02 and Yb3+,Tb3+: x Tb: 0.04 or x Yb: 0.10. For these materials, the exothermic α–β DSC signal was at higher temperature and also weaker than for the pure hexagonal ones; this will be discussed in more detail in the next chapter. Other impurities, e.g. crystalline NaF, were not observed. However, there might be amorphous NaF present since its presence has proven to be crucial to the formation of the hexagonal structure [21].
Thermal behaviour
The DSC curves of all NaYF4:Yb3+,R3+ revealed both an exothermic signal between ca. 400 and 450 °C and an endothermic one at ca. 670 °C (Fig. 2) due to the formation of the β and HT cubic phase of NaYF4:Yb3+,R3+, respectively. At least the α- to β-NaRF4 phase transition is irreversible (see the end of the chapter). In general, the temperature of this transition seems to decrease with decreasing R3+ dopant size. However, the temperature differences are not very large; the largest difference is 5 °C when the dopant concentration is the same: x R: 0.02 (R: Nd, Sm, Eu, Tb or Dy). Along with the concentration of the same R3+ dopant, the α- to β-NaRF4 transition temperature and its variation behave more irregularly than with different R3+ dopants. For instance, with Tb3+ doping, the largest difference is 18 °C (x Tb: 0.005–0.04 and x Yb: 0.20; Fig. 3). In contrast, the hexagonal-to-HT cubic phase transition temperature is rather constant regardless of the R3+ dopant and its concentration (Figs. 2, 3; Table 1). No clear reason was found for this difference (magnitude of variation) between the α- to β-NaRF4 and hexagonal-to-HT cubic transition temperatures. However, the stoichiometry and structure of β-NaRF4 are more well defined than those of α-NaRF4, so the hexagonal-to-HT cubic phase transition involves lesser and less varied changes than the α- to β-NaRF4 transition.
With the NaYF4:Yb3+ materials, the α- to β-NaRF4 transition temperature increases and the hexagonal-to-HT cubic one decreases with decreasing Yb3+ concentration (Table 1). At the same time, the specific enthalpy of both signals becomes substantially lower; this implies that the former transition is incomplete. This conclusion was confirmed by the XPD measurements; the pure hexagonal form (without any cubic) was obtained only with the highest Yb3+ concentration (when annealed at 500 °C). However, the Yb3+ concentration has a very weak effect on the α- to β-NaRF4 transition temperature of the NaYF4:Yb3+,Pr3+ materials. The reason for this inconsistence is still unclear, and further studies are needed to elucidate this observation. The hexagonal-to-HT cubic transition temperature of the Pr3+-doped materials decreases notably with increasing Yb3+ concentration.
With the NaYF4:Yb3+,Nd3+ materials, the α- to β-NaRF4 transition temperature attained the lowest limit with x Nd: 0.03, but the difference to the highest temperature was only 6 °C (x Nd: 0.002; Table 1). This suggests that 3 % is the optimum concentration of Nd3+ in NaYF4:Yb3+,Nd3+, when considering the structure alone and not the up-conversion luminescence. The hexagonal-to-HT cubic transition temperature behaves in the opposite way; it is the highest when x Nd: 0.03 and the lowest when x Nd: 0.002. Again the difference is small, only 4 °C. The specific enthalpies yielded similar results and the highest values were observed when x Nd: 0.03. These observations also supported the XPD measurements; the cubic form was observed when x Nd: 0.002 or 0.02. In general, it was interesting to find an optimum concentration at all since the difference between the ionic radius of Y and Nd is rather large, which suggests that the stability of β-NaRF4 would be the highest for the lowest dopant concentrations. A good example in this context is the much used laser material, Nd3+-doped Y3Al5O12 (Nd:YAG) [22].
The α- to β-NaRF4 transition temperatures of the Sm3+ and Dy3+-doped materials rise with the R3+ concentration (Table 1). However, the differences are quite small: only 4 and 8 °C, respectively. The hexagonal-to-HT cubic phase transition temperature is the same with both concentrations (the same R3+). The specific enthalpies of both transitions were smaller for the higher Sm3+ concentration; with Dy3+ doping, these were almost the same.
With Eu3+ doping, the α- to β-NaRF4 and hexagonal-to-HT cubic transition temperatures increase and decrease with decreasing Eu3+ concentration, respectively (Table 1). For the europium concentrations 0.03 or 0.04, the α- to β-NaRF4 transition temperature is the same and the specific enthalpies are notably higher than for materials with lower concentrations, suggesting that the dopant concentration may be close to optimal. As expected, also these observations support the XPD measurements where the cubic form was observed when x Eu was 0.002 or 0.02.
The transition temperatures of the NaYF4:Yb3+,Tb3+ materials vary irregularly when the Yb3+ concentration is constant (x Yb: 0.20; Fig. 3). When x Tb: 0.04, the specific enthalpy of the α- to β-NaRF4 transition signal is significantly weaker and temperature is higher than with other dopant concentrations (Table 1). With constant Tb3+ concentration (x Tb: 0.03), the α–β transition signal was at a radically higher temperature than with other Yb3+ concentrations, and the specific enthalpies of both signals were much lower with the lowest Yb3+ concentration (x Yb: 0.10). These two materials (x Yb: 0.20 + x Tb: 0.04 and x Yb: 0.10 + x Tb: 0.03) were the only ones with the cubic form present.
Based on the DSC results, the specific enthalpies of the α–β transition of the NaYF4:Yb3+,R3+ materials were eventually correlated with the intensity ratio between the XPD reflections 111 and 110 for the α- and β-NaRF4 forms, respectively (Fig. 4). The following correlation between the amount of the cubic form and the specific enthalpy of the cubic-to-hexagonal transition is tentatively suggested: the larger is the specific enthalpy, less cubic form is present as well. This enables the estimation of the amount of the cubic form using the specific enthalpy measured with DSC. So far, the cubic form has not been obtained if the specific enthalpy is >65 J g−1. Annealing and XPD measurements are thus not necessary to identify which materials would contain cubic form after annealing. This can save significantly both human and material resources when testing different dopants and dopant concentrations and avoiding the preparation of materials with the cubic form.
The DSC signal of the α–β transition was observed to broaden when the corresponding temperature rises. This may mean that there are various as-prepared products with almost the same compositions and thus with slightly different transition temperatures. The experimental conditions such as the packing density and crystallite size of the material affect the signal width as well, but these parameters were not studied in this work.
The nature of the phase transitions was studied by measuring the DSC signal also during the cooling step (Fig. 5) because the high-temperature forms can be quenched to room temperature retaining their structures. DSC signals were not obtained in contrast to heating measurements. This indicates that the α- to β-NaRF4 transition is irreversible, but the reason is not so clear though the generally better stoichiometry of the hexagonal phase is a plausible explanation. The absence of the signal for the HT cubic-β transition may be more easy to explain since the NaRF4 composition starts to decompose (slowly) immediately above the β-HT cubic transition. As shown by the TG curve (Fig. 6), the NaRF4 → NaF(g) + RF3 decomposition depletes the NaRF4 phase of NaF and the HT-cubic-β reverse transition is then prevented. The phase transitions may thus depend on the exact stoichiometry of the material. And the other way round, such phase transitions may be triggered by changes in composition. The TG curves may offer a similar explanation to the α–β transition since a slight mass change can be found close to the phase transition temperature. This change is much less than the one at higher temperature but evidently enough to prevent the α–β transition to be reversible.
In addition to the mass changes around phase transitions, the TG curves of NaYF4:Yb3+,R3+ (R: Pr, Nd, Sm, Eu, Tb, Dy or none) showed a total mass loss of ca. 2–4 % during heating to 500 °C (Fig. 5), attributed mainly due to the removal of water, ethanol and possible crystal water (all below 300 °C). Only small mass changes were observed close to the chosen heating temperature (500 °C).
Up-conversion luminescence
With NaYF4:Yb3+,R3+ materials studied here, up-conversion luminescence was obtained from the following rare earths: Pr3+, Nd3+, Eu3+, Tb3+, Yb3+ and also from the Er3+ and Tm3+ impurities in the Y3+/Yb3+ precursor. Up-conversion luminescence was not obtained from Sm3+ and Dy3+. The dopant concentrations with the strongest up-conversion luminescence varied significantly between Pr3+ and the other dopants: x Yb: 0.10 + x R: 0.001 (R: Pr3+), 0.20 + 0.04 (Nd3+), 0.20 + 0.03 (Eu3+), 0.15 + 0.03 (Tb3+) and 0.20 + 0 (none). The large difference in concentrations between Pr3+ and the other dopants, one order of magnitude, is due to, for example, cross-relaxation mechanisms. A detailed discussion on the up-conversion luminescence properties of the NaYF4:Yb3+,R3+ materials is out of scope of this work and will be published elsewhere in the future.
Up-conversion luminescence was observed of the Er3+ (and Tm3+) impurities with several NaRF4 materials because they are excited extremely effectively in the presence of the Yb3+ sensitizer [21, 23]. This should always be kept in mind because such impurities are common in the heavier rare earth oxide precursors due to the difficult separation of the heavier rare earths because of their very similar chemical properties. Er3+ emits at ca. 510–535, 535–565 and 630–685 nm due to the 2H11/2,4S3/2,4F9/2 → 4I15/2 transitions, respectively. Tm3+ emits at 460–485, 690–705 and 770–825 nm due to the 1G4,3F3,3H4 → 3H6 transitions, respectively. All of these Er3+ and Tm3+ impurity emissions were observed from the NaYF4:Yb3+,R3+ materials—in many cases with or without the actual dopant.
Conclusions
The structure of the NaYF4:Yb3+,R3+ (R: Pr, Nd, Sm, Eu, Tb, Dy or none) materials studied was mainly hexagonal, but trace amounts of the cubic form were present in some materials, as well. The amount of the cubic form, the temperature of the α- to β-NaRF4 transition and the specific enthalpy of this transition are intertwined. The rise in the temperature of the α- to β-NaRF4 transition DSC signal indicates that the cubic-to-hexagonal change becomes more difficult requiring a higher temperature or more energy. If the DSC signal weakens at the same time (lower specific enthalpy), even a higher temperature does not help and the structure does not change, at least not completely, to hexagonal. The specific enthalpy of the transition is the key factor. It enables to estimate the amount of cubic form in annealed NaYF4:Yb3+,R3+ materials prior to the annealing, which can save time and work. However, the theoretical reason behind this connection is still unclear and requires further studies, which will be carried out in the future. Finally, up-conversion luminescence was obtained of Pr3+, Nd3+, Eu3+, Tb3+, Er3+, and Tm3+ with the same Yb3+-doped NaYF4 host.
References
Auzel F. Upconversion and anti-stokes processes with f and d ions in solids. Chem Rev. 2004;104:139–73.
Antal T, Harju E, Pihlgren L, Lastusaari M, Tyystjärvi T, Hölsä J, Tyystjärvi E. Use of near-infrared radiation for oxygenic photosynthesis via photon up-conversion. Int J Hydrogen Energy. 2012;37:8859–63.
de Wild J, Meijerink A, Rath JK, van Stark WGJHM, Schropp REI. Upconverter solar cells: materials and applications. Energy Environ Sci. 2011;4:4835–48.
Ylihärsilä M, Valta T, Karp M, Hattara L, Harju E, Hölsä J, Saviranta P, Waris M, Soukka T. Oligonucleotide array-in-well platform for detection and genotyping human adenoviruses by utilizing upconverting phosphor label technology. Anal Chem. 2011;83:1456–61.
Krämer KW, Biner D, Frei G, Güdel HU, Hehlen M, Lüthi S. Hexagonal sodium yttrium fluoride based green and blue emitting upconversion phosphors. Chem Mater. 2004;16:1244–51.
Zhao J, Sun Y, Kong X, Tian L, Wang Y, Tu L, Zhao J, Zhang H. Controlled synthesis, formation mechanism, and great enhancement of red upconversion luminescence of NaYF4:Yb3+,Er3+ nanocrystals/submicroplates at low doping level. J Phys Chem B. 2008;112:15666–72.
Hyppänen I, Hölsä J, Kankare J, Lastusaari M, Pihlgren L, Soukka T. Preparation and up-conversion luminescence properties of NaYF4:Yb3+,Er3+ nanomaterials. Terrae Rarae. 2009;16:1–6.
Harju E, Hyppänen I, Hölsä J, Kankare J, Lahtinen M, Lastusaari M, Pihlgren L, Soukka T. Polymorphism of NaYF4:Yb3+,Er3+ up-conversion luminescence materials. Z Kristallogr Proc. 2011;1:381–7.
Thoma RE, Insley H, Hebert GM. Sodium fluoride–lanthanide trifluoride systems. Inorg Chem. 1966;5:1222–9.
PCPDFWIN v. 1.30, Powder Diffraction File, 1997, International Centre for Diffraction Data, entries 06-0342 (cubic NaYF4) and 28-1192 (hexagonal Na(Y0.57Yb0.39Er0.04)F4).
Mathews MD, Ambekar BR, Tyagi AK, Köhler J. J Alloys Compd. 2004;377:162–6.
Malinowski M, Joubert M-F, Jacquier B. Infrared to blue up-conversion in Pr3+ doped YAG and LiYF crystals. J Lumin. 1994;60–61:179–82.
Pollnau M, Hardman PJ, Clarkson WA, Hanna DC. Upconversion, lifetime quenching, and ground-state bleaching in Nd3+:LiYF4. Opt Commun. 1998;147:203–11.
Zhou Y, Lin J, Wang S. Energy transfer and upconversion luminescence properties of Y2O3:Sm and Gd2O3:Sm phosphors. J Solid State Chem. 2003;171:391–5.
Stręk W, Dereń PJ, Bednarkiewicz A, Kalisky Y, Boulanger P. Efficient up-conversion in KYb0.8Eu0.2(WO4)2 crystal. J Alloys Compd. 2000;300–301:180–3.
Stręk W, Bednarkiewicz A, Dereń PJ. Power dependence of luminescence of Tb3+-doped KYb(WO4)2 crystal. J Lumin. 2001;92:229–35.
Wermuth M, Riedener T, Güdel HU. Spectroscopy and upconversion mechanisms of CsCdBr3:Dy3+. Phys Rev B. 1998;57:4369–76.
Müller P, Wermuth M, Güdel HU. Mechanisms of near-infrared to visible upconversion in CsCdBr3:Ho3+. Chem Phys Lett. 1998;290:105–11.
Yi G, Lu H, Zhao S, Ge Y, Yang W, Chen D, Guo LH. Synthesis, characterization, and biological application of size-controlled nanocrystalline NaYF4:Yb,Er infrared-to-visible up-conversion phosphors. Nano Lett. 2004;4:2191–6.
Heer S, Kömpe K, Güdel HU, Haase M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide doped NaYF4 nanocrystals. Adv Mater. 2004;16:2102–5.
Hölsä J, Laihinen T, Laamanen T, Lastusaari M, Pihlgren L, Rodrigues LCV, Soukka T. Enhancement of the up-conversion luminescence from NaYF4:Yb3+,Tb3+. Phys B. 2014;439:20–3.
Geusic JE, Marcos HM, Van Uitert LG. Laser oscillations in Nd-doped yttrium aluminum, yttrium gallium and gadolinium garnets. Appl Phys Lett. 1964;4:182–4.
Kaczmarek SM, Leniec G, Typek J, Boulon G, Bensalah A. Optical and EPR study of BaY2F8 single crystals doped with Yb. J Lumin. 2009;129:1568–74.
Acknowledgements
Financial support is acknowledged from the Nordic Energy Research (AquaFEED project), the Academy of Finland (projects Energy Storage Luminophors 2 and Novel Rare Earth Optical Sensors and Materials—a bilateral project of Academy of Finland and CNPq, Brazil), the Graduate School of Materials Research (GSMR), as well as the University of Turku Graduate School (UTUGS), Turku, Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laihinen, T., Lastusaari, M., Pihlgren, L. et al. Thermal behaviour of the NaYF4:Yb3+,R3+ materials. J Therm Anal Calorim 121, 37–43 (2015). https://doi.org/10.1007/s10973-015-4609-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4609-x