Abstract
This study focuses on the preparation of camphene/palmitic acid mixture as novel phase change material for the first step of composite-based phase change materials. Camphene–palmitic acid-based composite phase change materials (PCMs) for thermal energy storage were prepared by vacuum impregnation method. The maximum incorporation percentage for camphene–palmitic into pyroclastic, fly ash, barite and marble powder were found to be 33.09, 55.5, 31.5 and 35.96 %, respectively. The differential scanning calorimetry analysis revealed that the composite PCMs were suitable applicants for building applications in terms of their appropriate phase change temperatures and extensive latent heat values. The SEM results showed that the homogeneity of the samples is good, and according to supporting materials porosity formation is observed. Thermal cycling test indicated that the form-stable composite PCMs have good thermal reliability and chemical stability although they were subjected to 2,000 melting/freezing cycling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In today’s technological developments and consume of energy resources, people are studying to find renewable energy sources and energy storage materials. In the aim of this, energy storage materials such as phase transition materials are good candidates for technological and environmental applications. The temperature of phase change materials (PCMs) remains constant or just shifts gently, while they absorb and release great amounts of thermal energy by changing from one phase to another [1–4]. Organic and inorganic PCMs can be used as phase change thermal energy storage materials. When organic PCMs compared with the inorganic PCMs, organic PCMs have lower heat of fusion than that of the inorganic PCMs. But the main advantages of the organic PCMs are that they have no subcooling and no corrosiveness. Besides these, they are not toxic and have stabilized performance, and many studies have been performed in this field [5–14].
In the whole of energy consumption, building sector is the major energy consumer with a total 40 % share [15–17]. In the last decade, the energy demand for buildings have increased popularity, due to enhancement of the building services and thermal comfort levels [18]. In addition, it is estimated that fossil fuels will continue to produce 75–80 % of the world’s primary energy by 2030 [19]. So, deficiency of fossil fuels and environmental apprehension has provided impetus to the development of sustainable building and renewable energy resources. One of the most potential sources of solar energy is believed to be direct solar energy [20]. But, the solar energy is interrupted, and its utilization requires efficient and appropriate energy storage system [21]. The phase change materials have attracted a large number of applications. PCMs that melt above 90 °C are used for absorption refrigeration, while PCMs that melt below 15 °C are used for storing coolness in air conditioning applications [22]. All other materials that melt between these two temperatures can be applied in solar water heater [23–26], solar air heating systems [27, 28], solar cookers [29, 30] and solar greenhouse [31, 32]. Besides these, PCMs have been considered for thermal storage in buildings since before 1980. With the progression of PCM implemented in wallboards, shutters and Trombe wall, under-floor heating systems and ceiling boards can be used as a part of the building for heating and cooling applications [33–41]. In this case, the heat indoors are stored in PCMs at the temperature higher than the melting point of PCMs, and the stored heat is released at the temperature lower than the melting point of PCMs at night. So, PCMs can decrease the temperature fluctuation indoor clearly and cut down the energy consumption in the buildings [42–47]. PCMs in building materials are generally enclosed in polymeric and metallic capsules. The encapsulating of PCM is expensive, and it may induce to seepage during the melting period of PCM as well as it may affect the mechanical strength of the building material. Thus, it is required to direct heat exchange between PCM and medium to provide higher thermal energy storage performance. Hence, mixing fatty acid with other organic and inorganic PCMs is an effective way to get a composite with a suitable phase change temperature and high latent heat. From this point of view, the PCMs including composite are promising materials that provide opportunity, no corrosion, quick heat transfer and suggest a large heat storage density [48–50]. Besides these, palmitic acid is one of the most important PCMs because of its attractive thermal and heat transfer characteristic, and camphene is bicyclic monoterpene, which is a minor constituent of many essential oils such as turpentine and cypress oil. Camphene can be an important organic phase change material for thermal energy storage that has a melting point at 37.90 °C with melting latent heat of 220.45 kJ kg−1 and a solidifying point at 28.7 °C with solidifying latent heat of 234.5 kJ kg−1. Consequently, these eutectic mixtures for supporting composite materials can be evaluated in buildings for thermal energy storage.
In this study, form-stable PCM consisting of camphene and palmitic acid was prepared. After this stage, camphene/palmitic acid (CP)-based composites were produced as novel potential PCMs for thermal energy storage in building practices. These composite-based PCMs were prepared by directly incorporation of camphene/palmitic acid in pyroclastic, fly ash, barite and marble powder as building materials. The CP and composite PCMs were characterized structurally by SEM and FTIR analyses techniques. Thermal energy storage properties and thermal reliability were determined by using DSC and thermal cycling test, respectively.
Materials and methods
Materials
All reagents were of analytical grade and were used without further purification. Camphene and palmitic acid were chosen to produce organic phase change material (PCM). Fly ash, barite, pyroclastic and marble powder are provided from Technology Faculty in Firat University.
The preparation of PCMs
The camphene and palmitic acid are weighted and uniformly mixed together in the beaker. After that, they are placed in a water bath with constant temperature of 80 °C and completely melted. Then, they are put in an ultrasonic cleaner for dispersion. Fatty acid mixture can be dispersed and mixed evenly by shock wave and micro-jet that are induced by ultrasonic. The above steps are repeated until they are uniformly mixed. After this step, vacuum operation was done to produce composite-based PCMs. Figure 1 demonstrates the process steps for making composite PCMs. The camphene/palmitic acid (CP) and building materials were embed in a vacuum furnace. As a beginning, the vacuum impregnation was done by vacuum furnace under vacuum pressure for 100 kPa for 6 h to depletion air from pores of building materials. After this step, CP was melted in 80 °C in the vacuumed furnace, and building materials were physically submerged in liquid CP for 4 h. Finally, the vacuum pump was switched off, and air was permitted to rejoin the furnace again to force the CP liquid to saturate into the pore space of the barite, fly ash, pyroclastic and marble powder composites, respectively [51].
Characterization of camphene–palmitic acid and composites
The morphology and microstructure of the camphene–palmitic acid and composite-based PCMs were observed using a scanning electronic microscope (SEM, LEO 440 model). The structural analysis of camphene–palmitic acid (CP) and camphene–palmitic acid-based composites was analyzed by using FTIR spectrometer. The spectra were recorded on a Perkin-Elmer infrared spectrometer as KBr pellets with a resolution of 4 cm−1 in the range of 400–4,000 cm−1. The thermal properties of PCMs were obtained using a differential scanning calorimeter (DSC, Shimadzu 60 WS), and DSC curve is tested with a heating rate of 5 °C min−1 in nitrogen atmosphere. The thermal stability of the composite PCMs was studied by means of thermogravimetry on a Shimadzu TA-60WS from room temperature to 600 °C with a heating rate of 15 °C min−1 in air atmosphere. The thermal reliability of the CP and the composite-based PCMs was evaluated with respected to change in the phase temperatures and latent heats after large number of thermal cycling. The thermal cycling consisted of exposing form-stable composite PCMs to a melting and freezing process. This was done by Applied Biosystem 96-well Thermal cycler.
Results and discussion
FTIR analysis of camphene/palmitic acid and camphene/palmitic acid composites
The FTIR spectra of the camphene, palmitic acid and their composite-based PCMs are shown in Figs. 2 and 3. Figure 2a shows the spectrum of the camphene, and the peak at 3,066 cm−1 signifies the stretching vibration of =C–H group, and the peak at 2,955–2,870 cm−1 represents the stretching vibration of its –CH3 groups. The peak at 1,656 cm−1 signifies the vibration of C=C group. The absorption peak at 1,484–1,359 cm−1 is assigned to the bending vibration of C–H groups. Figure 2b shows the spectrum of the palmitic acid. The peak at 2,951 cm−1 represents the symmetrical stretching vibration of its –CH2 group. The absorption peak at 28 cm−1 is assigned to the symmetrical stretching vibration of its –CH3 group. The peak at 1,695 cm−1 signifies the C=O stretching vibration. The absorption peak at 1,469–1,327 cm−1 corresponded to the deformation vibration of its –CH2 and –CH3 groups, respectively. It can be clearly seen from Fig. 2c that there are no shifts in the above main absorption peaks and in camphene/palmitic acid fatty acid mixture, and camphene and palmitic acid absorption peaks can be clearly seen. This shows that there is no chemical reaction between the functional groups of camphene and palmitic acid.
On the other hand, as can be seen in Fig. 3, the FTIR spectra of barite, fly ash, pyroclastic and marble powder after the incorporation of the camphene/palmitic acid (CP) showed new absorption bands of the fatty acid. In addition, there are no new peaks other than characteristic peaks of fatty acid and the building material (Fig. 3) of FTIR spectra of the composites. These results remark that there is no chemical interaction between the fatty acid mixture PCM and building materials.
Microstructure of camphene/palmitic acid and camphene/palmitic acid composites
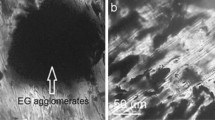
Figure 4 presents the SEM images of the camphene–palmitic acid (CP), pyroclastic, fly ash, barite and marble powder composite PCMs, respectively. The SEM images of the samples are taken at room temperature with the same magnification.
As can be observed from the SEM images, the CP have both granular and tube structure, where these two constitution dispersed in each other. From the Fig. 4d, it can be seen that barite composite PCM has small amount of pores in the structure and include different shapes and different sizes of disks and particulars. Fly ash composite PCM has great many pores and due to the high porosity, the structure has great specific surface area. There is no porosity observed in marble powder composite PCM from SEM images. And also the grain size of the particulars in marble powder-based PCM sample is nearly the same as observed in SEM micrographs. In Fig. 4b, the structure of the pyroclastic-based PCM is given, and we can observe grains with different sizes combined to each other in the structure. And when compared pyroclastic PCM with the CP, barite, fly ash and marble powder, only the pyroclastic-based PCM has the same structure as the others. It can be seen that porosity occurred between the grains consist in the structure.
Thermal properties and specific heat capacity of camphene/palmitic acid and camphene/palmitic acid composites
Thermal energy storage properties of camphene/palmitic acid and composite PCMs were investigated by using of DSC. The DSC curves, enthalpy results and the values of melting and solidification temperatures of camphene/palmitic acid and camphene/palmitic acid composites are presented in Fig. 5. From these curves, the melting and freezing temperatures of CP were determined as 70.88 ± 0.5 and 59.22 ± 0.3 °C. The melting temperatures of the composites were measured as 69.55 ± 0.4, 68.32 ± 0.2, 68.06 ± 0.5 and 69.14 ± 0.3 °C for pyroclastic, fly ash, barite and marble powder composite PCMs, respectively. And their freezing temperatures were measured to be 59.04 ± 0.4, 60.46 ± 0.2, 60.28 ± 0.6 and 59.91 ± 0.2 °C, respectively. However, there are little changes in the phase change temperatures of the composites; they are very close to fatty acids. These slight changes in phase change temperatures of the composite PCMs are conceivably due to the weak chemical interaction.
And although the latent heats of melting were found to be 209.65 J g−1 for CP, and 61.55, 37.08, 67.54, 58.02 J g−1 and freezing were found to be 189.02 for J g−1 for CP and 67.03, 34.41, 67.85 and 57.45 J g−1 pyroclastic, fly ash, barite and marble powder form-stable composites, respectively. Compared with camphene/palmitic acid (CP), the latent heats of the composite-based PCMs decrease slightly. The decrease in the latent heats is the result of the reduction in percentage of the CP in the composite PCMs. The percentages of CP (camphene/palmitic acid in this case) are calculated as follows:
The appropriate mass percentages of the CP in the composites are 33.09, 55.5, 31.5 and 35.96 %, for pyroclastic, fly ash, barite and marble powder-based PCMs, respectively. As the mass percentage of CP in the composite increases, the leakage formation occurred in composite PCMs. In addition to these, the mass-loss rates of the pyroclastic, fly ash, barite and marble powder composite PCMs are constant, when they are heated from room temperature to 80 °C and 60 min at 80 °C during thermogravimetric analysis. There is no camphene or palmitic acid loss from the composites even when they are heated over the melting temperature of the camphene/palmitic acid binary system. These results show that there were no seepage of the CP from the composites and can be used repeatedly in thermal energy storage system.
The specific heat capacity measurements of the CP and composite-based PCMs were made in air atmosphere with a heating rate of 10 °C min−1. Figure 6 is showing these specific heat values of CP and form-stable PCMs during heating. The measurement ranges of samples are between 64 and 82 °C. Alumina was used as standard material for the calculations. Heat capacity measurements of the CP and form-stable PCMs were determined according to the following steps:
-
1.
Isothermal at 64 °C for 10 min,
-
2.
Increasing the temperature from 64 to 82 °C with a heating rate of 10 °C min−1,
-
3.
Isothermal at 82 °C for 10 min.
After the measurements, the specific heat capacity values can be calculated using Shimadzu DSC-60A Cp software program by using the following Eq. [52]:
where (\( \delta \) Q/dt) is the heat flux given by DSC curve, m is the mass of sample, (dT/dt) is the heating rate of the sample, T is the temperature, and t is the time. The peak observed in Cp–T curves (Fig. 6) for each sample refers to the transformation of samples from solid phase to liquid phase. As can be seen in Fig. 6, the specific heat capacity of CP/barite is greater than that of the other composite-based PCMs, because the phase change material content in barite is bigger than in the other. In addition of this, increasing heat capacity in liquid or solid state would enhance the part of heat storage which can be used in a wider temperature range, rather than just to absorb or release heat energy near the melting point or freezing point [53].
Thermal stability camphene/palmitic acid composites
The thermal stability of camphene/palmitic acid, pyroclastic, fly ash, barite and marble powder-based PCMs is depicted in Fig. 7. From the TG curves, it may be thought that there is one step in the degradation of the composites. This step degradation occurs at the temperatures between 180 and 340 °C which is attributed to the thermal degradation of the camphene/palmitic acid (CP) molecular chains. However, the mass-loss rate of fly ash-based composite PCM is smaller than the other composite PCMs. The reason is that, less water molecules from the surrounding air were adsorbed in the pores of fly ash. Besides this, it can be seen from TG curves, the composite PCMs were not degraded or showed any mass loss at lower temperature than 180 °C. This consequence means that the composite PCMs have good thermal reliability in their working temperature range.
Thermal reliability of camphene/palmitic acid and camphene/palmitic acid composites
The composite-based PCMs must be chemically and thermally stable, and besides these, they have no or less changes in their chemical or thermal properties, after long-term utility period of composite PCMs.
The chemical stability of the composite PCMs after 2,000 thermal cycling was investigated by FTIR. Later, thermal treatment, no apparent difference can be occurred between the before and after thermal treatment of the composite-based PCMs, suggesting that the chemical structure of CP and form-stable composite PCMs were not affected by thermal cycling, and chemical degradation was not found during the thermal cycling period (Fig. 8).
Besides these, DSC curves for CP and its composite-based PCMs after 2,000 cycling are given in Fig. 9. Later, repeated 2,000 thermal cycling, the melting and freezing temperature of camphene/palmitic acid (CP) and the composites PCMs were changed to 68.82 and 59.2 °C for CP, 67.85 and 57.31 °C for pyroclastic, 66.89 °C and 58.14 °C for fly ash, 68.82 and 59.2 °C for barite and 67.34 and 58.81 °C for marble powder, respectively. These results indicated that the phase transition temperatures are in applicable level for thermal energy storage applications. As a result, they have good thermal reliability.
Conclusions
In this study, camphene/palmitic acid mixture is firstly synthesized in the literature as PCM by using the camphene. Besides this, in this work, fly ash, marble powder, barite and pyroclastic are firstly used as composite material. These composites are generally used in construction area as supporting material to prevent heat loss. The FTIR analysis results reveal that the CP in composites is physically adsorbed. The adsorption ratio of CP in composites were 33.09, 55.5, 31.5 and 35.96 %, for pyroclastic, fly ash, barite and marble powder, and the latent heat of the composite PCMs are 63,35, 37,08, 67,54 and 58,02 J g−1 for pyroclastic, fly ash, barite and marble powder form-stable composites, respectively. In addition, TG and thermal cycling tests showed that the form-stable composites are chemically and thermally stable and reliable. According to the SEM images, the dispersion of the supporting materials in the matrix is homogenous, and this observation is in comparison with the thermal analysis. And as expected, the homogeneity of the matrix is important for the use of these newly synthesized composites technological applications such as construction sector. Based on these results, it is finalized that CP and their composite-based materials can be regarded as promising PCMs for energy storage because of their sufficient thermal performance and thermal reliability.
References
Zeng J-L, Zhu F, Yu S, Zhu L, Cao Z, Sun L, Deng G, Yan W, Zhang L. Effects of copper nanowires on the properties of an organic phase change material. Sol Energy Mater Sol Cells. 2012;105:174–8.
Farid MM, Khudhair AM, Razack SAK, Al-Hallaj S. A review on phase change energy storage: materials and applications. Energy Convers Manag. 2004;45:1597–615.
Kenisarin M, Mahkamov K. Solar energy storage using phase change materials. Renew Sustain Energy Rev. 2007;11:1913–65.
Alkilani MM, Sopian K, Alghoul MA, Sohif M, Ruslan MH. Review of solar air collectors with thermal storage units. Renew Sustain Energy Rev. 2011;15:1476–90.
Li M, Wu Z, Kao H. Study on preparation, structure and thermal energy storage property of capric–palmitic acid/attapulgite composite phase change materials. Appl Energy. 2011;88:3125–32.
Gunther E, Schmid T, Mehling H, Hiebler S, Huang L. Subcooling in hexadecane emulsions. Int J Refrig. 2010;33(8):1605–11.
Farid MM, Khudhair AM, Razack SAK, Al-Hallaj S. A review on phase change energy storage: materials and applications. Energy Convers Manag. 2004;45:1597–615.
Fernandez AI, Martínez M, Segarra M, Martorell I, Cabeza LF. Selection of materials with potential insensible thermal energy storage. Sol Energy Mater Sol Cells. 2010;94:1723–9.
Demirel Y, Paksoy HÖ. Thermal analysis of heat storage materials. Thermochim Acta. 1993;213:211–21.
Paksoy HÖ. Determining thermal properties of heat storage materials using the twin bath method. Energy Convers Manag. 1996;37(3):261–8.
Mehling H, Hiebler S, Günther E. New method to evaluate the heat storage density in latent heat storage for arbitrary temperature ranges. Appl Therm Eng. 2010;30:2652–7.
Mehling H, Cabeza LF, Hippeli S, Hiebler S. PCM-module to improve hot water heat stores with stratification. Renew Energy. 2003;28(5):699–711.
Addeo A, Nicolais L, Busico V, Migliaresi C. The development of thermal energy storage systems exploiting solid–solid phase transitions. Appl Energy. 1980;6(5):353–62.
Daitoku T, Utaka Y. Separation characteristics of clathrate hydrates from a cooling plate for efficient cold energy storage. Appl Energy. 2010;87(8):2682–9.
Shi X, Ali Memon S, Tang W, Cui H, Xing F. Experimental assessment of position of macro encapsulated phase change material in concrete walls on indoor temperatures and humidity levels. Energy Build. 2014;71:80–7.
U.S.D.O. Energy Buildings energy data book office of energy efficiency and renewable energy. US Department of Energy, America 2007.
C.A.S. Department Hong Kong energy statistics in Census and Statistics Department Hong Kong, Hong Kong, Hong Kong Government 2008.
Waqas A, Ud Din Z. Phase change material (PCM) storage for free cooling of buildings a review. Renew Sustain Energy Rev. 2013;18:607–25.
Hayward T. Energy in 2009 from recession to recovery. BP Statistical Review of World Energy June 2010; 2010.
Sharma A, Tyagi VV, Chen CR, Buddhi D. Review on thermal energy storage with phase change materials and applications. Renew Sustain Energy Rev. 2009;13(2):318–45.
Borreguero AM, Luz Sánchez M, Valverde JL, Carmona M, Rodríguez JF. Thermal testing and numerical simulation of gypsum wallboards incorporated with different PCMs content. Appl Energy. 2011;88(3):930–7.
Farid MM, Khudhair AM, Razack Siddique Ali K, Al-Hallaj S. A review on phase change energy storage: materials and applications. Energy Convers Manag. 2004;45:1597–615.
Sharma A, Tyagi VV, Chen CR, Buddhi D. Review on thermal energy storage with phase change materials and applications. Renew Sustain Energy Rev. 2009;13:318–45.
Prakash J, Garg HP, Datta G. A solar water heater with a built-in latent heat storage. Energy Convers Manag. 1985;25(1):51–6.
Bansal NK, Buddhi D. An analytical study of a latent heat storage system in a cylinder. Sol Energy. 1992;33(4):235–42.
Chaurasia PBL, Phase change material in solar water heater storage system. In: Proceedings of the 8th international conference on thermal energy storage; 2000.
Morrison DJ, Abdel Khalik SI. Effects of phase change energy storage on the performance of air-based and liquid-based solar heating systems. Sol Energy. 1978;20:57–67.
Jurinak JJ, Adbel Khalik SI. On the performance of air-based solar heating systems utilizing phase change energy storage. Sol Energy. 1979;24:503–22.
Sharma SD, Buddhi D, Sawhney RL, Sharma A. Design, development and performance evaluation of a latent heat unit for evening cooking in a solar cooker. Energy Convers Manage. 1997;38(5):493–8.
Buddhi D, Sharma SD, Sharma A. Thermal performance evaluation of a latent heat storage unit for late evening cooking in a solar cooker having three reflectors. Energy Convers Manage. 2003;44(6):809–17.
Hung K, Abrams CF Jr, Coasts LL, Bowers CG, Jr. Development of greenhouse bulk drying systems for solar energy utilization and planted mechanization. AHARE paper no. 75-1018. St. Joseph, MI: American Society for Agricultural Engineering; 1975.
Kern M, Aldrich RA. Phase change energy storage in a greenhouse solar heating system. ASME paper no. 79-4028. St. Joseph, MI: American Society for Agricultural Engineering; 1979.
Swet J. Phase change storage in passive solar architecture. In: Proceedings of the 5th national passive solar conference. Massachusetts: Amhearst; 1980. p. 282–6.
Ghoneim AA, Klein SA, Duffie JA. Analysis of collector–storage building walls using phase change materials. Sol Energy. 1991;47(1):237–42.
Salyer IO, Sircar AK. Phase change material for heating and cooling of residential buildings and other applications. In: Proceedings of 25th intersociety energy conservation engineering conference; 1990. p. 236–43.
Shapiro MM, Feldman D, Hawes D, Banu D. PCM thermal storage in wallboard. In: Proceedings 12th passive solar conference, Portland; 1987. p. 48–58.
Harald Mehling. Strategic project “Innovative PCM-Technology” results and future perspectives. 8th expert meeting and work shop, Kizkalesi, Turkey, April 18–20, 2004.
Bakos G. Energy management method for auxiliary energy saving in a passive-solar-heated residence using low-cost off-peak electricity. Energy Build. 2000;31(3):237–41.
Lin KP, Zhang YP, Xu X, Di HF, Yang R, Qin PH. Modeling and simulation of under-floor electric heating system with shape stabilized PCM plates. Build Environ. 2004;39(12):1427–34.
Benard C, Gobin D, Gutierrez M. Experimental results of a latent heat solar roof used for breeding chickens. Sol Energy. 1981;6(4):347–54.
Gutherz JM, Schiler ME. A passive solar heating system for the perimeter zone of office buildings. Energy Sources. 1991;13:39–54.
Li M, Kao H, Wu Z, Tan J. Study on preparation and thermal property of binary fatty acid and the binary fatty acids/diatomite composite phase change materials. Appl Energy. 2011;88(1606–1):2.
Farid MM, Khudhair AM, Razack SAK, Al-Hallaj S. A review on phase change energy storage: materials and applications. Energy Convers Manage. 2004;45:1597–615.
Hasnain SM. Review on sustainable thermal energy storage technologies, part 1: heat storage materials and techniques. Energy Convers Manage. 1998;39:1127–38.
Farid MM, Kong WJ. Under floor heating with latent heat storage. Proc Inst Mech Eng. 2001;215:601–9.
Neeper DA. Thermal dynamics of wallboard with latent heat storage. Sol Energy. 2000;68:393–403.
Demirbas MF. Thermal energy storage and phase change materials, an overview. Energy Sources. 2006;1(1):85–95.
Sarı A, Karaipekli A. Fatty acid esters-based composite phase change materials for thermal energy storage in buildings. Appl Therm Eng. 2012;37:208–16.
Rady M. Granular phase change materials for thermal energy storage: experiments and numerical simulations. Appl Therm Eng. 2009;29:3149–59.
Shilei L, Neng Z, Guohui F. Impact of phase change wall room on indoor thermal environment in winter. Energy Build. 2006;38:18–24.
Nomura T, Okinaka N, Akiyama T. Impregnation of porous material with phase change material for thermal energy storage. Mater Chem Phys. 2009;115:846–50.
Sonia D, Rotaru P, Rizescu S, Bizdoaca NG. Thermal study of a shape memory alloy (SMA) spring actuator designed to insure the motion of a barrier structure. J Therm Anal Calorim. 2013;111:1255–62. doi:10.1007/s10973-012-2369-4.
Karagoz Z, Aksu Canbay C. Relationship between transformation temperatures and alloying elements in Cu–Al–Ni shape memory alloys. J Therm Anal Calorim. 2013;114:1069.
Acknowledgements
The authors gratefully acknowledge the financial support provided by Firat University Research Foundation (FUBAP FF.13.07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genc, Z.K., Canbay, C.A., Acar, S.S. et al. Preparation and thermal properties of heterogeneous composite phase change materials based on camphene–palmitic acid. J Therm Anal Calorim 120, 1679–1688 (2015). https://doi.org/10.1007/s10973-015-4478-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4478-3