Abstract
Osmolytes are cosolutes that induce stabilization of the structure of proteins. Polyols are osmolytes that have importance in a wide variety of biotechnological and industrial processes. In this work, a systematic study concerning the effect of polyols of different number of methylene and hydroxyl groups on the stability of α-chymotrypsinogen A is presented. Protein thermal stability measurements in buffer, ethylene glycol, glycerol, meso-erythritol, sorbitol and inositol was followed by fluorescence measurements. Under the selected conditions, the thermal denaturation of α-chymotrypsinogen A is a reversible transition between native and unfolded state that can be well described by a two-state model. Reversibility of the transition was confirmed by DSC, circular dichroism, UV–Vis and fluorescence measurements. The change in thermal stability of the protein in the presence of ethylene glycol, glycerol, erythritol, sorbitol and inositol shows that ethylene glycol is the only polyol that presents a destabilizing effect. The other cosolutes exert stabilizing effects that increase with the number of hydroxyl groups and depend on concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The conformational stability of proteins is controlled by intramolecular interactions between protein groups and intermolecular interactions between the protein and solvent molecules [1]. Hydrophobic and hydrophilic interactions between water and the protein have a strong effect on the stability of protein structure. Changes in pH, pressure, temperature and concentration of cosolutes may cause conformational changes in proteins. Although several models, such as preferential interaction, excluded volume, surface tension increment and Wyman binding functions, have been proposed to explain the experimental observations, the mechanism by which cosolutes induce changes in protein structure has not been clearly elucidated [2–6]. It is therefore of interest to study the thermodynamic behavior in different solvent systems as the results can contribute to obtain information about solute–solvent interactions and their effect on the stability of proteins [7]. Moreover, the information will help to find suitable conditions to achieve long-term stability of proteins.
α-Chymotrypsinogen A, a precursor of the enzyme chymotrypsin, is a globular protein with a molecular mass of 25.7 kDa. It contains 246 amino acid residues with five disulfide bonds and a secondary structure with a predominance of β-sheet [8]. Several studies on the stability of α-chymotrypsinogen A indicate that this protein undergoes a reversible thermal denaturation at acid pH [9–11]. Thermal denaturation studies show that α-chymotrypsinogen A unfolding proceeds through a two-state mechanism that involves the native and unfolded states provided that the transition is studied under reversible conditions [4, 12]. The denaturation temperature reported for the protein is strongly dependant on pH, ranging from 314 to 332 K [4, 5, 11].
It is known that polyols stabilize proteins, but the mechanism has not been elucidated. In particular, there is a lack of information concerning the effect of polyols on the conformational stability of α-chymotripsinogen A, especially about the effect of increasing the chain length and the number of hydroxyl groups of this cosolutes. For this reason, this paper presents an experimental systematic study of the effect of ethylene glycol, glycerol, meso-erythritol, sorbitol and myo-inositol on the thermal stability of α-chymotrypsinogen A at acid pH where the denaturation process is reversible.
Materials and methods
α-Chymotrypsinogen A type II, essentially salt-free, lyophilized powder (6× crystallized Sigma) was used without further purification. Ethylene glycol >99.0 % was obtained from Sigma-Aldrich, glycerol >99.0 % from Sigma, meso-erythritol >99.0 % from Sigma, d-sorbitol >99.0 % from Aldrich, and myo-inositol >99.0 % from Sigma. Water was doubly distilled, treated according to the literature and degassed before use. The final product exhibited a conductivity lower than 1.0 μS cm−1. Hydrochloric and phosphoric acids were analytical grade (Merck).
A stock protein solution was prepared dissolving a weighed amount of protein in HCl buffer 0.01 M pH 2.0. Polyol solutions in buffer of six different concentrations were prepared by mass. A sample of the stock solution was diluted with the polyol solution to obtain a final protein concentration of around 0.5 mg mL−1. Protein concentration was checked by UV using a Varian’s Cary 100 UV–Vis spectrophotometer with an extinction coefficient ε 282 = 1.97 mL mg−1 cm−1 at 280 nm [11, 13]. The same procedure was followed using a buffer of phosphate buffer 0.01 M pH 3.0.
Thermal denaturation profiles of the protein in the presence of polyol solutions were determined using a Cary Eclipse (Varian) fluorescence spectrometer. The spectra were measured with excitation at 280 nm and emission of 353 nm. Heating rate was of 1.0 K min−1. Even though the temperature dependence of the fluorescence intensity for α-chymotrypsinogen A does not show a classic sigmoidal shape, the curves exhibit a clear transition between two states making possible to determine the thermodynamic parameters.
Circular dichroism spectra were obtained in the far- and near-UV region using a spectrophotometer CHIRASCAN from Applied Photophysics Ltd. The spectra were recorded at 298.15 and 343.15 K in order to observe the change in structure in α-chymotrypsinogen. The denaturation profiles were obtained in the far UV at 236 and 225 and at 288 and 297 nm in the near UV, with a heating rate of 1.0 K min−1.
Reversibility studies of the denaturation process were performed by UV and fluorescence spectroscopy comparing spectra after successive heating of the sample [14]. The shape of the spectra, denaturation enthalpy and temperature were reproducible showing that under the experimental conditions, 0.5 mg mL−1 concentration of α-chymotrypsinogen A and 1.0 K min−1 heating rate, the thermal unfolding process of protein was reversible.
Differential scanning calorimetry was carried out using a VP-DSC MicroCal system (MicroCal, Northampton, MA, USA), and profiles were analyzed with the Origin 7.0 software. The protein concentration for all experiments was 0.5 mg mL−1. The liquid samples were previously degassed, and the heating rate was 1.0 K min−1.
Thermal denaturation parameters
Denaturation temperature T m and thermodynamic parameters of the unfolding process are obtained from the thermal denaturation curves [14–18]. The apparent equilibrium constant K U for a reversible denaturation process between native and unfolded states is determined from the relationship between the fraction of unfolded molecules X U and folded molecules X N during the reversible transition as described by Eq. 1:
Several properties, spectroscopic or calorimetric, can be used to determine the equilibrium constant and the free energy change for the unfolding process \( (\Delta G_{\text{U}}^{\text{o}} ) \) usually referred as the conformational stability of the protein [18].
When spectroscopic properties are used, the observed property during the thermal unfolding transition is given by:
where Y N and Y U are the values of the property for the native and unfolded species. The properties selected to follow unfolding depend on temperature, and it is assumed that they have a linear dependence that can be expressed by the following equations:
Y N0 and Y U0 are the values of the property for the native and unfolded species, at T = 0 and m N, m U are the experimental slopes. Combining Eqs. 1–5 the following relationship is obtained:
In Eq. 6, the free energy change \( \Delta G^{0} \) is represented by the integrated Gibbs–Helmholtz equation:
Normalized signals obtained by different experimental techniques can be globally fitted to Eqs. 6, 7 to yield a single set of thermodynamic parameters and specific sets of spectroscopic parameters if the thermal transition process can be represented by a two-state model.
Results and discussion
Thermal stability of α-chymotrypsinogen A at pH 2.0 and pH 3.0 was followed using different spectroscopic techniques in order to determine whether the transition can be assumed as a change between two states: a folded and an unfolded state or a multi-state denaturation process. The techniques used were UV–Vis absorbance, fluorescence and CD in the far and near UV (Fig. 1). Table 1 summarizes the thermodynamic parameters obtained from thermal unfolding process of α-chymotrypsinogen A using different spectroscopic techniques.
Denaturation temperatures and the shape of the unfolding curves obtained using the different techniques show consistency and indicate that the thermal unfolding of α-chymotrypsinogen A can be represented as a process between two states with a single transition between the native state and the unfolded state. A global analysis of the denaturation profiles indicates that a single denaturation temperature and enthalpy, essentially identical to those provided by individual analyses of the individual curves, describe the unfolding at either pH 2 or 3. This thermodynamic analysis confirms that the transition can be assumed as a change between two states.
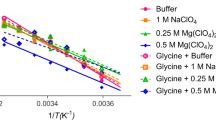
In order to examine the effect of polyols on protein stability, thermal denaturation profiles of α-chymotrypsinogen A in the presence of polyols and buffers were performed by fluorescence measurements. Figures 2 and 3 present the thermal unfolding curves of the protein in the presence of polyols and the selected buffers.
The effect of polyols on denaturation temperature clearly depends on the number of OH groups, as well as on concentration. Table 2 summarizes the data of thermal denaturation of α-chymotrypsinogen A in the presence of different concentrations of polyols in buffer. The results show that ethylene glycol exerts a destabilizing effect as it decreases the denaturation temperature of the protein as the polyol concentration increases. A stabilizing effect, which is reflected in the increase in the denaturation temperature, is observed with the other polyols that becomes stronger as polyol concentration increases. The stabilizing effect becomes larger as the number of hydroxyl groups in the polyol increases. Denaturation enthalpies do not follow a clear trend with the increase in polyol concentration, neither with change in denaturation temperature. Denaturation temperature on both buffers are consistent with those published by other authors [11, 19, 20].
Figure 4 shows the change in denaturation temperature of α-chymotrypsinogen A as a function of polyol concentration. It is clear that at equivalent concentrations, the stabilizing effect of the polyols increase in the following order:
Although the change in stability of the protein in polyols solutions was followed by fluorescence measurements, the calorimetry and van’t Hoff enthalpy was determined in concentrated solutions of polyols to ensure that the process is reversible in the presence of polyols. Table 3 shows the calorimetric and van’t Hoff enthalpy values as well as the relationship between the enthalpy changes. It can be seen that the relationship is very near to 1.0 confirming that the unfolding process of the protein is highly cooperative and can be represented by a reversible equilibrium between two states [21, 22].
The change in heat capacity of α-chymotrypsinogen A in buffer pH 2.0 and 3.0 and in the presence of the selected polyols was determined by differential scanning calorimetry at a concentration of 3.0 mol kg−1 except for inositol whose concentration was 0.5 mol kg−1 due to its low solubility. The ∆C p of protein unfolding at pH 2.0 and 3.0 was 13.3 kJ mol−1 K−1 and 13.3 kJ mol−1 K−1, respectively; these values agree with those obtained by other authors [11, 23].
In order to calculate the stability curves ∆G versus T of the protein, the denaturation temperature and enthalpy values obtained from fluorescence measurements as well as the denaturation heat capacity change obtained from DSC measurements were used. The denaturation enthalpy values from fluorescence measurements were not used to determine the heat capacity because a clear relation between denaturation enthalpies and denaturation temperatures is not observed. Heat capacity changes obtained from DSC were used to estimate in an approximate way the change in free energy for the process of thermal unfolding of α-chymotrypsinogen A in buffer and in the presence of polyols using the integrated Gibbs–Helmholtz equation [23]. Figure 5 shows the DSC curves for α-chymotrypsinogen A in the presence of polyol solutions at pH 2.0 and 3.0, and Figs. 6 and 7 show the stability curves of the protein in the selected cosolutes.
The free energy of stabilization ΔΔG of Chymotrypsinogen A in solution in the presence of polyols at the mean transition temperature of the protein, where \( \Delta G = 0 \) is shown in Tables 4 and 5. To evaluate the effect of the polyols on the stability of α-chymotrypsinogen A, the change in the stability of protein was determined as the change in free energy of the protein in the presence and the absence of polyol.
Results show that ethylene glycol is the unique polyol having a destabilizing effect on protein structure, and this effect becomes more pronounced as the concentration increases, exhibiting the characteristic behavior of monohydric alcohols [5, 20]. Glycerol has a small destabilizing effect at low concentrations, but as its concentration increases, a stabilizing effect is observed. Erythritol, sorbitol and inositol exhibit a stabilizing behavior that is more pronounced as concentration increases and is larger at pH 2.0. These changes in the change in free energy show that the effect of the presence of cosolutes is very important in stabilization of proteins [20].
A graphical representation of the change in stability of the protein at denaturing temperature in buffer pH 2.0 and 3.0, that is, 315.7 and 325.7 K, can be seen in Fig. 8. The results reflect the behavior observed in the denaturation temperature of α-chymotrypsinogen A in the presence of polyol solutions. The stabilizing effect of polyols on the protein increases with the number of hydroxyl groups, and the stabilizing behavior increases in the way observed for the denaturation temperature.
The effect of polyols on the secondary structure of α-chymotrypsinogen A was determinated by circular dichroism spectra in the far UV between 195 and 260 nm at 298.15 and 343.15 K, and it is shown in Fig. 9. The protein structural properties and the influence of the selected cosolutes were quantified with several deconvolution softwares CONTIN, SELCOM3, K2D and CDSSTR. The latter was chosen due to its higher accuracy with proteins that present a high degree of β-sheet structure [24–27]. The calculated structures showed a normalized standard deviation value NMRSD ≤ 0.085, presenting good consistency with databases [28]. Table 6 summarizes the structural data of α-chymotrypsinogen A in the presence of polyols.
The data of structural properties of the protein in buffer are in good agreement with results previously reported by other authors [29, 30]. The spectra show a typical behavior of a β-sheet protein with a high content of unstructured elements, with a local minimum at 229 nm and a global around 202 nm [13]. A comparison of the structural elements of the protein at 298.15 and 343.15 K indicates that no major loss of secondary structure takes place at the higher temperature, indicating that the unfolded state is probably a compact one.
The addition of polyols slightly modifies the content of the structural elements. Ethylene glycol exerts a small destabilizing effect on protein structure, and the most relevant effect on structural elements is related to an increase in the content of unordered regions. In contrast, glycerol, erythritol, sorbitol and inositol, which are stabilizing cosolutes, give rise to an increase in the content of α-helix elements at both pHs. Taking into account the results, it can be suggested that the polyols that act as stabilizers of the protein increase the helical content of α-chymotrypsinogen A [31], while the only polyol that exerts a clear destabilizing effect increases the content in disordered regions of the protein.
Conclusions
Comparing the thermal unfolding of chymotrypsinogen by different spectroscopic techniques, it is clear that the unfolding process is reversible, the transition can be described by a two-state process, and the cosolutes employed do not affect this mechanism. Further evidence that the transition occurs by a two-state model is that the relation between the calorimetric enthalpy determined by DSC and the van’ Hoff enthalpy is very close to 1 indicating a true two-state reversible equilibrium. According to the results obtained, the denaturation parameters were determined. Ethylene glycol is the only destabilizing polyol, and the effect becomes more pronounced as the concentration increases, while the other polyols exhibit a stabilizing behavior that is more pronounced as concentration increases. The results show that the stabilizing effect increases as the number of hydroxyl groups in the polyols becomes larger. According to the structural data, no loss of secondary structure of the protein after heating up 343.15 K at both pH is observed. Ethylene glycol exerts a small destabilizing effect on protein structure, and the most relevant effect on structural elements is related to an increase in the content of unordered regions. In the presence of glycerol, erythritol, sorbitol and inositol, the stabilizing polyols, an increase in the content of α-helix elements at both pHs is observed.
References
Romero CM, Lozano JM, Sancho J, Giraldo GI. Thermal stability of beta-lactoglobulin in the presence of aqueous solution of alcohols and polyols. Int J Biol Macromol. 2007;40:423–8.
Xie G, Timasheff S. Mechanism of the stabilization of ribonuclease A by sorbitol: preferential hydration is greater for the denatured than for the native protein. Protein Sci. 1997;6:211–21.
Davis-Searles PR, Saunders AJ, Erie DA, Winzor DJ, Pielak GJ. Interpreting the effects of small uncharged solutes on protein-folding equilibria. Annu Rev Biophys Biomol Struct. 2001;30:271–306.
Kaushik JK, Bhat R. Thermal stability of proteins in aqueous polyol solutions: role of the surface tension of water in the stabilizing effect of polyols. J Phys Chem. 1998;102:7058–66.
Gerlsma S. The effects of polyhydric and monohydric alcohols on the heat induced reversible denaturation of chymotrypsinogen A. Eur J Biochem. 1970;14:150–3.
Xie G, Timasheff SN. The thermodynamic mechanism of protein stabilization by trehalose. Biophys Chem. 1997;64:25–43.
Theodore BG, Herskovits T. On the structural stability and solvent denaturation of proteins. J Biol Chem. 1970;245:2588–98.
Hartley BS. Amino-acid sequence of bovine chymotrypsinogen-A. Nature. 1964;201:1284–7.
Brandts JF. The thermodynamics of protein denaturation i. the denaturation of chymotrypsinogen. J Am Chem Soc. 1964;86:4291–301.
Brandts JF. The thermodynamics of protein denaturation II. A model of reversible denaturation and interpretations regarding the stability of chymotrypsinogen. J Am Chem Soc. 1964;86:4302–14.
Jackson WM, Brandts JF. Thermodynamics of protein denaturation. Calorimetric study of the reversible denaturation of chymotrypsinogen and conclusions regarding the accuracy of the two-state approximation. Biochemistry. 1970;9:2294–301.
Poklar N, Vesnaver G, Lapanje S. Interactions of alpha-chymotrypsinogen A with alkylureas. Biophys Chem. 1996;57:279–89.
Chalikian TV, Volker J, Anafi D, Breslauer KJ. The native and the heat-induced denatured states of α-chymotrypsinogen A: thermodynamic and spectroscopic studies. J Mol Biol. 1997;274:237–52.
Romero CM, Albis A, Lozano JM, Sancho J. Thermodynamic study of the influence of polyols and glucose on the thermal stability of holo-bovine α-lactalbumin. J Therm Anal Calorim. 2009;98:165–71.
Cooper A. Thermodynamics of protein folding and stability. Protein A Compr Treatise. 1999;2:217–70.
Brandts JF, Hunt L. Thermodynamics of protein denaturation III. Denaturation of ribonuclease in water and in aqueous urea and aqueous ethanol mixtures. J Am Chem Soc. 1967;89:4826–38.
Haque I, Singh R, Moosavi-Movahedi AA, Ahmad F. Effect of polyol osmolytes on ∆GD, the Gibbs energy of stabilisation of proteins at different pH values. Biophys Chem. 2005;117:1–12.
Sancho J. The stability of 2-state, 3-state and more-state proteins from simple spectroscopic techniques… plus the structure of the equilibrium intermediates at the same time. Arch Biochem Biophys. 2013;531:4–13.
Miyawaki O, Tatsuno M. Thermodynamic analysis of alcohol effect on thermal stability of proteins. J Biosci Bioeng. 2011;111:198–203.
Gekko K, Morikawa T. Thermodynamics of polyol-induced thermal stabilization of chymotrypsinogen. J Biochem. 1981;90:51–60.
Privalov PL. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241.
Sturtevant JM. Biochemical applications of differential scanning calorimetry. Annu Rev Phys Chem. 1987;38:463–88.
Privalov PL, Khechinashvili NN. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974;86:665–84.
Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1:2876–90.
Greenfield NJ, Fasman GD. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969;8:4108–16.
Brahms S, Brahms J. Determination of protein secondary structure in solution by vacuum ultraviolet circular dichroism. J Mol Biol. 1980;138:149–78.
Reed J, Reed TA. A set of constructed type spectra for the practical estimation of peptide secondary structure from circular dichroism. Anal Biochem. 1997;254:36–40.
Mao D, Wachter E, Wallace BA. Folding of the mitochondrial proton adenosine triphosphatase proteolipid channel in phospholipid vesicles. Biochemistry. 1982;21:4960–8.
Freer ST, Kraut J, Robertus JD, Wright HT. Chymotrypsinogen: 2,5-Å crystal structure, comparison with α-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970;9:1997–2009.
Wang D, Bode W, Huber R. Bovine chymotrypsinogen A X-ray crystal structure analysis and refinement of a new crystal form at 1.8 A resolution. J Mol Biol. 1985;185:595–624.
Khan F, Khan RH, Muzammil S. Alcohol-induced versus anion-induced states of alpha-chymotrypsinogen A at low pH. Biochim Biophys Acta. 2000;1481:229–36.
Acknowledgements
This work was supported by Universidad Nacional de Colombia and COLCIENCIAS and by Grants BFU2010-16297, BFU2010-19451, PI078/08 and CTPR02/09 (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero, C.M., Abella, J.S., Velázquez, A. et al. Thermal denaturation of α-chymotrypsinogen A in presence of polyols at pH 2.0 and pH 3.0. J Therm Anal Calorim 120, 489–499 (2015). https://doi.org/10.1007/s10973-014-4374-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4374-2