Abstract
The addition of nanoparticles to a base fluid is one of the significant issues to enhance heat transfer. In this study, different nanofluids were developed by mixing a water base fluid with magnetic nanoparticles. Thermophysical properties such as thermal conductivity and viscosity of the obtained nanofluid were investigated. The effect of different nominal diameters of nanoparticles and concentrations of nanoparticles on the thermal conductivity and viscosity of nanofluids have been examined. Three different diameters of magnetic nanoparticles (about 37 nm, 71 nm, and 98 nm) have been tested in this experimental investigation. Experimental results indicate that thermal conductivity increases as volume fraction increases, and thermal conductivity of the nanofluid increases with a decrease of nanoparticle’s size. Moreover, the nanofluid dynamics viscosity ratio increases with an increase in particle concentration and nanoparticle’s diameter. This paper identifies several important issues that should be considered in future work.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanofluids are stable suspensions of nanoparticles (1–100 nm) in base fluids such as water, engine oil, and ethylene glycol that show many interesting properties, and significantly enhance the heat transfer characteristics of the pure fluid. Their distinctive features offer unprecedented potential for many applications. Nanofluid technology has emerged as a new heat transfer technique in recent years and has an amazing advantage over base cooling fluids in terms of heat transfer removal characterization. Energy supply, transportation, HVAC, microelectronics, etc., are some applications which relate to the potential benefits of nanofluids.
A literature survey reveals that thermophysical properties of nanofluids depend on various factors such as volume fraction [1, 2], temperature [3–5], nanoparticle material [6, 7], nanoparticle size [8–10], effect of particle shape/aspect ratio [11, 12], pH [7], and nature of base fluid [13–15], etc. Effective thermal conductivity and viscosity of magnetic Fe3O4/water nanofluid are reported by Sundar et al. [16]. Their experiment performed in the volume concentration ranging from 0.0 to 2.0 % and the temperature ranging from 20 to 60 °C. Their result showed that thermal conductivity and viscosity of the nanofluid were increased with an increase in the particle volume concentration and viscosity enhancement was greater compared to thermal conductivity enhancement at same volume concentration and temperature. In another work, Sundar et al. [17] investigated the effect of base fluid on thermal conductivity of an ethylene glycol and water mixture based on magnetic Fe3O4 nanofluid. Their nanofluids were prepared by dispersing nanoparticles into different base fluids consisting of various mass fractions of the ethylene glycol and water mixture. Their results indicated that thermal conductivity increases with the increase of particle concentration and temperature. Moreover, the maximum increase in thermal conductivity was 46 % at 2.0 vol% of nanoparticles dispersed in 20:80 % ethylene glycol and water mixture compared to other base fluids.
Wook Oh et al. [18] reported experimental data on the thermal conductivity enhancement in Al2O3 nanofluids with DI water and EG as base fluids using the modified 3× method. They showed that for DI water-based nanofluids, the incremental data of the thermal conductivity agreed well with those of Wang et al. [19], which show higher increment compared to the results of Lee et al. [20] and Das et al. [21]. Moreover, they showed that EG-based nanofluids had relatively low thermal conductivity values compared with those of Lee et al. [20] and Wang et al. [19].
Ghasempour et al. [22] studied thermal properties and rheological behavior of water-based Al2O3 nanofluid in concentrations ranging from 3 to 50 % in mass and temperatures ranging from 293 to 323 °K. They observed thermal conductivity and viscosity enhancement are in the range of 1.1–87 % and 18.1–300 %, respectively. Moreover, their results revealed that thermal conductivity increases nonlinearly with concentration, but linearly with increase in temperature.
The study of the effect of particle size, temperature, and mass fraction on the thermal conductivity ratio of alumina (Al2O3)/water nanofluids was examined by Teng et al. [23]. Their nanofluids prepared by the direct synthesis method served as the experimental sample, and nanoparticles, each of a different nominal diameter (20, 50, and 100 nm), were dispersed into four different concentrations (0.5, 1.0, 1.5, and 2.0 mass%). They concluded that high thermal conductivity ratios related to small nanoparticle size and higher temperatures. Low concentrations of COOH-functionalized DWCNTs/water nanofluid and MgO–water nanofluid were presented by Hemmat Esfe et al. [24, 25]. They measured thermal conductivity and viscosity of nanofluids at volume fractions less than 1 % and found corresponding correlations.
The effect of dispersion method on thermal conductivity and stability of nanofluid was investigated by Nasiri et al. [26]. They utilized five different carbon nanotube (CNT) structures: single-wall CNTs (SWNTs); double-wall CNTs (DWNTs); few-wall CNTs (FWNTs); and two multiwall nanotubes (MWNTs). These variations were synthesized to prepare nanofluids with three different dispersion methods: functionalization; SDS/ultrasonic probes; and an SDS/ultrasonic bath. Their results indicated that the best stability and thermal conductivity are associated with functionalized nanofluids.
Yu et al. [27] performed experimental investigations on thermal conductivity and the viscosity of aluminum nitride nanofluid. Their results showed that at a volume fraction of 0.1, thermal conductivity enhancement ratios are 38.71 and 40.2 %, respectively, for ethylene glycol and propylene glycol as the base fluids. Moreover, they reported that for <5.0 vol% for Newtonian behavior, and for >5.0 vol% for obvious shear-thinning behavior, for ethylene glycol and propylene glycol.
Lee et al. [28] prepared an ethylene–glycol based nanofluid containing ZnO nanoparticles by a one-step physical method known as pulsed-wire evaporation. They concluded that a higher nanoparticle concentration is beneficial to thermal conductivity, while it has a detrimental effect on viscosity. In another work Hemmat Esfe et al. [29] presented an experimental investigation on the effect of temperatures and particle volume concentration on the dynamic viscosity of ZnO–EG nanofluid. The mean diameter of zinc oxide nanoparticles was 18 nm. They found that, in general, nanofluid dynamic viscosity increases considerably with particle volume fraction but does not significantly decrease with temperature increase. Their results also showed that Einstein’s formula and some others originating from classical linear fluid theory seems to be limited to nanofluids with low particle fractions.
Until now, there has been much research on the characteristics of dispersion and properties of different nanofluids, but there is little research on Fe–water nanofluids in the literature.
Review of the aforementioned articles [1–29] shows that various parameters are studied in different works. Different aspects of nanofluid were studied by each experiment and therefore different results were obtained, each useful. This study considered the effect of some parameters such as nanoparticle concentrations and nanoparticles diameter for (oxide-coated) iron-water nanofluids on the thermal conductivity and dynamic viscosity of Fe/water nanofluids.
The intent is to discover an exact understanding of thermal conductivity behavior and the role each of these parameters has on increasing or decreasing thermal conductivity. Research on the diameter of iron nanoparticles in water and its effect on thermal conductivity has not been reported in the literature. Also, according to the information available to the authors, limited reports on the influence of temperature and diameter of nanoparticles in nanofluids with metal particles have been published to date. Much research and experiments were carried out with iron oxide consisting of measuring thermal conductivity changes with changes in volume fraction [30–33], temperature [34, 35], and the kind of base fluid [36]. Therefore, this research is a prior study on the role of iron nanoparticle size in the base fluid and its interaction with temperature will be examined.
Preparation of nanofluids
Preparation of nanofluids is the first step in experimental studies with nanofluids. Nanofluids are not simply liquid–solid mixtures. Some special requirements are essential, such as even and stable suspension, durable suspension, negligible agglomeration of particles, and no chemical change in the fluid.
In the current study, (oxide-coated) Fe nanoparticles were chosen because of their widely known thermal properties.
The test nanofluids were obtained by dispersing Fe nanoparticles in DI water as base liquid at ambient conditions. Fe nanoparticles, with sizes of about 37, 71, and 98 nm were prepared. To stabilize the suspension, a specific type of activator is used to cover the nanoparticles. The amount of activator is proportional with mass percentage of iron nanoparticles and water.
Fe volume concentrations of 0.01 (1.0 %), 0.005 (0.5 %), 0.0025 (0.25 %), 0.00125 (0.125 %), 0.000625 (0.0625 %), and 0.000313 (0.0313 %) were used for the investigation.
In this work, Fe–water nanofluid was prepared utilizing a two-step method. In general, there are two fundamental methods to obtain nanofluids:
-
(1)
Single-step direct evaporation method In this method, direct evaporation and condensation of the nanoparticulate materials in the base liquid is obtained to produce stable nanofluids.
-
(2)
Two-step method In this method, nanoparticles are obtained by different methods and then are dispersed into the base liquid.
The two-step method is the most economic method to produce nanofluids on a large scale, because nanopowder synthesis techniques have already been scaled to industrial production levels.
Generally, ultrasonic equipment is used to disperse the particles and reduce their agglomeration. Sonication is the act of applying ultrasound energy to agitate particles, for various purposes. Nanofluids were prepared by dispersing a specific amount of Fe in DI water using an ultrasonic vibrator for 120 min to obtain a uniform and stable dispersed solution and break down intermolecular interactions of the nanoparticles. After 16 h, no sedimentation was observed in any samples of nanofluid.
Thermal conductivity of nanofluid
Thermal conductivity is an important parameter in enhancing the heat transfer performance of a base fluid. Since the thermal conductivity of solid metals is higher than that of fluids, the suspended particles are expected to increase thermal conductivity and heat transfer performance. Many researchers have reported experimental studies on the thermal conductivity of nanofluids. The temperature oscillation method [37], the steady-state parallel-plate method [38], and transient hot-wire method [39] have been employed to measure the thermal conductivity of nanofluids. However, the transient hot-wire method has been extensively used by many researchers because of high accuracy and high rapidity. A detailed review on different techniques for measurement of thermal conductivity of nanofluids is available in the literature [40].
The thermal conductivity of magnetic nanofluids with various concentrations was measured using a KD2 Pro thermal property analyzer (Decagon Devices, Inc., USA). The experimental setup for measuring thermal conductivity consists of a KD2- Pro digital recorder, a handheld controller, and a nanofluids container consisting of a handheld microcontroller and sensor needles. The complete description of this instrument has been published elsewhere. All the measurements were performed at an ambient temperature. The accuracy of the instrument was checked by measuring the thermal conductivity of glycerin provided by suppliers and comparing it with the instrument reading of standard samples. The accuracy of the device is ±5 %. The experiments are repeated three times for each case and the average value is reported.
Thermal conductivity behavior
Experimental studies show that thermal conductivity of nanofluids depends on many factors such as particle volume fraction, particle material, particle size, particle shape, base fluid material, and temperature. Amount and types of additives and the acidity of the nanofluid were also shown to be effective in thermal conductivity enhancement. Particle volume fraction is a parameter investigated in almost all experimental studies, and the results are usually in qualitative agreement. Most of the researchers report increasing thermal conductivity with increasing particle volume fraction and the relation found is usually linear. Particle size is another important parameter in the thermal conductivity of nanofluids.
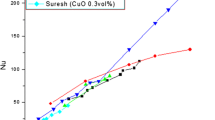
Figure 1 shows the effect of nanoparticle diameter and volume fraction of nanoparticles on thermal conductivity ratio of water-based nanofluid containing iron nanoparticles. The nominal sizes of the nanoparticles were 37, 71, and 98 nm, respectively. It is evident from this figure that the thermal conductivity of nanofluids increases with decreasing particle size and increases as volume fraction increases. This trend is theoretically supported by two mechanisms of thermal conductivity enhancement: Brownian motion and liquid layering around nanoparticles.
As a result of experimental findings under the same concentration conditions, it was concluded that thermal conductivity increases with decreasing particle size. It should be noted that these results are in agree with the aforementioned studies. Smaller particle size is directly correlated with greater surface area of solid–liquid interface. It helped to enhance thermal conductivity. Therefore, particle diameter affected not only the suspension state, but also thermal conductivity of nanofluids. The relationship between the relative thermal conductivity enhancement and the volume fraction is nearly linear and can be reproduced using models from Hamilton and Crosser [41] and Yu–Choi [42]. This linear behavior between the thermal conductivity enhancement and volume concentration can be attributed to large regions of particle-free liquid with high thermal resistance created by highly agglomerated nanoparticles. Our results for Fe nanoparticles (98 nm) were also compared with the experimental data of Yu–Choi [42] and Hamilton and Crosser’s model [41].
The results in Fig. 2 have shown that both formulas proposed by Yu–Choi [42] and Hamilton and Crosser’s model [41] severely underestimate nanofluid thermal conductivity, especially for a particle fraction higher than 0.004.
Proposed model
Figure 3 illustrates the curve fitting of experimental data to the dynamic viscosity of nanofluid in terms of nanoparticle diameter and volume fraction. Experimental data were used to curve fit nanofluid thermal conductivity and dynamic viscosity as a function of nanoparticle diameter and volume fraction. A simple equation is proposed to correlate the relative thermal conductivity (\( \frac{{k_{{{\text{nf}}}} }}{{k_{{{\text{bf}}}} }} \)) as a function of d p and φ:
In this model, a nanofluid is considered as a two-phase fluid and is treated as a solid–liquid mixture. This equation is based on the assumptions that the thermal conductivity of the nanofluid is a function of base fluid thermal conductivity, particle concentration, and nanoparticle diameter and that the nanoparticles can be modeled as rigid spherical particles. This assumption seems incorrect for system nanofluids which present specific properties and interactions not yet completely understood. No other study on relative thermal conductivity (\( \frac{{k_{{{\text{nf}}}} }}{{k_{{{\text{bf}}}} }} \)) of metallic nanofluid as a function of d p and φ has been found.
Dynamic viscosity behavior
Figure 4 shows the results for the dynamics viscosity ratio (defined as the ratio of nanofluid/water viscosity) obtained for Fe–water under ambient conditions and particle volume fractions varying from 0.000313 to as high as 0.01. As it is expected, the nanofluid dynamics viscosity ratio increases with increasing b particle concentration and nanoparticle diameter.
The results in Fig. 5 have shown that the formula proposed by Wang et al. [19] overestimated nanofluid viscosity, and the formula proposed by Einstein [43] severely underestimated nanofluid viscosity, especially for a particle fraction higher than 0.004. It appears difficult to draw a conclusive statement about such a result, which may be due to various factors such as the methods used for nanofluid preparation as well as the differences in measurement technique.
Proposed model
Experimental data were used to curve fit the nanofluid dynamic viscosity as a function of nanoparticle diameter and volume fraction as shown in Fig. 6. A simple equation is proposed to correlate the dynamic viscosity as a function of d p and φ:
This model is dedicated to Fe-water nanofluid with specific solid volume fractions lower 1 %. In this model, a nanofluid is considered as a two-phase fluid and is treated as a solid–liquid mixture. This equation is based on the assumption that the dynamic viscosity of the nanofluid is a function of the base fluid dynamic viscosity, particle concentration, and nanoparticle diameter. No other studies on dynamic viscosity of water-based nanofluids containing Fe nanoparticles as a function of d p and φ has been reported.
Conclusions
Many interesting properties of nanofluids have been reported in past decades. This paper presents an experimental study of Fe–water nanofluids to investigate the effective thermal conductivities and dynamic viscosity of water-based nanofluids containing Fe nanoparticles.
Well-dispersed water-based Fe nanofluids were obtained by dispersing Fe nanoparticles into a base liquid. The thermal conductivity and dynamic viscosity data have been obtained for different nominal diameters of nanoparticles and different concentrations of nanoparticles. It has been found that generally, thermophysical properties of nanofluids such as viscosity and thermal conductivity depend on the nominal diameter of nanoparticles and concentrations of nanoparticles.
We also curve fit nanofluid thermal conductivity and dynamic viscosity as a function of nanoparticle diameter and volume fraction. Based on the results, key findings and conclusions from the present simulation are as follows:
-
The thermal conductivity increases as volume fraction increases and thermal conductivity of the nanofluid increases with decreasing nanoparticle size.
-
The nanofluid dynamics viscosity ratio increases with increasing particle concentration and nanoparticle diameter.
More comprehensive models need to be developed. Particle dispersions, clustering, and temperature must be taken into account in the model development for nanofluids. To reach universal models for thermal properties, more complete experiments involving a wide range of nanoparticle sizes would be conducted in the future.
References
Chandrasekara M, Sureshb S, Senthilkumara T. Mechanisms proposed through experimental investigations on thermophysical properties and forced convective heat transfer characteristics of various nanofluids, a review. Renew Sustain Energy Rev. 2012;16:3917–38.
Barbés B, Páramo R, Blanco E, Pastoriza-Gallego MJ, Piñeiro MM, Legido JL, Casanova C. Thermal conductivity and specific heat capacity measurements of Al2O3 nanofluids. J Therm Anal Calorim. 2013;11:1615–25.
Patel HE, Sundararajan T, Das SK. An experimental investigation into the thermal conductivity enhancement in oxide and metallic nanofluids. J Nanopart Res. 2010;12:1015–31.
Hemmat Esfe M, Saedodin S, Mahian O, Wongwises W. Thermal conductivity of Al2O3/water nanofluids: measurement, correlation, sensitivity analysis, and comparisons with literature reports. J Therm Anal Calorim. 2014;117:675–81.
Mintsa HA, Roy G, Nguyen CT, Doucet D. New temperature dependent thermal conductivity data for water-based nanofluids. Int J Therm Sci. 2009;48:363–71.
Hwang YJ, Ahn YC, Shin HS, Lee CG, Kim GT, Park HS, et al. Investigation on characteristics of thermal conductivity enhancement of nanofluids. Curr Appl Phys. 2006;6:1068.
Gowda R, Sun H, Wang P, Charmchi M, Gao F, Gu Z, et al. Effects of particle surface charge, species, concentration, and dispersion method on the thermal conductivity of nanofluids. Adv Mech Eng. 2010;2010:1–10.
Hemmat Esfe M, Saedodin S, Bahiraei M, Toghraie D, Mahian O, Wongwises W. Thermal conductivity modeling of MgO/EG nanofluids using experimental data and artificial neural network. J Therm Anal Calorim. 2014;118(1):287–294. doi: 10.1007/s10973-014-3771-x.
Anoop KB, Sundararajan T, Das SK. Effect of particle size on the convective heat transfer in nanofluid in the developing region. Int J Heat Mass Transf. 2009;52:2189–95.
Vajjha RS, Das DK. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int J Heat Mass Transf. 2009;52:4675–82.
Timofeeva EV, Routbort JL, Singh D. Particle shape effects on thermophysical properties of alumina nanofluids. J Appl Phys. 2009;106:014304.
Cherkasova AS, Shan JW. Particle aspect-ratio effects on the thermal conductivity of micro- and nanoparticle suspensions. ASME J Heat Transf. 2008;130:082406.
Liu MS, Lin MCC, Huang IT, Wang CC. Enhancement of thermal conductivity with carbon nanotube for nanofluids. Int Commun Heat Mass Transf. 2005;32:1202–10.
Yu W, Xie H, Chen W. Experimental investigation on thermal conductivity of nanofluids containing graphene oxide nanosheets. J Appl Phys. 2010;107:094317.
Gallego MJP, Lugo L, Legido JL, Pineiro MM. Thermal conductivity and viscosity measurements of ethylene glycol-based Al2O3 nanofluids. Nanoscale Res Lett. 2011;6:1–11.
Syam Sundar L, Singh Manoj K, Sousa Antonio C M. Investigation of thermal conductivity and viscosity of Fe3O4 nanofluid for heat transfer applications. Int Commun Heat Mass Transf. 2013;44:7–14.
Syam Sundar L, Singh MK, Sousa ACM. Thermal conductivity of ethylene glycol and water mixture based Fe3O4 nanofluid. Int Commun Heat Mass Transf. 2013;49:17–24.
Oh DW, Jain A, Eaton JK, Goodson KE, Lee JS. Thermal conductivity measurement and sedimentation detection of aluminum oxide nanofluids by using the 3× method. Int J Heat Fluid Flow. 2008;29:1456–61.
Wang X, Xu X, Choi SUS. Thermal conductivity of nanoparticle-fluid mixture. J Thermophys Heat Transf. 1999;13:474–80.
Lee S, Choi SUS, Li S, Eastman JA. Measuring thermal conductivity of fluids containing oxide nanoparticles. J Heat Transf. 1999;121:280–9.
Das SK, Putra N, Thiesen P, Roetzel W. Temperature dependence of thermal conductivity enhancement for nanofluids. J Heat Transf. 2003;125:567–74.
Ghanbarpour M, Bitaraf Haghigi E, Khodabandeh R. Thermal properties and rheological behavior of water based Al2O3 nanofluid as a heat transfer fluid. Exp Therm Fluid Sci. 2014;53:227–35.
Teng TP, Hung YH, Teng TC, Mo HE, Hsu HG. The effect of alumina/water nanofluid particle size on thermal conductivity. Appl Therm Eng. 2010;30:2213–8.
Hemmat Esfe M, Saedodin S, Mahian O, Wongwises S. Heat transfer characteristics and pressure drop of low concentrations of COOH-functionalized DWCNTs/water nanofluid in turbulent flow. Int J Heat Mass Transf. 2014;73:186–194.
Hemmat Esfe M, Saedodin S, Mahmoodi M. Experimental studies on the convective heat transfer performance and thermophysical properties of MgO–water nanofluid under turbulent flow. Exp Therm Fluid Sci. 2014;52:68–78.
Nasiri A, Shariaty-Niasar M, Rashidi A, Amrollahi A, Khodafarin R. Effect of dispersion method on thermal conductivity and stability of nanofluid. Exp Therm Fluid Sci. 2011;35:717–23.
Yu W, Xie H, Li Y, Chen L. Experimental investigation on thermal conductivity and viscosity of aluminum nitride nanofluid. Particuology. 2011;9:187–91.
Gj Lee. KyuKim C, Lee MK, KyuRhee C, Kim S, Kim C. Thermal conductivity enhancement of ZnO nanofluid using a one-step physical method. Thermochim Acta. 2012;542:24–7.
Hemmat Esfe M, Saedodin S. An experimental investigation and new correlations of viscosity of ZnO–EG nanofluid at various temperatures and different solid volume fractions. Exp Therm Fluid Sci. 2014;55:1–5.
Gavili A, Zabihi F, Isfahani TD, Sabbaghzadeh J. The thermal conductivity of water base ferrofluids under magnetic field. Exp Therm Fluid Sci. 2014;41:94–8.
Yasar RM, Mathias A, Lars F, Bernd W. Thermal, electrical and magnetic studies of magnetite filled polyurethane shape memory polymers. Mater Sci Eng. 2007;444:227–35.
Zhu H, Zhang C, Liu S, Tang Y, Yin Y. Effects of nanoparticle clustering and alignment on thermal conductivities of Fe3O4 aqueous nanofluids. Appl Phys Lett. 2006;89:23123.
Abareshi M, Goharshadi E, Zebarjad S, Fadafan HK, Youssefi A. Fabrication characterization and measurement of thermal conductivity of Fe3O4 nanofluids. J Magn Magn Mater. 2010;322:3895–901.
Fertman VE, Golovicher LE, Matusevich NP. Thermal conductivity of magnetite magnetic fluids. J Magn Magn Mater. 1987;65:211–4.
Parekh K, Lee HS. Magnetic field induced enhancement in thermal conductivity of magnetite nanofluid. J Appl Phys. 2010;107:09A310.
Yu W, Xie H, Chen L, Li Y. Enhancement of thermal conductivity of kerosene-based Fe3O4 nanofluids prepared via phase-transfer method. Colloids Surf A. 2010;355:109–13.
Cahill DG. Thermal conductivity measurement from 30 to 750 K: the 3u method. Rev Sci Instrum. 1990;61:802–8.
Kurt H, Kayfeci M. Prediction of thermal conductivity of ethylene glycolwater solutions by using artificial neural networks. Appl Energy. 2009;86:2244–8.
Challoner AR, Powell RW. Thermal conductivities of liquids: new determinations for seven liquids and appraisal of existing values. Proc R Soc Lond A. 1956;238:90–106.
Paul G, Chopkar M. Manna IA, Das PK: techniques for measuring the thermal conductivity of nanofluids: a review. Renew Sustain Energy Rev. 2010;14:1913–24.
Hamilton R, Crosser O. Thermal conductivity of heterogeneous two component systems. Ind Eng Chem Fundam. 1962;3:187–91.
Yu W, Choi SUS. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: a renovated Maxwell model. J Nanopart Res. 2003;5:167–71.
Einstein A. Eineneuebestimmung der molekuldimensionen. Ann Phys Leipzig. 1906;19:289–306.
Acknowledgements
The authors would like to acknowledge the assistance provided by the Nanofluid Laboratory of Semnan University Science and Technology Park for providing necessary instruments to carry out the sample preparation and helping in analyzing the samples to complete the article in time. We would like to especially thank Mr. Hafezi, Mr. Moulaie, Mr. Maroofi, and Mr. Makki for their support and cooperation. The fourth author would like to thank the National Science and Technology Development Agency, the Thailand Research Fund and the National Research University Project for supporting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hemmat Esfe, M., Saedodin, S., Wongwises, S. et al. An experimental study on the effect of diameter on thermal conductivity and dynamic viscosity of Fe/water nanofluids. J Therm Anal Calorim 119, 1817–1824 (2015). https://doi.org/10.1007/s10973-014-4328-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4328-8