Abstract
Thin films of BiFe1−xCoxO3 (BFCO, x = 0–0.05) were prepared using the sol–gel method and deposited on a fluorine-doped tin oxide (FTO)/glass substrate. The crystal structure, surface morphology, dielectric properties, polarization, and optical characteristics of the BFCO thin films were investigated. X-ray diffraction (XRD) and Raman spectroscopy analyses show that Co doping induces lattice distortion. Scanning electron microscopy (SEM) images demonstrate that BFCO films with x = 0.03 possess uniform fine grains, which are crucial for their ferroelectric properties. From XPS pattern, it can be observed that Co doping can inhibit the conversion of Fe3+ into Fe2+, and BiFe0.97Co0.03O3 films exhibit greatly reduced oxygen vacancy concentration. Therefore, BiFe0.97Co0.03O3 film was found to have the lowest leakage current density (J = 7.18 × 10−7 A/cm2). The film demonstrates outstanding residual polarization at room temperature, with a value of Pr = 152.1 μC/cm2, more than twice the magnitude of that in pure BFO (Pr = 72.33 μC/cm2). Moreover, the dielectric properties of BFCO films show a significant improvement when compared to those of pure BFO samples. This enhancement is attributed to the Co doping-induced structural transition, along with a reduction in grain size and a decrease in the concentration of oxygen vacancies. Additionally, the BiFe0.97Co0.03O3 film exhibits a narrower band gap (Eg = 1.69 eV) in comparison to the BFO film (Eg = 1.87 eV). Consequently, an expansion in the range of photovoltaic applications for BFO films can be achieved.
Graphical Abstract

Fig 1. a–f Ferroelectric hysteresis loops of the BFCO thin films. Fig 2. a UV–Vis absorption spectra of the BFCO thin films. b Tauc curves of the BFCO thin films.
Highlights
-
BiFe1−xCoxO3 (BFCO, x = 0–0.05) thin films on a fluorine-doped tin oxide (FTO)/glass substrate were prepared through the sol–gel method.
-
BiFe0.97Co0.03O3 was found to have the lowest leakage current density (J = 7.18 × 10−7 A/cm2), with excellent remnant polarization at room temperature which is more than twice as large as that of pure BFO.
-
The bandgap (Eg = 1.69 eV) was reduced by doping BFO with Co.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multiferroic materials simultaneously possess two or more ferromagnetic orders, such as ferroelectricity, ferromagnetism, ferroelasticity, and ferrotoroidicity, which result in a diverse range of physical phenomena [1, 2]. These properties not only open up new avenues of research in condensed matter physics but also hold significant promise for a range of applications, including new information storage media, spintronics, ultrafast optoelectronic devices, piezoelectric nanogenerators and ferroelectric random access memories [3,4,5]. Bismuth ferrite (BiFeO3), known as BFO, is widely recognized as the most significant single-phase multiferroic material at room temperature due to its simultaneous exhibit of ferroelectricity and ferromagnetism [6, 7].
BFO possesses high Curie temperature (Tc ~ 830 °C) and high Néel temperature (TN ~ 370 °C). In addition, the theoretical ferroelectric polarization value is as high as 100 μC/cm2, which makes it one of the most promising multiferroics [8,9,10,11,12]. With the emphasis on new environmentally friendly functional materials, BFO lead-free films have become a research focus. However, due to the high-temperature volatilization of Bi3+ ions and the fluctuation of valence state of Fe ions (from Fe3+ to Fe2+) during the films’ preparation, BFO films usually show high concentrations of oxygen vacancy defects, resulting in large leakage current and poor ferroelectric properties [13,14,15,16,17]. To improve the ferroelectric properties, researchers have employed various methods such as ion substitution, structural domain control, chemical modification processes, and composites [18,19,20,21,22]. Among them, ion doping substitution is a simpler, lower cost, and more efficient method. Transition metal ions such as Cu, Zn, Ti, Mn, Co, etc. can be used to replace Fe3+ or Bi3+, causing a certain degree of lattice distortion and altering the carrier concentration, leading to high-performance [23,24,25,26]. As the ionic radii of Fe3+ and Co2+ are similar, a small amount of Co2+ can replace Fe3+ ions in BiFeO3. Substitution of lower-priced elements requires charge compensation, which can be met by the creation of anionic (oxygen) vacancies or an increase in cationic valence (Fe2+-Fe3+), thus suppressing valence fluctuations from Fe3+ to Fe2+. Thus, low-priced dopants can lead to a decrease in the band gap due to an increase in the defect-induced energy level or state density within the band gap. Low (2+) ion substitution also provides the possibility for the production of defective dipoles, which can therefore lead to drastic changes in various physical properties [27]. Co2+ serves as a dopant substitution element and its substitution role may be of great interest. Xue et al. reported that by co-doping with Gd and Co, the ferroelectric properties of the BFO thin film were further enhanced (Pr = 101 μC/cm2, Ec = 345 kV/cm) [28]. Ghanshyam et al. successfully prepared a Bi0.99Sm0.01Fe0.99Co0.01O3 thin film through the sol–gel method, achieving a low leakage current density of 10−7 A/cm [29]. Sinha et al. synthesized Co-doped BFO thin films via the sol–gel method, and the results showed that as the Co2+ doping level increases in the BFO lattice, the size of the pristine BiFeO3 NPs gradually decreases, and Co doping enhances the magnetic and dielectric properties to a greater extent [30]. Saha et al. induced ferromagnetic properties in Co modified BF-PT and provided guidance for understanding the electromechanical response in BFO-based alloys [31]. Wani et al. used sol–gel methods to prepare pure BFO and Co-doped BFO nanostructured materials, systematically studying the influence of Co substitution on the structure, morphology, dielectric, and optical properties of BFO. The results showed that BiFe1−xCoxO3 (x = 0.00, 0.03, 0.05, 0.10) all displayed a perovskite structure. With the increase of doping level, the average grain size enlarges, and the induced strain initially decreases and then strengthens [32]. As a result, Co doping significantly enhanced the physical properties of BFO films. The single-ion substitution method has also become an efficient method for obtaining excellent multiferroic properties in BFO films. These results are of extraordinary significance for the application of BFO.
In order to demonstrate the impact of Co doping on the ferroelectric properties of BFO thin films, we prepared thin films of BiFe1−xCoxO3 (BFMO) with Co doping concentrations (x = 0–0.05) on fluorine-doped tin oxide (FTO)/glass substrates using the sol–gel method. By controlling the doping level, the ferroelectric and dielectric properties can be adjusted to achieve specific functional applications. As doping increases, the oxygen vacancy content decreases leading to improved ferroelectric properties, dielectric properties, and photovoltaic performance.
2 Experiment
Using the sol–gel technique, spin coated films of BiFe1−xCoxO3 (BFCO) with various Co doping levels (x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05) were prepared on FTO/glass substrates. The precursor raw materials were analytically pure Fe(NO3)3·9H2O, Bi(NO3)3·5H2O and Co(NO3)2·6H2O. A 4% excess of bismuth is used to compensate for the effects caused by bismuth volatilization during high-temperature annealing. The precursor solution was prepared by weighing according to the desired stoichiometric ratio and dissolving in organic solvents (ethylene glycol monomethyl ether and glacial acetic acid) with a volume ratio of 1:3 (overall Bi concentration of 0.25 M). The mixture was continuously stirred at a constant speed for 1 h at room temperature to ensure complete dissolution. Citric acid monohydrate (C6H8O7, as a chelating agent; 0.25 M), ethylene glycol (C2H6O2, serving as a dispersant, 12 ml), and ethanolamine (C2H7NO, stabilizer and a viscosity modifier, 0.5 ml) were then sequentially added to the aforementioned mixture. The resulting solution was stirred continuously for 2.5 h at room temperature to obtain a homogeneous precursor with a solution concentration of 0.25 M with respect to Bi. The precursor solution was aged for 48 h to promote hydrolysis and polymerization within the solution. The FTO/glass substrates were sequentially cleaned with acetone, alcohol, and deionized water through ultrasonic cleaning. The precursor solutions with different Co contents were dropped onto the substrate and spin-coated at 2000 rpm for 15 s to obtain the initial wet film. To remove water and organic solvents, the deposited thin film were placed in a constant temperature oven set at 85 °C for a duration of 10 min. Subsequently, it was transferred to a muffle furnace at 550 °C for 10 min to eliminate any remaining organic solvents and initiate the crystallization process of the film. By performing 14 consecutive annealing cycles, the thermal treatment process of the wet film was completed, ultimately resulting in the desired thickness of the BFCO thin film. Subsequently, the coated film was annealed at 550 °C for 30 min to facilitate the full growth of BFO grains. To fulfill the electric properties measurement requirements, Pt dot electrodes with an area of 0.126 mm2 were deposited through sputtering with the utilization of a shadow mask.
The crystallographic structures of the BFCO thin films were evaluated using X-ray diffraction (XRD) with an Ultima IV diffractometer in grazing incidence mode (angle of incidence = 0.5°). Raman spectroscopy, performed on the prepared film samples using LabRAM HR and a laser wavelength of 633 nm, was employed to analyze their molecular structure. The grain microstructure and cross-sections of the films were observed using scanning electron microscopy (SEM) with a Zeiss Sigma300 instrument. Furthermore, the sample’s elemental composition was analyzed through energy-dispersive X-ray spectroscopy (EDS). XPS (ESCALAB 259xi) was used to further examine the chemical elements and surface compositions of the samples. The polarization-electric field hysteresis loops and the leakage currents were measured using a ferroelectric analyzer (Aix ACCT, TF Analyzer 2000). The optical bandgap was determined using a Hitachi U4100 spectrophotometer, which measured the absorption spectra in the ultraviolet-visible (UV–Vis) range. The dielectric frequency spectra were obtained by analyzing the impedance using a precision analyzer (Agilent 4294A).
3 Results and discussion
3.1 Phase structure and micromorphology
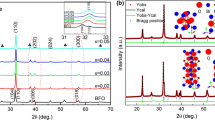
The XRD patterns of the BFCO films are illustrated in Fig. 1. Peaks were located at about 26.6°, 33.8°, and 37.9°, which correspond to SnO2 (JCPDS no.41-1445). The other diffraction peaks match the standard values reported in JCPDS No. 72-2112, and the R3c rhombic perovskite BFCO structure is detected. No peaks associated with impurities were detected beyond the 20–60° range in relation to the perovskite structure. This is due to the thermal annealing process and appropriate content of bismuth and iron ions. Figure 1a indicates that all six samples possessed an identical single-phase structures, and the results show that the doping did not affect the lattice symmetry [33]. Figure 1b depicts a magnified partial view of the XRD pattern, specifically highlighting the range of ~31–33°. The diffraction peaks at (012) and (110) orientations in pure BFO demonstrate a bimodal characteristic. Upon the Co2+ substitution, the (110) peak exhibits a reduced angular displacement and diminished intensity, suggesting the partial replacement of Fe ions by the doped ions within the BF lattice structure. This results in a single peak formed by the merging of the (012) and (110) peaks. Additionally, the similar merging of XRD peaks and angular shifts could suggest that the doping of Co2+ might induce distortion in the lattice structure of the BFCO thin films. This is attributed to the mismatch in ionic radii, as Co2+ ions possess a larger radius (0.740 Å) in comparison to Fe3+ ions (0.645 Å). This results in slight changes in the interplanar spacing and grain size within the BFCO lattice as the Co doping content increases. The diffraction peak positions remain almost constant when the content of Co exceeds 0.04, which may be due to the fact that Co is a variable valence ion. In this regard, it is noteworthy that the enhancement of ferroelectric properties in thin films may be strongly correlated with lattice deformation and ion valence states [34, 35]. Table 1 lists the changes in lattice parameters (a and c) and volume. The variations in a and c are related to the doping level of Co, indicating a significant alteration in the film structure. The increase in the volume of the BFO crystal unit cell is attributed to the larger ion radius of Co2+ (0.740 Å) compared to that of Fe3+ (0.645 Å).
The sample was examined through Raman scattering analysis in Fig. 2, which possesses a strong sensitivity to the crystal structure and provides complementary information to that of XRD characterization. To accurately determine the peak position for each Raman vibration mode, the Raman spectra of the BFCO films from 50 to 800 cm−1 were fitted using a Lorentz model. The rhomboid perovskite structure consists of 4 A1 vibration modes and 9 E vibration modes [36, 37]. There are nine distinct Raman spectroscopic patterns observed in the films. The vibrations of Bi-O primarily occurs in the low-wavenumber region corresponding to the A modes, while the E modes in the high-wavenumber region are related to the vibrations of Fe-O [38]. Ion doping has a significant effect on the vibrational intensity and position of Bi-O and Fe-O modes, with the intensity and position of the A1-1 and A1-2 peaks of the Co-doped samples were different compared to the pure BFO. With the increase of Co doping concentration, the A1-1 and A1-2 vibration modes tend to merge into a single mode. This is due to the change in the length of the Bi-O bond and the angle of the Bi-O-Bi bond, caused by the substitution of Co2+ ions for Fe3+ ions. The change in the E mode confirms that Co partially replaces Fe in the BFO lattice. In addition, the intensity of the A1-4 and E-9 vibration peaks in the BFCO thin film gradually increases, and the intrinsic vibration mode of Fe-O bond partially changes to that of the Co-O bond. Therefore, the structural distortion of the FeO6 octahedron increases. On the other hand, due to the Jahn-Teller effect, the mismatch of radii between Co and Fe ions leads to severe distortion of the iron oxide octahedron [39, 40]. The results indicate that Co doping leads to changes in the bond length and bond angle of the Fe-O bond and indirect changes in the Bi-O bond, which cause a change in the lattice of the film, consistent with the XRD results. These data are summarized in Table 2.
Synthesis conditions and doping are known to alter surface microstructure and grain growth, thereby affecting material properties. The surface morphology of BFCO and partial section morphology of BFCO films (x = 0, 0.03) are displayed in Fig. 3. As shown in the figure, the six films have good crystallization, and appear flat and smooth without cracks; nevertheless, some pinhole defects were scattered on the sample surface due to volatilization of organic substances and Bi ions during annealing. Some grain clusters appeared in the BFCO film with Co doping, which may be due to the obvious effect of the introduction of Co ion on grain growth, especially the sample of x = 0.03 (Fig. 3d) [41, 42]. The average grain size of BFCO-0 (Fig. 4a) and BFCO-3 thin films (Fig. 4b) is 90 nm and 78 nm, respectively, according to the histogram of particle size distribution. The results demonstrate that the optimal level of Co ion doping exerts a favorable influence on the enhancement of grain growth. For BFO films, grain size is closely related to lattice distortion and electrical properties, which also confirms the XRD correlation analysis. In the cross-sectional morphology of BFCO-0 and BFCO-3 films shown in Fig. 3a, d, respectively, it can be observed that there is a clear layered structure without mutual diffusion between the film and the FTO substrate. The thicknesses of the BFCO-0 and BFCO-3 films were measured to be 1.4 μm and 1.3 μm, respectively. Due to the use of specific FTO/glass substrates, all films have a specific thickness of 1.1 μm of FTO layer underneath. The improved compactness of the film structure also has an impact on the reduction of its leakage current density [43, 44]. The composition of BiFe0.97Co0.03O3 was analyzed by EDS, and its elemental energy spectrum and mapping map are shown in the Fig. 3g, h. In addition to the adventitious carbon coating used for SEM characterization, only the elements Bi, Fe, Co, and O were detected in the sample. Moreover, these elements exhibited uniform distribution throughout the sample. The atomic content of each element in the prepared BiFe0.97Co0.03O3 thin film sample is basically consistent with the stoichiometric ratio of BFCO, which directly indicates that Co has been successfully incorporated into the BFO lattice. The above results combined with XRD analysis directly confirmed the presence of Co ions and the successful Co doping of BFO.
The ferroelectric performance of the films is influenced not only by its growth orientation but also by any defects present, such as oxygen vacancies [45]. The surface elemental chemical states of the thin films were determined by conducting XPS measurements, as depicted in Fig. 5. The characteristic peaks are clearly discernible, and detailed information regarding the element content is provided in Table 3. Since the experimental samples themselves do not contain the element C, the binding energy was first calibrated by a charge shift based on C1s = 284.8 eV. In Fig. 5a, the presence of a double peak in the low binding energy region of Fe indicates the coexistence of both Fe2+ and Fe3+ in the thin film. Moreover, the ratio of Fe3+ to Fe2+ directly impacts the electronic and valence states of the film. In the thin films, due to the presence of Fe2+, a certain number of oxygen vacancies will be generated to maintain charge balance. Thus, the concentration of Fe2+ ions is correlated with the number of oxygen vacancies [46, 47]. Through the analysis results, the surface Fe2+ content of BFCO films (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) was found to be 71.5%, 71.4%, 73.0%, 64.2%, 73.4%, and 79.4%, respectively. In the BiFe0.97Co0.03O3 film, the Fe2+ content is the lowest, which also indicates that Co doping is beneficial in inhibiting the conversion of Fe3+ to Fe2+.
In Fig. 5b, corresponding to the Co2p orbital satellite peaks (namely Co2p3/2 and Co2p1/2), the presence of both divalent Co and trivalent Co is observed. It is found that the multivalent Co undergoes a valence state transition after heat treatment. Quantitative analysis reveals that the Co2+/Co3+ ratio of the BiFe0.97Co0.03O3 film is 37.1/62.9. The presence of Co2+ leads to the formation of oxygen vacancies. The oxygen vacancies combine with defect dipoles to form defect complexes (\({{\rm{C}}{\rm{o}}}_{{\rm{F}}{\rm{e}}}^{{\prime} }-{{\rm{V}}}_{{\rm{O}}}^{\bullet \bullet }\)) and help balance the charge concentration. However, with the introduction of Co3+, there is a gradual increase in the conversion of Co2+ to Co3+, effectively inhibiting the generation of oxygen vacancies. The details of this conversion are shown in the following equations.
The O1s spectrum is shown in Fig. 5c and consists of three parts. The peak at ~531 eV corresponds to lattice oxygen (O2−), while the peak in the middle at ~532 eV represents surface-adsorbed oxygen originating from the surface metal atoms and chemisorbed species [48]. The last peak at ~533 eV corresponds to adsorbed oxygen. All BFCO films contain oxygen vacancies, which directly affect their ferroelectric properties [49]. As the Co doping level increases, the concentration of oxygen vacancies in BFCO thin films gradually decreases. In the BiFe0.97Co0.03O3 thin film, the concentration of oxygen vacancies is reduced to 7.6%. This is attributed to the charge balance effect between Fe2+ and Co3+. When the Co content increases to 0.05, the oxygen vacancy content in the thin film increases significantly to 11.5%, indicating that the Fe2+ and oxygen vacancy content is the lowest in the BiFe0.97Co0.03O3 thin film. Through XRD combined with XPS analysis, it is demonstrated that Co ions are efficiently doped into the BFO lattice, and there is a valence state transition of Co2+, resulting in the presence of a large number of Co3+ ions in the BFCO thin film. From this, it can be concluded that a certain amount of Co doping can effectively suppress the formation of oxygen vacancies, and the impact on the thin film leakage current density will be discussed in detail in the following sections [50].
3.2 Physical properties
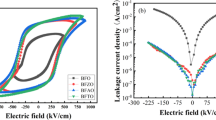
Figure 6a–f shows the hysteresis loops and polarization current curves of BiFe1−xCoxO (x = 0.00–0.05) thin films, while details of the Pr values are presented in Table 4. The red curve in the figure is the I-E curve from which the switching current (Is) and the polarization leakage current (IL) of all film samples are obtained. The Is value reflects the difficulty of ferroelectric domain reversal. The larger the Is value, the easier the ferroelectric domain reversal and the stronger the spontaneous polarization of the film. The IL value represents the bias of the polarization leakage current and reflects the degree of polarization leakage. The higher the IL value, the greater the polarization contribution from non-intrinsic leakage. The minimum Is value of BFCO-0 is 1.07 × 10−3 mA and the minimum IL value of BFCO-3 is 3.4 × 10−4 mA, which corresponds to the lowest leakage current density of BFCO-3. From the current curve, it can be seen that the ferroelectric domain inversion of BFO films is significantly enhanced by appropriate Co doping. Compared to BFCO thin films, BFO exhibits an unsaturated circular hysteresis loop with a low residual polarization value of 72.33 μC/cm2. It can be seen that the BFCO film exhibits a rectangular P-E loop and a higher Pr value than the BFO film. The remnant polarization value of the BFCO film increases with the increase of dopant concentration. The residual polarization value of BFCO films also increases with the increase in dopant concentration, indicating that the doping of Co can effectively enhance the ferroelectric performance. Among them, the BiFe0.97Co0.03O3 thin film sample has the highest residual polarization value of Pr ≈ 152.1 μC/cm2, with a coercive field of Ec ≈ 554.7 kV/cm. This could be attributed to the increased susceptibility of ferroelectric domains to switching under high electric fields. We speculate that the ultra-high polarization of the BFCO thin film may be attributed to three reasons. Firstly, the substitution of Co2+ influences the lattice distortion caused by the mismatch in the ionic radii of Fe and Co, thus increasing the tendency favorably for spontaneous polarization. Secondly, the large residual polarization value may be attributed to the increase in thin film density due to the reduction in grain size, which in turn reduces the leakage current density. As the leakage current decreases, the residual polarization gradually increases. Thirdly, combined with XPS analysis, it is shown that the doping of Co at the Fe sites in BFO inhibits the generation of oxygen vacancies, thereby improving the ferroelectric performance of the thin film [51, 52]. When the Co content exceeds x = 0.03, the residual polarization value of the thin film shows a decreasing trend. This phenomenon is likely due to the increase in leakage current with the increase in grain size and defect density, which in turn leads to a deterioration in its ferroelectric behavior.
In fact, the P-E data of the BFCO thin films may be influenced by the leakage current density and nonlinear dielectric characteristics, and hence further analysis is required [53]. The ferroelectric test was conducted to determine the intrinsic polarization of the BFCO thin films, as shown in Fig. 7a. It is well known that ∆P = Psw−Pnsw = 2Pr, where Psw represents the switching polarization and Pnsw represents the non-switching polarization. At the highest voltage, the pulse residual polarization values (∆P) of BFCO (x = 0–0.05) thin films are: 108.31, 156.69, 195.10, 223.33, 106.54, and 85.25 μC/cm2, respectively. From the analysis of the graphs, it can be observed that the trend of ∆P values is consistent with the P-E data. However, it is evident that the ∆P value is smaller than the 2Pr value of the BFCO thin film, indicating that the influence of leakage current on the ferroelectric behavior of the material cannot be ignored.
The insulation properties of BFCO thin films were tested within the range of 0–250 kV/cm, and a curve of the sample leakage current density varying with the applied electric field was plotted. As a result of the aforementioned factors, including oxygen vacancies, the concentration of Fe2+ ions and grain boundaries, the current density of thin films made of BFCO is low at low electric fields but increases rapidly at high electric fields, as illustrated in Fig. 7b. The J values of BiFe1−xCoxO3 (x = 0.00–0.05) films are 2.45 × 10−5, 8.79 × 10−5, 3.27 × 10−5, 7.18 × 10−7, 2.64 × 10−4 and 2.81 × 10−3 A/cm2, respectively The sample with x = 0.01 reduced the leakage current density by two orders of magnitude compared with pure BFO samples. Therefore, a certain amount of Co ion doping can effectively reduce the leakage current density of BFO thin films. The origin of the high leakage current in BFO-based materials is the generation of oxygen vacancies. As an electron trapping center, oxygen vacancies can enhance leakage by activating conduction with an externally applied electric field [54]. XPS confirms that Co doping suppresses electron hopping between Fe3+ and Fe2+ and the generation of oxygen vacancies. At the same time, \({{\rm{Co}}}_{{{\rm{Fe}}}^{3+}}^{2+}\) combines with \({V}_{O}^{\bullet \bullet }\) to form defect dipoles that restrict the movement of oxygen vacancies. As another factor that suppresses the increase of leakage current, grain boundaries reduce significantly as Co ions enter the BFO lattice, resulting in large insulation boundaries. Therefore, the grain boundaries also contribute to the reduction of leakage current behavior by serving as a barrier for current conduction. When x = 0.02–0.05 mol%, the carrier density in the BFCO thin film increases as the oxygen vacancy content increases, resulting in an increase in J value [55].
In Fig. 7c, to better understand the leakage mechanism of BFCO film, it is necessary to further investigate the conduction mechanism of the sample. For volumetric conduction, leakage includes the effects of both free carriers generated by heat and injected carriers. The leakage current curve was fitted using the volumetric confinement conduction mechanism, and the relationship between log(J) and log(E) was analyzed [56]. The conduction mechanism was determined by the slope of the linear correlation, where α = 1 for Ohmic conduction and α ≥ 2 for space charge-limited conduction (SCLC). In the BFCO films with x = 0.02, 0.03, 0.04, and 0.05, the leakage current is mainly caused by the Ohmic conduction mechanism, and the curve fitting slopes are close to 1 (1.57, 1.74, 1.26, and 1.47, respectively). This indicates that intrinsic mobile electrons or holes in BFCO film contribute to conduction. For the thin film samples with x = 0.00 and 0.01, the Ohmic conduction mechanism is still the main mechanism, but SCLC is also observed, which may be due to the unequal density of injected carriers and equilibrium carriers that result in a space charge effect [57].
In order to study the dielectric properties of thin films with different Co doping levels, the dielectric constant and dielectric loss values of BFCO thin films were obtained as the frequency varied as shown in Fig. 8. Under the action of an alternating electric field, the space charge undergoes changes, with a relatively large εr value, which remains largely unchanged at higher frequencies [58]. As the frequency increases, the oxygen vacancy-related dipoles fail to respond promptly to the changes, resulting in a decreasing trend in the εr value. At 1 kHz, the εr value of BFO is 30.04, while the εr value of BiFe0.97Co0.03O3 is 74.89. The variation in εr value is attributed to the reduction in the number of defects such as oxygen vacancies. Figure 8b demonstrates that at lower testing frequencies, the dielectric loss values (tanδ) of all samples decrease with increasing frequency. However, when the frequency exceeds 100 kHz, there is a tendency for the dielectric loss to increase. This is due to the presence of a resonance frequency between ion hopping and the external field, resulting in the transfer of electrical energy to oscillating ions. At the same time, with the increase of Co content, the tanδ value decreases to 0.076 in BiFe0.97Co0.03O3. In the low frequency region, it is mainly leakage current loss, while in the high frequency region, polarization relaxation dominates [59, 60]. The minimum tanδ value of BiFe0.97Co0.03O3 is related to the lowest leakage current and oxygen vacancy concentration. Overall, the results indicate that Co doping significantly alters the dielectric properties of BFO.
Using UV-visible absorption spectroscopy to study the optical properties of samples enables changes in the band structure and band gap of the samples to be explored, as shown in Fig. 9. The charge transfer of Bi-O occurs around 250–360 nm, while the charge transfer band of Fe-O corresponds to the peak around 490 nm. In Fig. 9a, all BFCO films show a noticeable absorption peak, with the maximum absorption wavelength around 500 nm, confirming the visible light response of all samples [61]. With the increase of Co doping concentration, the light absorption of the sample shows a red shift, and the width of the absorption peak increases. New energy levels appear when dopants are added, resulting in an increase in the density of states of the energy band, which corresponds to the generation of localized states in the energy band gap, thereby altering the density of states in the energy band. Estimation of the optical band gap for the corresponding sample is based on the Tauc equation and calculated using the formula ahv = A (hvᅳEg)n. Here, BFO is a direct band gap material, and the value of n is 1/2 [62]. Plotting the (ahv)2−hv curve and extending its linear segment to intersect the x-axis will give the extrapolated optical band gap in Fig. 9b. The bandgap widths of BiFe1−xCoxO (x = 0.00–0.05) are approximately 1.87 eV, 1.93 eV, 1.90 eV, 1.82 eV, 1.85 eV, and 1.69 eV, respectively. With the doping of Co, the band gap width shows a decreasing trend. This may be due to the lattice distortion of FeO6 octahedra caused by Co doping, which in turn causes a slight strain in the sample structure and a reordering of molecular orbitals, thereby changing the range of visible light absorption. The research results can provide a reference for photodetectors and infrared detectors based on BFO thin films [63, 64].
4 Conclusion
This study systematically investigates the influence of Co ion substitution at the Fe site on BiFeO3 (BFO) thin films through the preparation of BFCO thin films (x = 0–0.05) on FTO/glass substrates using the sol–gel technique. XRD analysis confirms the perovskite rhombohedral structure of the BFMO thin films, with no secondary phases detected. SEM analysis combined with the XRD results indicates that Co doping leads to the formation of smaller and more uniformly distributed grains, resulting in reduced grain boundary resistance. This facilitates the reversal of ferroelectric domains and enhances polarization. The presence of Co is confirmed by EDS and XPS, which reveals a reduction in the concentrations of Fe2+ ions and oxygen vacancies. Consequently, this reduction contributes to the decrease in leakage current. Among the BFCO thin films, the BiFe0.97Co0.03O3 thin film exhibits the highest remnant polarization (Pr = 152.1 μC/cm2) and the lowest leakage current density (7.18 × 10−7 A/cm2). The remnant polarization of the BFO thin film significantly increases with an increase in the applied electric field, while Ohmic conduction becomes the dominant leakage mechanism. Additionally, the bandgap of the BFO thin film decreases as the Co doping level increases, reaching a minimum value of 1.69 eV at x = 0.05. These findings demonstrate the effectiveness of Co doping in simultaneously reducing the bandgap and enhancing the ferroelectric behavior of BFO. Consequently, BFCO thin films hold promise as potential materials for the development of efficient ferroelectric photovoltaic devices.
References
Wang J, Neaton JB, Zheng H, Nagarajan V, Ogale SB, Liu B et al. (2003) Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 299:1719–1722
Van Aken BB, Rivera JP, Schmid H, Fiebig M (2007) Observation of ferrotoroidic domains. Nature 449:702–705
Hajra S, Oh Y, Sahu M, Lee K, Kim HG, Panigrahi BK, Mistewicz K, Kim HJ (2022) Piezoelectric nanogenerator based on flexible PDMS-BiMgFeCeO6composites for sound detection and biomechanical energy harvestingt. Energy Fuels 5:6049–6058
Kumar N, Shukla A, Kumar N, Sahoo S, Hajra S, Choudhary RNP (2018) Structural, electrical and ferroelectric characteristics of Bi(Fe0.9La 0.1)O3. Ceram Int 44:21330–21337
Liu YQ, Wang Y, Ma J, Li S, Pan H, Nan CW, Lin YH (2022) Controllable electrical, magnetoelectric and optical properties of BiFeO3 via domain engineering. Prog Mater Sci 127:100943
Eerenstein W, Mathur N, Scott JF (2006) Multiferroic and magnetoelectric materials. Nature 442:759–765
Fiebig M (2005) Revival of the magnetoelectric effect. J Phys D: Appl Phys 38:R123–R152
Wu JG, Fan Z, Xiao DQ, Zhu JG, Wang J (2016) Multiferroic bismuth ferrite-based materials for multifunctional applications: ceramic bulks, thin films and nanostructures. Prog Mater Sci 84:335–402
Spaldin NA, Cheong SW, Ramesh R (2010) Multiferroics: past, present, future. Phys Today 63:38
Ramesh R, Spaldin N (2007) Multiferroics: progress and prospects in thin films. Nat Mater 6:21–29
Catalan G, Scott JF (2009) Physics and applications of bismuth ferrite. Adv Mater 21(24):2463–2485
Yin LI, Mi W (2020) Progress in BiFeO3-based heterostructures: materials, properties and applications. Nanoscale 12(2):477–523
Blázquez Martínez A, Godard N, Aruchamy N, Milesi-Brault C, Condurache O, Bencan A, Glinsek S, Granzow T (2021) Solution-processed BiFeO3 thin films with low leakage current. J Eur Ceram Soc 41(13):6449–6455
Makhdoom AR, Akhtar MJ, Rafiq MA, Hassan MM (2012) Investigation of transport behavior in Ba doped BiFeO3. Ceram Int 38:3829–3834
She S, Yu J, Tang W, Zhu Y, Chen Y, Sunarso J, Zhou W, Shao Z (2018) Systematic study of oxygen evolution activity and at ability on La1−xSrxFeO3-δ perovskite electrocatalysts in alkaline media. ACS Appl Mater Interfaces 10:11715–11721
Xue M, Tan G, Xia A, Chai Z, Lv L, Ren H, Ren X, Li J (2019) Multi-doped bismuth ferrite thin films with enhanced multiferroic properties. Ceram Int 45:12806–12813
Yu C, Viola G, Zhang D, Zhou K, Koval V, Mahajan A, Wilson RM, Tarakina NV, Abrahams I, Yan H (2018) Phase evolution and electrical behaviour of samarium-substituted bismuth ferrite ceramics. J Eur Ceram Soc 38:1374–1380
Biswas PP, Pal S, Subramanian V, Murugavel P (2019) Large photovoltaic response in rare-earth doped BiFeO3 polycrystalline thin films near morphotropic phase boundary composition. Appl Phys Lett 114(17):173901
Bhatnagar A, Roy Chaudhuri A, Heon Kim Y, Hesse D, Alexe M (2013) Role of domain walls in the abnormal photovoltaic effect in BiFeO3. Nat Commun 4(1):2835
Walker J, Bryant P, Kurusingal V, Sorrell C, Kuscer D, Drazic G, Bencan A, Nagarajan V, Rojac T (2015) Synthesis-phase-composition relationship and high electric-field-induced electromechanical behavior of samarium-modified BiFeO3 ceramics. Acta Mater 83:149–159
Tan G, Chai Z, Zheng Y, Yue Z, Yang W, Guo M, Ren H, Xia A, Lv L, Liu Y (2018) Tunable structural transition and multiferroic properties of the composite thin films through the structural transition of magnetic layer. J Eur Ceram Soc 38:4463–4475
Kumar N, Shukla A, Choudhary RNP (2020) Effects of milling time on structural, electrical and ferroelectric features of mechanothermally synthesized multi-doped bismuth ferrite. Appl Phys 126:181
Yang SJ, Zhang FQ, Xie XB, Sun HJ, Zhang LP, Fan SH (2018) Enhanced leakage and ferroelectric properties of Zn-doped BiFeO3 thin films grown by sol-gel method. J Alloy Compd 734:243–249
Sharma GN, Dutta S, Pandey A, Singh SK, Chatterjee R (2017) Microstructure and improved electrical properties of Ti-substituted BiFeO3 thin films. Mater Res Bull 95:223–228
Priya AS, Geetha D (2021) Impact of (Zr, Cu) ion substitution on the optical, dielectric, and impedance behavior of BiFeO3. Braz J Phys 51:40–46
Liu Y, Tan GQ, Guo MY, Chai ZJ, Lv L, Xue MT, Rena XX, Li JC, Ren HJ, Xia A (2019) Multiferroic properties of La/Er/Mn/Co multi-doped BiFeO3 thin films,. Ceram Int 45:11765–11775
Qi X, Dho J, Tomov R, Blamire MG, MacManus-Driscoll JL (2005) Greatly reduced leakage current and conduction mechanism in aliovalent ion doped BiFeO3. Appl Phys Lett 86(6):062903
Xue X, Tan G, Liu W, Hao H, Ren H (2015) Structural, electrical, and magnetic properties of multiferroic Bi1−xGdxFe0.97Co0.03O3 thin films. J Alloy Compd 622:477–482
Arya G, Yogiraj J, Negi NS, Shah J, Kotnala RK (2017) Observation of enhanced multiferroic, magnetoelectric and photocatalytic properties in Sm-Co codoped BiFeO3 nanoparticles. J Alloy Compd 723:983–994
Sinha AK, Bhushan B, Jagannath, Gupta N, Sen S, Prajapat CL, Nuwad J, Bhatt P, Mishra SK, Meena SS, Priyam A (2020) Effect of cobalt-doping on dielectric, magnetic and optical properties of BiFeO3 nanocrystals synthesized by sol-gel technique. Solid State Sci 102:106168
Saha S, Singh RP, Rout A, Mishra A, Ali A, Basumatary H, Ranjan R (2023) Inducing ferromagnetism and magnetoelectric coupling in the ferroelectric alloy system BiFeO3–PbTiO3 via additives. J Appl Phys 133:064101
Wani WA, Kundu S, Ramaswamy K, Venkataraman H (2020) Structural, morphological, optical and dielectric investigations in cobalt doped bismuth ferrite nanoceramics prepared using the sol-gel citrate precursor method. J Alloy Compd 846:156334
Eerenstein W, Mathur ND, Scott JF (2006) Multiferroic and magnetoelectric materials. Nature 442(7104):759–765
Deng XL, Zeng ZX, Gao RL (2020) Study of structural, optical and enhanced multiferroic properties of Ni doped BFO thin films synthesized by sol-gel method. J Alloy Compd 831:154857
Qi J, Liu H, Feng M, Xu H, Liu HW, Wang C, Wang AP, Lü WM (2020) Enhanced hydrogen evolution reaction in Sr doped BiFeO3 by achieving the coexistence of ferroelectricity and ferromagnetism at room temperature. J Energy Chem 53:93–98
Li Z-J, Hou Z-L, Song W-L, Liu X-D, Cao W-Q, Shao X-H, Cao M-S (2016) Unusual continuous dual absorption peaks in Ca-doped BiFeO3 nanostructures for broadened microwave absorption. Nanoscale 8:10415–10424
Singh MK, Jang HM, Ryu S, Jo MH (2006) Polarized Raman scattering of multiferroic BiFeO3 epitaxial films with rhombohedral R3c symmetry. Appl Phys Lett 88:042907
Singh M, Jang H, Jo M, Ryu S (2006) Polarized Raman scattering of multiferroic BiFeO3 epitaxial films with rhombohedral R3c symmetry. Appl Phys Lett 88:042907
Wang Y, Wang J (2009) Modulated charged defects and conduction behaviour in doped BiFeO3. thin films, J Phys D: Appl Phys 42(16):162001
Bai L, Sun MJ, Ma WJ, Yang JH, Zhang JK, Liu YQ (2020) Enhanced magnetic properties of Co-doped BiFeO3 thin films via structural progression. Nanomaterials 10:1798
Yue ZW, Tan GQ, Yang W, Ren HJ, Xiao A (2016) Enhanced multiferroic properties in Pr-doped BiFe0.97Mn0.03O3 films. Ceram Int 42:18692–18699
Guo M, Tan G, Yang W, Liu Y, Ren H, Xia A, Lv L, Xue M (2018) Multiferroic properties of Bi0.89Ho0.08Sr0.03Fe0.97−xMn0.03NixO3 thin films modulated by F-N tunneling effects. Ceram Int 44:12282–12291
Ren XX, Tan GQ, Liu Y, Li JC, Xue MT, Ren HJ, Xia A, Liu WL, Liu Y (2020) Oxygen vacancy and grain boundary resistance regulate the intrinsic ferroelectric properties of Bi0.96Sr0.04Fe0.98−xMnxCo0.02O3 thin film. J Eur Ceram Soc 44:5431–5440
Chai Z, Tan G, Yue Z, Xue M, Liu Y, Lv L, Ren H, Xia A (2018) Structural transition, defect complexes and improved ferroelectric behaviors of Bi0.88Sr0.03Gd0.09Fe0.94Mn0.04Co0.02O3/Co1−xMnxFe2O4 bilayer thin films. Ceram Int 44:15770–15777
Zhang Y, Qi J, Wang Y, Tian Y, Zhang J, Hu T, Wei M, Liu Y, Yang J (2018) Tuning magnetic properties of BiFeO3 thin films by controlling Mn doping concentration. Ceram Int 44:6054–6061
Verma P, Singh P, Roy PK (2021) Influence on properties of Bi0.9Sm0.1FeO3 multiferroic system with Mg substitution at Fe-site. J Solid State Chem 302:122432
Mocherla PSV, Karthik C, Ubic R, Ramachandra Rao MS, Sudakar C (2013) Tunable bandgap in BiFeO3 nanoparticles: the role of microstrain and oxygen defects. Appl Phys Lett 103:022910
Kossar S, Amiruddin R, Rasool A, Giridharan NV, Dhayanithi D, Santhosh Kumar MC (2020) Ferroelectric polarization induced memristive behavior in bismuth ferrite (BiFeO3) based memory devices. Superlattice Microst 148:106726
Jin LH, Tang XW, Wei RH, Yang BB, Yang J, Song WH, Dai JM, Zhu XB, Sun YP (2016) BiFeO3(00 l)/LaNiO3/Si thin films with enhanced polarization: an all solution approach. RSC Adv 6:78629–78635
Liang XL, Dai JQ, Zhang GD (2022) Great ferroelectric properties and narrow bandgaps of BiFeO3 thin films by (Mg, Mn) modifying. Appl Surf Sci 586:152751
Yu BF, Li MY, Hu ZQ, Pei L, Guo DY, Zhao XZ, Dong SX (2008) Enhanced multiferroic properties of the high-valence Pr doped BiFeO3 thin film. Appl Phys Lett 93:182909
Dong G, Tan G, Luo Y, Liu W, Xia A, Ren H (2014) Charge defects and highly enhanced multiferroic properties in Mn and Cu co-doped BiFeO3 thin films. Appl Surf Sci 305:55–61
Naganuma H, Inoue Y, Okamura S (2008) Evaluation of electrical properties of leaky BiFeO3 films in high electric field region by high-speed positive-up-negativedown measurement. Appl Phys Express 1:061601
Wang J, Luo L, Han C, Yun R, Tang X, Zhu Y, Nie Z, Zhao W, Feng Z (2019) The microstructure, electric, optical and photovoltaic properties of BiFeO3 thin films prepared by low-temperature sol-gel method. Materials 12:1444
Qi X, Dho J, Tomov R, Blamire MG, MacManus-Driscoll JL (2005) Greatly reduced leakage current and conduction mechanism in aliovalent-ion-doped BiFeO3. Appl Phys Lett 86:062903
Wang C, Takahashi M, Fujino H, Zhao X, Kume E, Horiuchi T, Sakai S (2006) Leakage current of multiferroic (Bi0.6Tb0.3La0.1)FeO3 thin films grown at various oxygen pressures by pulsed laser deposition and annealing effect. J Appl Phys 99:054104
Dai Y, Guo Q, Cui C, Yang L, Li C, Li X (2018) Role of ferroelectric/ferromagnetic layers on the ferroelectric properties of magnetoelectric composite films derived by chemical solution deposition. Mater Res Bull 99:424–428
Zhu J-H, Dai J-Q, Xu J-W, Li X-Y (2018) Effect of Zn and Ti Co-doping on structure and electrical properties of BiFeO3 ceramics. Ceram Int 44:9215–9220
Chai ZJ, Tan GQ, Yue ZW, Xue MT, Liu Y, Lv L, Ren HJ, Xia A (2018) Structural transition, defect complexes and improved ferroelectric behavior of Bi0.88Sr0.03Gd0.09Fe0.94Mn0.04Co0.02O3/ Co1−xMnxFe2O4 bilayer thin films. Ceram Int 44:15770–15777
Lin D-Y, Chen H-Z, Kao M-C, Zhang P-L (2020) Ferroelectric and electrical properties optimization of Mg-doped BiFeO3 flexible multiferroic films. Symmetry 12(7):1173
Ramachandran B, Dixit A, Naik R, Lawes G, Rao MSR (2010) Charge transfer and electronic transitions in polycrystalline BiFeO3. Phys Rev B 82:012102
Guo BL, Deng HM, Zhai XZ, Zhou WL, Meng XK, Weng GE, Chen SQ, Yang PX, Chu JH (2017) Cr doping-induced structural phase transition, optical tuning and magnetic enhancement in BiFeO3 thin films. Mater Lett 186:198–201
Zhang S-T, Zhang Y, Lu M-H, Du C-L, Chen Y-F, Liu Z-G, Zhu Y-Y, Ming N-B, Pan XQ (2006) Substitution-induced phase transition and enhanced multiferroic properties of Bi1−xLaxFeO3 ceramics. Appl Phys Lett 88:162901
Chai Z, Tan G, Yue Z, Yang W, Guo M, Ren H, Xia A, Xue M, Liu Y, Lv L (2018) Ferroelectric properties of BiFeO3 thin films by Sr/Gd/Mn/Co multi-doping. J Alloy Compd 746:677–687
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 52073129 and 51762030).
Author information
Authors and Affiliations
Contributions
JZ wrote the main manuscript text and GCZ and XJZ prepared figures 1–3. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Dai, JQ., Zhang, GC. et al. Outstanding ferroelectric properties in the narrow bandgap cobalt-substituted BiFeO3 spin-coated films. J Sol-Gel Sci Technol (2024). https://doi.org/10.1007/s10971-024-06443-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-024-06443-4