Abstract
Rods of silver (Ag)-dysprosium (Dy3+) co-doped in silica xerogels were synthesized via sol–gel method. The doped matrices, as a function of Dy3+ ions concentration, were characterized using ultraviolet-visible and near-infrared (UV–VIS-NIR) and photoluminescence (PL) spectroscopy. The formation of fine spherical Ag nanoparticles was confirmed by the field-emission scanning electron microscopy (FE-SEM). In addition to the surface plasmon resonance (SPR) bands of Ag nanoparticles centered at 423 nm, the UV–VIS-NIR spectra revealed absorption peaks due to Dy3+ active centers. From the photoluminescence results, three emission peaks at 477, 518 and 576 nm were observed when the samples were excited by 350 nm source. The results from the CIE chromaticity diagram gave an indication of the white light emissivity at the excitation wavelength of 350 nm and thus these samples can be a promising candidate for white light emitting applications.
Graphical abstract

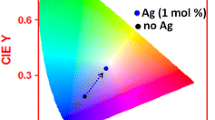
CIE diagram of the photoluminescence spectra of Ag-Dy3+ co-doped silica xerogels
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, sufficient work has been devoted to enhance white light emitting diodes (LEDs) pours into the benefits of high efficiency, higher brightness, environmental friendliness, lower energy consumption, and lengthen the life-time compared to conventional lighting systems. Resulted in recent advancements in lighting sources, white LEDs have demonstrated significant potential for use as indicators in a wide range of equipment, besides the wide range of local applications. White LEDs are commercial productions by combining blue and yellow bad effects including color change and minimizes lightening on the long way. The reason of this is the decay of the individual substance vary. For this reason, there is a lot of interest in developing single-component phosphors that emit white light on exposing to ultraviolet light [1, 2].
Rare earth (RE) ion-containing functional materials have enormous potential for use in display technology, lamp phosphors, and white light sources. These materials are widely used as monochromatic devices because of their intense emission capability, flexible emission colors with different activators, and high thermal and chemical stability. As optical materials capable of emitting in the visible and near-infrared ranges, the absorption and emission of various trivalent REs’ f-f or f-d transitions has got considerable attention. According to their remarkable photoluminescence in a variety of matrices, trivalent dysprosium (Dy3+) ions are important active centers. Because their emission wavelength is highly dependent on the type of host matrix, the emission colors produced can be used to produce both blue and yellow emissions, which is useful for white light generation. To produce overall white light, the yellow-to-blue emission intensity ratio (Y/B) can be adjusted. Generally speaking, Dy3+ has three visible emission peaks: the blue one around 480 nm caused by the 4F9/2 → 6H15/2 electronic transition, the yellow one around 575–580 nm caused by the hypersensitive 4F9/2 → 6H13/2 transition (J = 2), and the red one around 670 nm caused by the 4F9/2 → H11/2 transition. The hypersensitive transition of Dy3+ ion can be significantly influenced by the surrounding environment, and thus variable ratio of Y/B emission intensities can be adapted through appropriate host matrix selection [3, 4].
Combining lanthanide ions with noble metals like silver or gold proved to be an efficient way of increasing the photoluminescence of REs in various types of glasses. First, the improved photoluminescence performance was due to the influence of Ag nanoparticles with 420 nm absorbance on local surface plasmon resonance (SPR). This optical phenomenon was explained as a collective excitation of conduction band electrons that produced high electromagnetic fields near Ag nanoparticles, increasing excitation efficacy and radiative decay rate, and thus efficiently increasing lanthanide ion absorbance coefficient. Others, on the other hand, explained photoluminescence enhancement as classical energy transfer from non-plasmonic very fine molecule-like Ag particles or isolated Ag+ ions to lanthanide ions [5]. Several methods have been used to synthesize elements such as Ag co-doped with lanthanide elements, including the sol–gel method, which gives advantages such as almost well organized and good dopant dispersion [6].
The recent research focus on the preparation of Ag-Dy3+ co-doped silica matrices, and studying the surface plasmon resonance effects of Ag nanoparticles on the Dy3+ ions properties in the direction of white LED applications.
2 Experimental part
2.1 2-1 liquid samples
A 50 ml as a starting solution of ~5.0 × 10−3 mol\l silver nitrate in water was prepared. (0.0425 g of AgNO3, purity >99%in 50 mL deionized H2O). then 25 mL of that solution was taken and add it to another 100 mL of H2O (now ~1.0 × 10−3 mol\l).In another hand a solution of 1% sodium citrate (0.5 g of C6H5O7Na3, purity >99% in 50mLof H2O) was prepared. Then 62.5 mL solution of AgNO3 was Heated until it begins to boil at 97 C. At a moment of boiling add 2.5 mL of 1% sodium citrate solution drop by drop, as soon as boiling commences. We Continue heating for 9 min until color change is evident (pale yellow) Fig. 1a, then the solution was removed from the heating source and the absorption spectrum is taken after the solution is cooled [7]. Dysprosium chloride hexahydrate (DyCl3.6H2O, 99.9% from Aldrich) was used as the source of Dy3+ ions. A different concentration of Dy3+ solutions was prepared by dissolved DyCl3.6H2O in deionized water to obtain 1.0 × 10−1 mol\L; then this concentration was reduced to (2.0 × 10−2, 35 × 10−3, 55 × 10−3, and 125 × 10−4) mol\L by dilution equation (see Fig. 1b).
2.2 2-2 bulk samples
The Ag-Dy+3 co-doped silica xerogels were prepared under optimum conditions: pH = 1.5, molar ratio (R) of TEOS:Ethanol:H2O = 1:5:10 at room temperature. TEOS (purity >98%) and absolute Ethanol (C2H5OH) were mixed. For Ag-Dy3+ co-doped, a DyCl3 solutions, with different Dy3+ ions concentrations and Ag NPs solution, were added to the mixture of TEOS and absolute EthOH before the hydrolysis and denoted as sol(Ι), then left for 10 min on a magnetic stirrer to be homogenized. The mixture of deionized water and absolute EthOH denoted as sol(Π) was slowly added to the sol(Ι) for the hydrolysis. The final solution was left for 30 min on a magnetic stirrer at room temperature, and for 2 h under magnetic stirrer at 60 °C. After this step, 0.5 mL of N,N-dimethylformamide was added. The resultant solution was poured in a closed glass tube and kept under room temperature (Fig. 1d).
2.3 Characterization of the synthesized samples
X-ray diffraction (XRD) analyses were utilized for examination of the obtained materials structure by means of a Philips X-ray diffractometer, model-6000, supplied by Shimadzu with Cu Kα radiation(λ = 1.5406 Å) powered at 40 kV and 30 mA in the 2θ range from 5° to 80°. Ultraviolet-visible-near-infrared (UV–Vis-NIR) optical absorption spectra of the synthesized materials were recorded by a Centra-5 UV–VIS Spectrometer, which used a double beam (tungsten lamp and deuterium lamp) as a pumping source. Field Emission-Scanning electron microscope (FE-SEM) produces images of a sample were obtained by means of a—(Inspect F 50 FE- SEM)was performed on the prepared samples at the optimum conditions were studied in order to confirm the formation of nanoparticles. A fluorescence spectrophotometer SHIMADZU RF-5301PC Spectrofluorophotometer, Japan, in the EM wavelength range (200–900) nm, with a Xenon CW lamp as an excitation source, was employed for the recording of photoluminescence (PL) excitation and emission spectra of the synthesized samples.
3 Results and discussions
3.1 Results of chemical reduction method
In order to investigate the phase structure of synthesized Ag nanoparticles (NPs), the XRD pattern in Fig. 2 shows two diffraction peaks at 29° and 32° corresponding to the crystalline structure of silver having [200] and [111] plane, respectively [8, 9].
The surface plasmon resonance was observed and determined by UV–Visible spectrometry. Figure 3 shows the absorption spectrum of Ag NPs prepared by chemical reduction method at 5 min reduction period after boiling point and concentration of 5.0 × 10−3 M. The peak of the surface plasmon resonance absorbance band was observed at 423 nm.
The particle size of the Ag NPs was estimated by the FE-SEM. Firstly, a glass microscope slide was coated (coat-drop method) by silver nanoparticles colloidal. From the first look at the FE-SEM image, a uniform distribution of silver nanoparticles observed in the form of nanosphere with particle sizes ranging from 26.43 to 54 nm as shown in Fig. 4.
3.2 Result of dissolving process
Eight absorption bands of Dy3+ ions were observed at the room-temperature absorption spectra of the prepared DyCl3 solutions at different concentrations of Dy3+ ions and located at 317, 352, 365, 374, 446, 750, 810 and 911 nm as shown in Fig. 5. The absorption band at 317 nm can be assigned to the 6H15/ 2 → 4M17/2 and 6P3/2 Dy3+ ion transitions. The bands at 352, 365 and 446 nm, are related to the 6H15/2 → 4I13/2, 4F7/2, 6H15/2 → 4M15/2, 6P7/2 and 6H15/2 → 4I11/2 transitions of Dy3+ ions, respectively. The bands at 750, 810 and 911 nm are ascribed to the 6H15/2 → 6F3/2, 6H15/2 → 6F5/2 and 6H15/2 → 6F7/2 transitions of Dy3+ active ions, respectively [2, 3, 10,11,12]. It is clear that the absorbance increased as Dy3+ concentration increases, and this is due to the increasing contribution of these ions in the absorption process according to Beer-Lambert law.
The photoluminescence spectra of Dy3+ excited with source 350 nm at room temperature are shown in Fig. 6. These spectra are composed of two peaks, 577 and 481 nm, which are attributed to the transitions 4F9/2 → 6H13/2 and 4F9/2 → 6H15/2 [11, 13]. As the Dy3+concentration increased, there was a significant change in the position or shape of the peaks.
3.3 Result of sol–gel technique
The absorption spectra of Ag-Dy3+ co-doped in silica xerogels exhibits band detected at 213 nm is attributed to the formation of defects in the silica matrix, with absorption bands ranging from 210 to 250 nm [14]. The SPR abortion band of Ag NPs is centered at 429 nm [15, 16]. These five absorption bands of Dy3+ ions are located at 354, 575, 686, 810 and 911 nm and observed. The bands at 354 and 575 are related to the 6H15/2 → 4I13/2, 4F7/2 and 6H15/2 → 6F3/2. The bands at 686, 873 and 979 nm are ascribed to the 6H11/2 → 4F9/2, 6H15/2 → 6F7/2 and 6H15/2 → 6F11/2 transitions of Dy3+ ions. The peak of each band is increased as a result of a corresponding increase of concentration. This is due to the fact that the probability of absorption process is increased as the contribution of Ag-Dy3+ in the silica host increases according to Beer-Lambert law (see Fig. 7).
Simultaneous blue, yellow and green emissions are detected at 477, 576 and 518 nm, respectively, by the photoluminescence (PL) spectra of Ag-Dy3+ co-doped silica xerogels excited by 350 nm, as shown in Fig. 8. The blue emission detected at 475 nm originates from the magnetic dipole transition with parity of 4F9/2 → 6H15/2, whereas the yellow emission seen at 575 nm is caused by the 4F9/2 → 6H13/2 hypersensitive electric dipole transition. Photoluminescence intensity decreases at high concentration of Dy3+ due to the effect of aggregation of these ions in the structural pores of the silica matrix. Therefore, the surface plasmon resonance band of Ag NPs will improve the emission of Dy3+ throughout the energy transfer and hence the possibility of obtaining emission of white light will increase too. In other words, the interpretation of the obtained emission white light can be attributed to the combined influences of the quantum size effect of very small Ag NPs and their SPR in conjugation with active centers of Dy3+ [17,18,19].
3.4 Evaluation of white light emission
The white light emissivity of the prepared samples was evaluated using CIE chromaticity program and based on the data of photoluminescence (PL) spectra. It appears to be a curved triangle that contains all of the visible colors. The chromaticity diagram is obtained by choosing PL spectra from the worksheet or the active graph. Multiple PL spectra can be chosen and each PL spectrum corresponds to a point in the chromaticity diagram. Figure 9 shows the best performance of chromaticity for white light observed at excitation wavelength (λex) of 350 nm with the coordinates at about (0.333, 0.333) for all concentrations of Dy3+ ions.
From the chromaticity diagram, it can be noticed that both the x- and y-axis are ranging from 0 to approximately 0.8. Wavelength range of 380–700 nm trace around the curved edge. White surrounds the central point, called B, shows the typical screen range. The screen triangle’s corners where the full intensity of red, green and blue is likely to occur. A common claim for a good screen is that red should be around (0.735, 0.265), green should be around (0.274, 0.717), and blue should be around (0.167, 0.009).
4 Conclusions
The sol–gel method was successfully used to prepare a transparent medium hosts from Ag-Dy3+ co-doped silica xerogels. The effect of SPR band of Ag NPs will improve emission of Dy3+ ion to obtain white light. When exposed to UV light at 350 nm, an appropriate mixture of green, yellow and blue emissions produced from Ag-Dy3+ co-doped silica xerogel that emits white light. Based on CIE calculated chromaticity coordinates, it is a promising candidate for white LED applications.
Data availability
Authors declare that all data and materials included in this paper are available on demand
References
Deng Y, Song W, Dong W, Dai R, Wang Z, Zhang Z, Ding Z (2014) “White light emission of Eu3+/Ag co-doped Y2Si2O7”. J Rare Earths 32:779–786
Zhang Z, Han C, Shi W, Kang Y, Wang Y, Zhang W, Wang D (2015) “Enhanced novel white emission in Ca3(PO4)2:Dy3+ single-phase full-color phosphor by charge compensation”. J Mater Sci Mater Electron 26:1923–1931
Jena P, Gupta SK, Natarajan V, Sahu M, Satyanarayana N, Venkateswarlu M (2015) “Structural characterization and photoluminescence properties of sol-gel derived nanocrystalline BaMoO4:Dy3+“. J Lumin 158:203–210
Jena P, Gupta SK, Natarajan V, Padmaraj O, Satyanarayana N, Venkateswarlu M (2015) “On the photo-luminescence properties of sol-gel derived undoped and Dy3+ ion doped nanocrystalline Scheelite type AMoO4 (A = Ca, Sr and Ba)”. Mater Res Bull 64:223–232
Li JJ, Wei RF, Liu XY, Guo H (2012) “Enhanced luminescence via energy transfer from Ag+ to RE ions (Dy3+, Sm3+, Tb3+) in glasses”. Opt Express 20:10122–10127
Fatemah F (2021) Al-Harbiand Jaber Mohamed El Ghoul 2,3, “ Sol–Gel Synthesis of Dy Co-Doped Ag:V Nanoparticles for Optoelectronic Applications”. Condens Matter 6:24–35
Al-Mashhadni TA, Al-Maliki FJ (2022) Optimized Characteristics of Silver Nanoparticles Synthesized by Chemical Reduction and Embedded in Silica Xerogels”. Iraqi J Appl Phys 18(3):25–30
Eka Putra AE, Sulfiana E, Amaliyah N, Hayat A, Arsyad H (2019) “Hazardous Content Removal and Silver Nanoparticle Recovery from Liquid Radiography Waste Using Microwave Plasma”. IIETA 29(6):369–373
Han JW, Gurunathan S, Jeong JK, Choi YJ, Kwon DN, Park JK, Kim JH (2014) “Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line”. Nanoscale Res Lett 9(1):459
Lim TY, Wagiran H, Hussin R, Hashim S, Saeed MA (2014) “Physical and optical properties of dysprosium ion doped strontium borate glasses”. Phys B 451:63–67
Kang J-G, Gwag JS, Sohn Y (2015) Synthesis and characterization of Dy(OH)3 and Dy2O3 nanorods and nanosheets. Ceram Int 41:3999–4006.
Lakshminarayana G, Yang R, Mao M, Qiu J (2009) “Spectral analysis of RE3+ (RE = Sm, Dy, and Tm): P2O5–Al2O3–Na2O- glasses”. Opt Mater 31:1506–1512
Kumar JS, Pavani K, Babu AM, Giri NK, Rai SB, Moorthy LR (2010) “Fluorescence characteristics of Dy3+ ions in calcium fluoroborate glasses”. J Lumin 130:1909–1916
Danchova N, Gutzov S (2013) Time evolution of samarium doped silica sol-gel materials followed by optical spectroscopy. J Sol-Gel Sci Technol 66:248–252
Wu S, Schell AW, Lublow M, Kaiser J, Aichele T, Schietinger S, Polzer F, Kühn S, Guo X, Benson O, Ballauff M, Lu Y (2013) Silica-coated Au/Ag nanorods with tunable surface plasmon bands for nanoplasmonics with single particles. Colloid Polym Sci 291:585–594
Zhou Z, Liu G, Wei Q, Yang H, Liu Q (2016) Luminescence properties of Ag nanoclusters doped SiO2-PbF2 oxyfluoride glasses. J Lumin 169:695–700
Sahu IP, Bisen DP, Brahme N, Tamrakar RK, Shrivastava R (2015) “Luminescence studies of dysprosium doped strontium aluminate white light emitting phosphor by combustion route”. J Mater Sci Mater Electron 26:8824–8839
Sk. Mahamuda, Swapna K, Packiyaraj P, Rao AS, Prakash GV (2014) Lasing potentialities and white light generation capabilities of Dy3+ doped oxy-fluoroborate glasses”. J Lumin 153:382–392
Marzouk MA, Abo-Naf SM (2019) ” Structure characterization and photoluminescence of sol-gel synthesized AgDy-codoped silica phosphor“. J Non-Crystalline Solids 505:292–300
Acknowledgements
All entities with a contribution to this work were acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Mashhadani, T.A., Kadhim, F.J. Photoluminescence properties of sliver-dysprosium co-doped silica obtained by sol–gel method. J Sol-Gel Sci Technol 106, 553–560 (2023). https://doi.org/10.1007/s10971-023-06098-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06098-7