Abstract
The low cost, accessibility, and ease of implementation of SiO2 hydrophilic coatings should encourage further promotion as practical surface treatments. The calcination process, however, poses an obstacle to convenience. Herein, a calcination-free, antifogging SiO2 superhydrophilic coating was prepared at room temperature by sol–gel method with the guidance of particle gradation theory, which was used to regulate the size and concentration of colloid nanoparticles. The surface micromorphology, roughness, and water contact angle (WCA) of coatings were characterized and measured using scanning electron microscope (SEM), atomic force microscope (AFM), and contact angle measuring equipment. The antifogging capability of superhydrophilic coating was also examined. It has been found that hydrophilicity of coatings can be significantly improved by reasonable particle gradation design. The closer the particle packing pattern is to the hexagonal close-packing model, the better the hydrophilicity of coatings. When the concentration ratio of particle diameter 60.29, 9.26, and 3.68 nm is 15:4:1, the coating exhibits exceptional hydrophilicity (WCA, 2.3°) and outstanding anti-fogging performance. An implication of this study is that a versatile and easily manipulated strategy is presented here for designing surface microstructures that are sensitive to roughness.
Graphical Abstract

Highlights
-
The calcination-free SiO2 superhydrophilic coating was successfully prepared by the sol-gel method.
-
Reasonable matching of colloidal silica particle size can effectively improve the coating hydrophilic performance.
-
The silica coating exhibits exceptional hydrophilicity (WCA, 2.3°) and outstanding anti-fogging performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of the central aspects of materials surface science and engineering is the wetting behavior of solid surfaces, which has been of increasing interest. Surface engineering has improved solid surface performance and extended its functionalities, especially in transparent glass for its wide range of applications [1,2,3]. Superhydrophilic coatings endowing the substrates with antifogging, self-cleaning, and antireflective properties have broad potential for application in optics [4, 5], biomedicine [6,7,8] and other fields [9, 10]. While hydrophilicity is undoubtedly the most important criterion for coatings applied to glass substrates, ease of preparation, workability and low-cost are also important factors that should not be overlooked. In response to these demands, there are lots of ongoing efforts to find more optimum materials or advanced methods to improve hydrophilicity.
A TiO2-coated glass showed surprisingly light-induced superhydrophilicity under UV irradiation in 1997 [11], opening up the field for further research. Such coatings, however, are very limited in their practical application due to the indispensable of UV light irradiation. In order to achieve superhydrophilicity under lightless conditions, the researchers introduced SiO2 [2, 12,13,14,15] or ZnO [16, 17] into titanium dioxide film. On the other hand, a major obstacle to the widespread of actual application of TiO2 films is the control of costs when applied in large quantities. In contrast, silica nanoparticles have aroused the interest of researchers due to their affordability, easy availability of raw materials and variety of shapes. Silica nanoparticles have since been used in a number of applications for preparation of superhydrophilic coatings [18,19,20,21,22]. He et al. [21, 23] employed colloidal silica nanoparticles with special morphological structures, such as raspberry-like, mulberry-like, to prepare superhydrophilic coatings on the glass slide surface. The results showed that nanoparticles with unique structures facilitate hydrophilicity, yielding a coating with a maximum transmittance of 97% and excellent antifogging properties. Typically, nanoparticles are deposited on a surface by photocatalytic lithography method [24], chemical vapor deposition [25], electrochemical polymerization [26, 27], or sol–gel [19, 21, 28]. Whereas other methods involve complicated process, stringent conditions, or costly instruments, the sol–gel method should be more widely adopted due to its simplicity, versatility, and low-cost. However, there is still an easily negligible but inevitable procedure, calcination treatment, which poses an impediment to convenience, when preparing SiO2 superhydrophilic coatings using the sol–gel method. In addition, electrostatic layer-by-layer (LBL) assembly, a relatively facile strategy, has also been applied to fabricate silica superhydrophilic surfaces. For example, Cebeci [29] assembled polycation and SiO2 nanoparticles to produce a superhydrophilic coating using the LBL method. An LBL-assembled nanofilm containing branched polyethyleneimine and silsesquioxane-modified nanoparticles was reported by Lin [30]. In their study, they found a static water contact angle less than 1° was obtained by 20 bilayers with a homogeneous film thickness, and surface roughness increased with layer thickness.
Prior studies have noted the importance of chemical composition and surface micromorphology and architecture to a hydrophilic coating. Correspondingly, related researches mainly focused on the modification of functional groups on coating surfaces and the construction of complex micromorphological surfaces, i.e., the hierarchical structures with higher surface roughness were constructed via different chemical and physical methods [31, 32]. Most recent studies on silica superhydrophilic coating have focused on chemical modification. There is less literature on surface structure design. In addition, silicon alkoxides, such as tetraethyl orthosilicate (TEOS), can be hydrolyzed and condensed in ethanol solvents with the help of water and base catalysts [33]. It is possible to attain fine control of particle size, a constrained size distribution, a smooth spherical morphology of resulting silica particles. Therefore, the sol–gel process combined with the Stöber method is well suited for the design of surface structures for superhydrophilic silica coatings.
Based on the guidance of particle gradation theory, a calcination-free, antifogging, and superhydrophilicity silica coating was fabricated on glass slide surface via sol–gel method in this study. The diameter of colloidal particles for preparing SiO2 coating was designed by the particle gradation theory. The influence of particle concentration ratio on surface properties, including morphology, wettability, roughness, and antifogging performance, have been discussed. There is considerable potential for eyewear and windshields to benefit from this simple, convenient, and low-cost superhydrophilic silica coating.
2 Experimental section
2.1 Materials
Standard microscope glass slides (Jiangsu huida medical instruments co., Ltd. China) of 75 mm × 25 mm × 1 mm were employed as the substrates. Tetraethyl orthosilicate (TEOS, 99+%) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Aqueous ammonia (NH3·H2O, 25%, AR), absolute ethanol (C2H5OH, 99.5%, AR), acetic acid (C2H5OH, 99.8%), hydrogen peroxide (H2O2, 30%, AR), were obtained from Sigma-Aldrich. Deionized water (>18 MΩ cm) was applied in rinsing procedures and later all experiments. All these reagents mentioned above were used without further purification.
2.2 Glass slides pretreating
Prior to the deposition procedure, commercially available glass substrates were successively cleaned by sonication in Piranha solution (98 wt% H2SO4: 30 wt% H2O2) and deionized water and for 10 min, respectively. The glass substrates were washed with deionized water and dried under N2 flow condition.
2.3 Preparation of colloidal particle solution
A simple sol–gel method known as the Stöber method was employed to prepare monodisperse colloidal silica nanospheres with varied sizes. A typical preparation procedure of SiO2 precursor solution is as follows. The SiO2 solution was prepared from deionized water, absolute ethanol and TEOS in a mole ratio of 1:1:0.31. An appropriate amount of ammonia or acetic acid was added to regulate the pH value of solutions. The resultant solution was kept in 45 °C for 4 h under constant magnetic stirring to obtain a hydrolysis solution of TEOS. After that, the solution was aged in shade for 3 days in order to form colloidal solution containing desired size nanoparticles. It is worth mentioning that the different size of colloidal particles was achieved mainly by modulating the pH value of solution.
2.4 Fabrication of superhydrophilic coating on glass slides
Superhydrophilic silica coatings were fabricated by the sol–gel dip-pulling method, and the fabrication process of coating was shown in Fig. 1. The specific experimental procedure is as follows. 10 mL of large diameter silica sol was placed in a beaker and diluted to the required concentration by adding appropriate amount of absolute ethanol. The concentration of dilute solution depends on the result of particle gradation theory (shown in Section 2.6). After then, the solution was stirred for 5 min at room temperature and sonicated for 15 min, respectively, to obtain a homogeneous distribution state. Subsequently, the pretreated glass slide was immersed in the diluted solution for 1 min. It was followed by pulling up slowly at a speed of 2 mm/s and drying under ambient condition for 2 h. The medium and small colloidal particles also sequentially cycled above process with varied concentrations. Up to this point, the superhydrophilic silica coating with excellent antifogging property formed.
2.5 Characterization and test
The diameter distribution of SiO2 nanoparticles were tested by zeta potential analyzer on a Malvern Zetasizer Nano ZSP equipment over different reaction conditions. The surface morphological properties of deposited silica coating were evaluated by the scanning electron microscopy (SEM) images obtained from a Merlin Compact CARL ZEISS equipment operated at 10 kV. In order to determine 3D topographical information and root-mean-square roughness (RMS) values of coating, atomic force microscopy (AFM), Dimension FastScan (Bruker), was utilized. Water contact angles (WCAs) of coating surface were measured at ambient temperature with Korea Phoenix 300 contact-angle system. The deionized water droplet (3 μL) was used to drop onto as-prepared specimen surface. The images were taken and contact angles were measured from shape analysis of the sessile drop. Each contact angle value was averaged from five different positions on the coating surface. The antifogging properties of a tri-grading nanoparticles structural SiO2 coating were examined by cooling a half-coated standard glass slide at −22 °C for 3 h in a refrigerator, and then exposing it to humid laboratory air (relative humidity about 50%).
2.6 Design of tri-grading particles coating
It is well-known that the wetting behavior of a substrate surface is determined by both its physicochemical properties and roughness. For a given glass substrate condition, the hydrophilic/hydrophobic state of substrate can be improved by adjusting their surface functional groups or roughness. During the course of fabricating superhydrophilic coating via the sol–gel method, the size, shape, and stacking state of colloidal particles usually play important roles in hydrophilic performance of the coating. Hence, the hydrophilic performance of coatings can be further improved, if the particle size can be reasonably designed and the ratio of varied particles can be regulated.
As a result of the above considerations, we first prepared a coating consisting of monodisperse particles in order to observe the packing state between colloidal particles, as shown in Fig. 2(a). Based on the SEM image of silica nanoparticles film, it can be seen that the film is complete, without cracks or exposed glass substrate, and the particles are uniform in size and placement without agglomeration. Further observation of the morphology of particles showed that they were all spherical in shape and slightly flattened. In addition to SiO2 particle size and stacking state affecting the surface roughness, size-matching was crucial to determining the superhydrophilicity of coatings, especially at micro/nanometer scales. Therefore, the silica particles used were assumed to be ideal spheres and the superhydrophilic coating was prepared via tri-grading particles stacking to promote space utilization. When tri-grading particles are stacked, three different diameter particles are sequentially deposited, one after the other, from the largest to the smallest. It should be noted that it is possible to control the stacking type of silica particle by adjusting the concentration ratio of colloidal solution. The roughness factor is generally understood to mean the ratio of the actual surface area to the geometric projected surface area, which is positively correlated with surface roughness. As shown in Fig. 2(b–e), two models were illustrated to approximate the perfect particle stacking condition: primitive cubic packed model (PCP) and hexagonal close-packing model (HCP). According to particle stacking theory, the PCP model has a void fraction of 47.64% and a roughness factor of 1.92, while the HCP model has a void ratio of 25.59% and a roughness factor of 1.95. With a roughness factor of 1.81 for monodisperse particle coating, the roughness factor for coatings prepared by tri-grading particle strategy is significantly higher, indicating that a theoretically rational particle design is indeed effective in boosting the hydrophilicity of coatings.
The diameter relationship between particles in Fig. 2(b–e) can be further expressed. Taking the uppermost layer of particles as the reference plane which has the greatest effect on the surface roughness. Under PCP condition, the connection between three particle sizes can be elaborated as d2 ≤ 0.414d1 and d3 ≤ 0.108d1, while HCP is d2 ≤ 0.156d1 and d3 ≤ 0.062d1, where d1, d2, d3 is the diameter of biggest, medium and smallest particles respectively. The relationship of particle sizes was the key to obtain a coating with excellent hydrophilic performance. Three monodisperse nanoparticles with mean sizes of 60.29, 9.26, and 3.68 nm were used to prepare the coatings (as shown in Fig. 3). These particles were selected mainly because their diameters satisfy requirements of the two above relationships, but are also closer to the HCP model. According to the particle gradation theory, the characteristic structure of packing model can be explained as follow equations:
where φ1, φ2, φ3, and ε1, ε2, ε3 are the volume fraction and the void ratio of the biggest, medium and the smallest particles, respectively. However, particle stacking was often somewhere between the PCP model and the HCP model under actual conditions. Hence, the void ratio of tri-grading particle coatings was in the range of 47.64% to 25.95%. The upper and lower limits correspond to the minimum porosity of PCP model and the maximum porosity of HCP model. In addition, two other void ratio conditions of 40% and 30% were set between upper and lower limits. For convenience of calculation, equal values of ε1, ε2, and ε3 are usually assumed. For each of the four conditions of void ratio, the three particle volume fraction ratios were obtained using Eq. (1). As a result of their positive relationship, volume fraction ratios were used as solutions concentration ratios. In order of 47.64%, 40%, 30%, and 25.95%, the coating samples obtained for the corresponding concentrations were named S1–S4, as well as a blank glass substrate named S0. The detailed recipes for preparation of samples were given in Table 1.
3 Results and discussion
3.1 Characterization of silica coating
The size distribution of colloidal particles used for coating preparation is critical for hydrophilicity. Three colloidal SiO2 nanoparticle size distribution curves were measured with a Zeta potential analyzer, shown in Fig. 3. One can see that a distinct narrow single peak is visible in all three particle size distribution curves, indicating well-defined particle size distributions without agglomeration. The average size of three particles was 3.68 nm (Particle 1), 9.26 nm (Particle 2), and 60.29 nm (Particle 3).
Pretreated glass slides were dip-coated with silica colloidal solution at predetermined concentration ratios to obtain samples S1–S4. Surface morphology of the coating samples were characterized by SEM and AFM, as shown in Figs. 4, 5, and 6. Figure 4 displays that the surface of all four glass slides are filled with silica nanoparticles, with no obvious unfilled areas or creaks, indicating a crack-free, densely silica hydrophilic coating can be obtained by drying at room temperature. Between the four coating samples, there was no significant difference in surface morphology. In contrast to the image of monodisperse coating (Fig. 2(a)), however, one of the most prominent difference is the changes in microscopic morphology of coatings. The number of irregularly shaped nanoparticles in samples S1–S4 increases significantly, and the surface of particles is rougher. As can be seen in high magnification morphology Fig. 5, the voids between larger particles are clearly filled with smaller particles (as shown by the red arrow in the Fig. 5), and the coating microstructure changes significantly with the change of stacking form. The porosity gradually decreases and the bigger particles are packed by smaller particles. Based on color shades in Fig. 6, no significant differences are evident between the four samples, indicating similar surface roughness values. What stands out in Fig. 6, however, is the variation in morphological appearance of silica particles, which is consistent with the SEM image observations. Adsorption of smaller particles on the surface of large is a major influence on these changes.
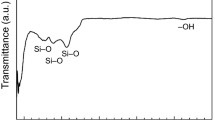
It is now well established that functional groups can impair or strength wettability of surfaces. An infrared spectroscopy (FT-IR) analysis was performed to characterize the composition of coatings drying at ambient conditions, as shown in Fig. 7. The prominent peaks located at 805 cm−1 and 1075 cm−1 are caused by the symmetric and antisymmetric stretching vibrations of Si-O-Si bond, respectively, stemming from the process of sol–gel. In the spectrum, the extensive stretching and bending vibration absorption peak of hydroxyl group are individually found at around 3450 cm−1 and 1633 cm−1, which provides hydrophilic properties to the surface. Nonetheless, the peaks located at 2981 cm−1, 2906 cm−1, and 1385 cm−1 corresponds to the antisymmetric stretching vibration of C–H bonds and the antisymmetric flexural vibration of -CH3 bonds demonstrating the presence of -OC2H5 groups, and they are hydrophobic. According to the relative intensity of the absorption peak, some alkoxy groups have not yet been hydrolyzed completely. The results reveal that the -OH groups are widely distributed on the coating surface, but the alkoxy groups are still present, reducing hydrophilicity.
3.2 Hydrophilicity
A solid surface’s wetting behavior by liquids plays an essential role in a solid–liquid–gas system and is one of its most fundamental properties. Water contact angel is widely recognized and applied to quantitatively describe the interactions between a liquid and a solid surface. Surface wetting state of a solid may be classified on the basis of WCA into hydrophilic (<90°) and hydrophobic (>90°). A superhydrophilic surface, defined as one that allows for complete spreading of water droplets on a solid surface, usually has a WCA less than 5°. Hydrogen bonds with water are formed rapidly on the surface of the coating due to the abundance of hydroxyl groups, enabling the water to spread swiftly.
Figure 8 illustrates the results of a comparative test of WAC of samples S0–S1 depending on the stacking model of silica particles. As seen in Fig. 8(a), the WCA of S0 (blank glass slide) is 57.0° indicating the nature of hydrophilic of substrate, which is determined by the material itself. According to Fig. 8(b–e), the WCA of samples S1–S4 is considerably reduced after a silica coating is applied to the slide surface. It might refer to the fact that an effective hydrophilic coating has been produced on the slide surface by dip-coating method. The WCA of samples S1–S4 are 8.8°, 7.4°, 6.5°, and 2.3°. From Fig. 8(b–e), we can clearly see that the WCA values are steadily declining from sample S1 to sample S4, and the sample S4 has reached superhydrophilicity. It can therefore be concluded that the WCA gradually decreases as the stacking style of colloidal nanoparticles transforms from PCP to HCP. Additionally, AFM is used to characterize the three-dimensional morphology of coating surfaces in order to study how microstructure affects hydrophilicity. Figure 9 illustrates a more detailed picture of small particles filling voids between larger particles, which is in accordance with previous observations. The roughness values of samples S1–S4 were obtained in the range of 500 × 500 nm. The variation rules of WAC and RMSsr depending on preparing conditions are summarized in Table 2. The value of RMSsr has gradually increased from S1 to S4, further supporting the above theoretical calculation for coating design. The relationship between coating hydrophilicity and their surface roughness can be revealed by Wenzel model. It usually can be expressed as cosθw = r cosθY, where θw is the apparent contact angle of water on a rough surface; θY is the intrinsic contact angle as measured on a smooth surface and r is the roughness factor. It should be noted that as a rule r ≥ 1 and θY is fixed for determined substrates. The Wenzel equation indicates that given a hydrophilic surface, the rougher the surface, the smaller the value of θw, and the more hydrophilic the coating.
3.3 Antifogging test
Condensation of small water droplets will form a fog on the slide surface, scattering incident light and reducing transparency and visibility, shown in the top half of Fig. 10. As a result of fogging, the “antifogging” under glass slides are blur and indistinguishable. The superhydrophilic coating, an effective antifogging measure, makes positive contact with small droplets and quickly forms a transparent water film after the droplets spread. The generated film ensures scattering losses of incident light are so limited that the “antifogging” remains clearly visible, just as shown in the bottom half of Fig. 10(b–d). Further comparison of the antifogging performance of the four samples reveals that they all improved to varying degrees, especially in S3 and S4 where there is hardly any evidence of fog formation. This confirms the results above that they have a rougher surface and a lower WAC. The surface of a coating that reaches or is very close to the superhydrophilic level effectively prevents the formation of fog and guarantees the transparency of glass. Further, the progressively clearer “antifogging” from S1 to S4 also demonstrates that coating hydrophilicity increases as the silica colloidal nanoparticle stacking model changes from PCP to HCP.
On the basis of above results, it could be found that a proper design of silica colloidal nanoparticle stacking model does indeed markedly upgrade coating hydrophilicity. The rougher coating surface might be the main factor in the enhanced hydrophilic performance. Four samples, however, show relatively low surface roughness due to the small size (<100 nm) of the particles. Coating surface roughness may increase further if larger diameter colloidal nanoparticles are used. A complicated surface architecture is more likely to be beneficial in improving coating hydrophilicity. According to the tri-grading nanoparticle coating characterization, however, there is no longer a need to increase the number of grades, since smaller particles have so little impact on structure or composition. Correspondingly, the design concept can also be applied to organic hydrophilic coatings. A versatile and easily manipulated strategy is presented here for designing surface microstructures that are sensitive to roughness.
4 Conclusions
We report a fabrication strategy to achieve calcination-free, antifogging, superhydrophilic silica coatings by sol–gel method. The design of diameter and concentration of colloidal nanoparticles with guidance of the particle gradation theory is the most crucial step. As a result of this study, we identified that a rational design for colloidal nanoparticle packing enhances the surface roughness of coatings prepared by sol–gel, and the closer particle packing type is to the hexagonal close-packing model, the better hydrophilicity of SiO2 coatings. The superhydrophilic SiO2 coating shows a minimum WCA of 2.3° with excellent antifogging characteristic, when the concentration ratio of three particles is 15:4:1. This convenient, low-cost preparation method may make superhydrophilic SiO2 coating more promising for practical applications.
References
Otitoju TA, Ahmad AL, Ooi BS (2017) Superhydrophilic (superwetting) surfaces: a review on fabrication and application. J Ind Eng Chem 47:19–40. https://doi.org/10.1016/j.jiec.2016.12.016
Zheng S, Wang D, Tian Y, Jiang L (2016) Superhydrophilic coating induced temporary conductivity for low-cost coating and patterning of insulating surfaces. Adv Funct Mater 26(48):9018–9025. https://doi.org/10.1002/adfm.201602843
Yao L, He J (2014) Recent progress in antireflection and self-cleaning technology—from surface engineering to functional surfaces. Prog Mater Sci 61:94–143. https://doi.org/10.1016/j.pmatsci.2013.12.003
Lu X, Wang Z, Yang X, Xu X, Zhang L, Zhao N, Xu J (2011) Antifogging and antireflective silica film and its application on solar modules. Surf Coat Technol 206(6):1490–1494. https://doi.org/10.1016/j.surfcoat.2011.09.031
Kim K, Dhungel SK, Jung S, Mangalaraj D, Yi J (2008) Texturing of large area multi-crystalline silicon wafers through different chemical approaches for solar cell fabrication. Sol Energy Mater Sol Cells 92(8):960–968. https://doi.org/10.1016/j.solmat.2008.02.036
Lobo AO, Corat MA, Ramos SC, Matsushima JT, Granato AE, Pacheco-Soares C, Corat EJ (2010) Fast preparation of hydroxyapatite/superhydrophilic vertically aligned multiwalled carbon nanotube composites for bioactive application. Langmuir 26(23):18308–18314. https://doi.org/10.1021/la1034646
Weng X, Ji Y, Ma R, Zhao F, An Q, Gao C (2016) Superhydrophilic and antibacterial zwitterionic polyamide nanofiltration membranes for antibiotics separation. J Membr Sci 510:122–130. https://doi.org/10.1016/j.memsci.2016.02.070
Lu T, Xu X, Liu X, Sun T (2017) Super hydrophilic PVDF based composite membrane for efficient separation of tetracycline. Chem Eng J 308:151–159. https://doi.org/10.1016/j.cej.2016.09.009
Liang S, Kang Y, Tiraferri A, Giannelis EP, Huang X, Elimelech M (2013) Highly hydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes via postfabrication grafting of surface-tailored silica nanoparticles. ACS Appl Mater Inter 5(14):6694–6703. https://doi.org/10.1021/am401462e
Hancock MJ, Piraino F, Camci-Unal G, Rasponi M, Khademhosseini A (2011) Anisotropic material synthesis by capillary flow in a fluid stripe. Biomaterials 32(27):6493–6504. https://doi.org/10.1016/j.biomaterials.2011.05.057
Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T (1997) Light-induced amphiphilic surfaces. Nature 388(6641):431–432. https://doi.org/10.1038/41233
Wang J, Wang D, Wang J, Zhao W, Wang C (2011) High transmittance and superhydrophilicity of porous TiO2/SiO2 bi-layer films without UV irradiation. Surf Coat Technol 205(12):3596–3599. https://doi.org/10.1016/j.surfcoat.2010.12.033
Tricoli A, Righettoni M, Pratsinis SE (2009) Anti-fogging nanofibrous SiO2 and nanostructured SiO2-TiO2 films made by rapid flame deposition and in situ annealing. Langmuir 25(21):12578–12584. https://doi.org/10.1021/la901759p
Wang X, Ding H, Lv G, Zhou R, Ma R, Hou X, Zhang J, Li W (2022) Fabrication of superhydrophilic self-cleaning SiO2–TiO2 coating and its photocatalytic performance. Ceram Int 48(14):20033–20040. https://doi.org/10.1016/j.ceramint.2022.03.278
Li X, He J (2013) Synthesis of raspberry-like SiO2-TiO2 nanoparticles toward antireflective and self-cleaning coatings. ACS Appl Mater Inter 5(11):5282–5290. https://doi.org/10.1021/am401124j
Chen Y, Zhang C, Huang W, Yang C, Huang T, Situ Y, Huang H (2014) Synthesis of porous ZnO/TiO2 thin films with superhydrophilicity and photocatalytic activity via a template-free sol–gel method. Surf Coat Technol 258:531–538. https://doi.org/10.1016/j.surfcoat.2014.08.042
Wu J, Xia J, Lei W, Wang B-p (2011) A one-step method to fabricate lotus leaves-like ZnO film. Mater Lett 65(3):477–479. https://doi.org/10.1016/j.matlet.2010.10.029
Chen H, Li X, Li D (2022) Superhydrophilic–superhydrophobic patterned surfaces: from simplified fabrication to emerging applications. Nanotechnol Precis Eng 5 (3). https://doi.org/10.1063/10.0013222
Ye L, Zhang Y, Song C, Li Y, Jiang B (2017) A simple sol–gel method to prepare superhydrophilic silica coatings. Mater Lett 188:316–318. https://doi.org/10.1016/j.matlet.2016.09.043
Du X, Li X, He J (2010) Facile fabrication of hierarchically structured silica coatings from hierarchically mesoporous silica nanoparticles and their excellent superhydrophilicity and superhydrophobicity. ACS Appl Mater Interfaces 2(8):2365–2372. https://doi.org/10.1021/am1003766
Liu X, He J (2007) Hierarchically structured superhydrophilic coatings fabricated by self-assembling raspberry-like silica nanospheres. J Colloid Interface Sci 314(1):341–345. https://doi.org/10.1016/j.jcis.2007.05.011
Liu X, He J (2009) Superhydrophilic and antireflective properties of silica nanoparticle coatings fabricated via layer-by-layer assembly and postcalcination. J Phys Chem C 113(1):148–152. https://doi.org/10.1021/jp808324c
Li X, He J (2012) In situ assembly of raspberry- and mulberry-like silica nanospheres toward antireflective and antifogging coatings. ACS Appl Mater Inter 4(4):2204–2211. https://doi.org/10.1021/am3002082
Lai Y, Lin C, Wang H, Huang J, Zhuang H, Sun L (2008) Superhydrophilic–superhydrophobic micropattern on TiO2 nanotube films by photocatalytic lithography. Electrochem Commun 10(3):387–391. https://doi.org/10.1016/j.elecom.2007.12.020
Liu H, Feng L, Zhai J, Jiang L, Zhu D (2004) Reversible wettability of a chemical vapor deposition prepared ZnO film between superhydrophobicity and superhydrophilicity. Langmuir 20(14):5659–5661. https://doi.org/10.1021/la036280o
Çağlar A, Cengiz U, Yıldırım M, Kaya İ (2015) Effect of deposition charges on the wettability performance of electrochromic polymers. Appl Surf Sci 331:262–270. https://doi.org/10.1016/j.apsusc.2015.01.103
Godeau G, Darmanin T, Guittard F (2016) Switchable surfaces from highly hydrophobic to highly hydrophilic using covalent imine bonds. J Appl Polym Sci 133 (11). https://doi.org/10.1002/app.43130
Topçu Kaya AS, Cengiz U (2019) Fabrication and application of superhydrophilic antifog surface by sol–gel method. Prog Org Coat 126:75–82. https://doi.org/10.1016/j.porgcoat.2018.10.021
Cebeci FÇ, Wu Z, Zhai L, Cohen RE, Rubner MF (2006) Nanoporosity-driven superhydrophilicity: a means to create multifunctional antifogging coatings. Langmuir 22(6):2856–2862. https://doi.org/10.1021/la053182p
Lin X, Hwangbo S, Jeong H, Cho Y-A, Ahn H-W, Hong J (2016) Organosilicate based superhydrophilic nanofilm with enhanced durability for dentistry application. J Ind Eng Chem 36:30–34. https://doi.org/10.1016/j.jiec.2016.02.017
Moazzam P, Tavassoli H, Razmjou A, Warkiani ME, Asadnia M (2018) Mist harvesting using bioinspired polydopamine coating and microfabrication technology. Desalination 429:111–118. https://doi.org/10.1016/j.desal.2017.12.023
Kim JH, Shim TS, Kim S-H (2016) Lithographic design of overhanging microdisk arrays toward omniphobic surfaces. Adv Mater 28(2):291–298. https://doi.org/10.1002/adma.201503643
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26(1):62–69. https://doi.org/10.1016/0021-9797(68)90272-5
Acknowledgements
This work was financially supported by the science foundation of the National Key Laboratory Foundation of Science and Technology on Advanced Composites in the Special Environments and Shenzhen Science and Technology Program (Grant No. KQTD2016112814303055).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Sun, Y., Zeng, G. et al. Facile fabrication and antifogging test of a calcination-free SiO2 superhydrophilic coating. J Sol-Gel Sci Technol 105, 662–672 (2023). https://doi.org/10.1007/s10971-023-06042-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06042-9