Abstract
The aim of this research is the study of self-cleaning and antibacterial activity of SiO2/TiO2 thinfilm contains copper in it on ordinary ceramic tiles substrates. Four solutions using the sol-gel method with various volume ratio of Si/Ti precursors (i.e., 1/9, 2/8, 3/7, and 4/6) were prepared. Then, they were deposited on the ceramic tiles via the dip-coating technique and heated through thermal treatment at 650° for an hour. The XRD results show anatase and rutile structures are the main phases in the composite films of Cu/SiO2/TiO2 coatings. The rms roughness obtained by AFM for ceramic tile was initially ~20 nm, which reduced to <2 nm after deposition. The SEM images, depicted a uniform and crack-free thin-film coating with a thickness <30 nm. Stearic acid coating was applied on the samples as a pollution and was completely removed after being subjected to 24 hrs of UV illumination (400–800 μW/cm2) in an environment with low and variable humidity (21–37 RH). About 76° reduction of water contact angle (WCA) was observed during photocatalytic test for the 1/9 (optimum) sample. In addition, a 33° decrease in WCA was measured in the light-induced hydrophilicity experiment. The 1/9 sample that reacted mostly under UV illumination in both photocatalytic and light-induced hydrophilicity was concluded as the optimum one. To improve the antibacterial property of the coating, copper nitrate trihydrate was added to the 1/9 solution. Moreover, antibacterial test was performed through “viable cell count” method by E. coli bacteria. The results of antibacterial test showed a 99% and 99.99% reduction in the number of E. coli bacteria during 1 and 24 hrs intervals, respectively. This is, surprisingly, to the best of our knowledge, the same as the results that can be obtained using silver nanoparticles.

Graphical abstract
Highlights
-
Self-cleaning take place before 24 hours under a very weak UV illumination.
-
Elimination of 99% and 99.99% of E. coli bacteria within 1 and 24 hours of using Cu instead of Ag.
-
Self-clean and antibacterial thin-film coating which is commercially affordable rather than using detergent materials.
-
No visible changes have been observed in the design or color of ceramic tiles.
-
Applicable to use in different environments with variable climate conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Today, smart and advanced functional coatings are being extensively used in the areas of marine, automobile manufacture, environmental tools, and building materials. The use of surfaces with special wettability, like self-cleaning and anti-bacterial surfaces, has become more common in both industrial and academic research fields. Since cleaning the different surfaces in a building requires a considerable amount of energy, time, detergents, and financial resources, one is led to find an alternative self-cleaning for eliminating the pollution on these surfaces [1]. Furthermore, there is a big competition among companies to produce highly efficient anti-bacterial surfaces like ceramic tiles. The use of these surfaces is essential, especially for use in schools, restaurants, industrial facilities, public places, playrooms, and particularly hospitals [2,3,4].
One way to induce the self-cleaning property is to use photocatalyst materials. The production of photocatalytically active building materials that are activated by UV radiation makes it possible to obtain a self-cleaning and self-sterilizing surface that can degrade some organic pollutants in the surrounding. Among the photocatalytic materials, we benefit the optical properties of TiO2 nanoparticles. TiO2 is one of the most interesting ones that has been widely used as a target for various microorganisms. It is chemically stable, non-toxic, low cost, and most importantly photo stable [5], high photoactive, broad-spectrum active, and antibacterial, which makes it an ideal photocatalyst. Titanium dioxide has a variety of crystal phases such as anatase, rutile, and brookite. Among these crystal phases, the anatase and rutile phases exhibit a high photocatalytic activity [6, 7].
Even though the role of pure titanium dioxide in textiles is now known to everyone, it is suggested to add silicon dioxide to the surface coating formulation in order to improve the efficiency of titanium dioxide photocatalytic property and surface hydrophilicity. Silica plays an important role; increasing the surface area close to titanium dioxide, and acidity of the surface [8]. One strategy to avoid the release of TiO2 from the surface to the environment is to fix the photocatalyst in a SiO2 matrix [9, 10]. It is found that after adding SiO2, the just produced water contact angle (WCA) is small, and it is also very hydrophilic in the dark. The SiO2/TiO2 composite film has been found not only to improve hydrophilicity but also photocatalytic activity [11]. These characteristics, i.e., bonding role, acidity, and enhance photocatalytic behavior makes the SiO2 preferable for our study [8, 12, 13].
It is well known that metal ions such as silver [8, 14, 15], copper [16,17,18,19,20], and zinc have antibacterial property, a phenomenon known as the oligodynamic effect. If these metals are combined with the TiO2 film on the glazing sanitary ware, it would be possible to develop sanitary ware which has photocatalysts that produce sterilization under ultraviolet light and the metal ions have antibacterial effects even in the dark [21, 22].
Silver nanoparticles either interact with the cell membrane or enter the cell, cause the cell to die. The analogous mechanism for copper nanoparticles is yet unknown, however, it is believed by some to be similar to that of silver [20]. Copper is one of the trace elements, and also vital for living microorganisms. It is a constituent of enzymes that are involved in processes such as electron transport and the redox cycle. The concentration of copper generally found in microorganisms is in the millimolar range; a higher concentration of free ion Cu2+ can be toxic for cells, although its toxicity is reduced when combined with organic compounds. Copper disrupts amino acid biosynthesis pathways by producing reactive oxygen species (such as H2O2). H2O2 disrupts the amino acid biosynthetic pathways by damaging the iron-sulfur enzymes, which happen to be the primary intracellular targets of copper’s toxicity, and the DNA by generating free radicals [20]. In this work, we follow an approach to obtain a self-clean and antibacterial coating on ordinary ceramic tiles substrates. To the best of our knowledge, copper has not been shown 99.99% antibacterial property in the self-cleaning coating on ceramic tiles substrates. This value is reported for silver; therefore, the use of copper instead of silver was investigated in this work. Furthermore, removing the contaminations on the surface in weak UV illumination and low humidity conditions, are the other goals.
2 Experimental details
Ordinary ceramic tiles were chosen as the substrates and were cleaned using water, acetic acid, and ethanol, respectively. The TiO2/SiO2 composite films were made using the sol-gel method. 1 ml of Tetraethylorthosilicate (TEOS) in 6 ml ethanol is hydrolyzed for 1 h containing 0.01 ml HCl, as TEOS precursor solution. Then, 3 ml Titanium Butoxide was dissolved in a 17 ml ethanol solution. Next, after mixing TEOS with varied amounts of Titanium butoxide precursor solution, additional amounts of HCl catalyst were added (Titanium Butoxide/HCl = 1:0.5 in volume ratio). Therefore, four different solutions were obtained with various volume ratio, i.e., 1/9, 2/8, 3/7, and 4/6 [11]. Then, using the dip-coating method, the substrates were coated and heat treated at 650° in a cubic furnace for an hour.
To study morphology, composition, thickness, and crystal phase in the structures, methods such as AFM (NT-MDT AFM), SEM, cross-section SEM (SEM, Hitachi S4460), and XRD (XRD, Philips, X’Pert) were used. To determine hydrophilicity and photocatalytic activity, the WCA of the surfaces were measured. After employing different experiments, optimum solution (i.e., 1/9 sample) was chosen to add the antibacterial material.
To investigate the light-induced hydrophilicity and photocatalytic activity, the samples were placed under a UVA lamp (400–800 μW/cm2, 365 nm wavelength) in a low humid environment (~21–37 RH) for 24 hrs. The WCA was taken three times every 2 h and monitored after 24 hrs illumination. The wettability of a solid surface is measured by the contact angle (CA) between the edge of the distilled water droplet and the surface below it, that is denoted by θ [23, 24].
To study the photocatalytic activity, the obtained coatings were covered by stearic acid using the spin-coating technique, and were kept in the normal lab conditions for 24 hours, so that the stearic acid was fully dried. The CA of the water droplet was measured at two different stages in the sample: before applying the stearic acid and during the UV irradiation. The CA of samples before applying the contamination (stearic acid), and after covering with stearic acid are denoted by θ0 and θ, respectively. Then, the samples were subjected to UV beam and monitored up to 24 hrs.
Finally, the antibacterial activity of the 1/9 (Cu/SiO2/TiO2) sample was determined by a viable cell count experiment, performed on one pathogen, namely Escherichia coli, which represents a gram-negative bacteria. In fact, E. coli plays a role in the corruption process, but if found excessively in the human body, it can cause diarrhea and fever [2]. During the test 105 CFU/ml of E. coli bacteria was applied on the 1/9 (SiO2/TiO2), 1/9 (Cu/SiO2/TiO2), and also control sample (i.e., a bare ceramic tile). Afterward, bacteria were gathered from the samples and control surface after 1 and 24 hrs. To investigate the adherence of coating to the ceramic tiles’ substrate, a common tape test was performed to study the peeling of the coating.
3 Results and discussion
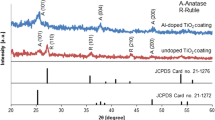
As mentioned in the introduction, one way to obtain self-cleaning surfaces is to benefit from the photocatalytic activity of TiO2. Therefore, an X-ray diffraction pattern was obtained to ascertain of existing the main crystal phases of TiO2 in the coatings. XRD analysis of 1/9 (SiO2/TiO2) sample is chosen as a representative, which is depicted in Fig. 1. It shows a big amorphous hump and two other sharp peaks. The amorphous hump indicates the SiO2 presence as the main material exists in the soil of the ceramic tile. The sharp peaks of TiO2 signify formation of well-developed crystals in the films that are corresponding to rutile and anatase phases, respectively. Therefore, as the presence of these crystal structures of TiO2 is verified, photocatalytic activity is expected [6, 7, 25].
The other main item of making a surface easy to clean, is to profit the hydrophilic surface. Therefore, changes of the surface roughness before and after coating on the ceramic tile substrates were monitored. AFM images of the control sample are depicted in both 2D and 3D formats, in Fig. 2. The rms roughness of control sample was found to be about 20 nm. AFM illustrations of four different coatings of SiO2/TiO2 in 1/9, 2/8, 3/7, and 4/6 ratios in 2D and 3D images are shown in Fig. 3. Their rms roughness were measured less than or equal to 2 nm; it indicates that the surface roughness was decreased after deposition (Table 1).
Due to the residue [26, 27] of ceramic tile some spherical particles has been formed during the ceramic production, that has shown in the SEM image of Fig. 4. The SEM image of 1/9 (SiO2/TiO2) sample as evident is shown in Fig. 5. The number of residue (impurities) decreased after coating the control sample with a self-cleaning layer, drastically. The dark color is the obtained thin-film coating and the presence of white points on the surface of 1/9 sample could be agglomerations of Ti particles [2]. According the obtained cross-section SEM image, thickness of the self-clean coating for all samples were measured smaller than 30 nm (Fig. 6).
In agreement with the self-cleaning of the surface, light-induced hydrophilicity experiment was performed. The WCA of the control sample was measured 39°, while immediately after applying the self-cleaning coating, it became smaller than 10°(a surface with CA < 90° is considered as hydrophilic, and has a strong adhesion to water droplet, which spreads and wets a large surface area, if the droplet lies almost flat and the CA is less than about 20 degrees, the surface is recognized as superhydrophilic. While the one with CA > 90° is termed hydrophobic that is highly water-repellent [23, 28,29,30]). Then, WCA was increased to near ~40–50° after 2 days of remaining in lab. conditions and without any sort of protection. WCA of 1/9 (SiO2/TiO2) sample reduced regularly from ~46° at the beginning of the experiment to ~13° after 24 hrs UV light irradiation. The obtained values for all other samples are reported in Table 2 and Fig. 7. Obviously, reduction of WCA corresponding to 1/9 (SiO2/TiO2) sample is the most one in comparison with the other samples. This reduction of angle to <15° is a sign of hydrophilicity or almost super-hydrophilicity of the surface. As the roughness of the surface dropped, the surface’s hydrophilicity increased, and the surface became self-cleaning by sheeting water droplet, reducing the CAs to very low values under irradiation, and then dirt will be washed away.
Hydrophilic coatings have a feature when exposed to light they are able to break down dirt, a process known as ‘photocatalysis,’ though it is the coating, not the incident light; that functions as a catalyst and cause the surface to be clean by eliminating the dirt. To study the photocatalytic activity of samples, CA of distilled water droplet before and after stearic acid coating were measured. According to Fig. 8, when TiO2 is activated by UV light can decompose the stearic acid in 5 h. According the diagram, at the end of experiment, the WCA of samples had a remarkable reduction compared to the beginning. This reduction indicates the elimination of the surface pollution (here, stearic acid) during the first 5 h of a weak UV illumination (Table 3). Moreover, after 5 h the decrease in the WCA is a sign of light-induced hydrophilicity. A negative value of the WCA means that the measured value at the end of the test (i.e., after the stearic acid elimination) is less than the initial one. It was predicted that after 5 h, the light-induced hydrophilicity should progress.
Four solutions with various volume ratio of Si/Ti, were prepared to study the effect of silicon on hydrophilicity and photocatalytic activity [11]. As mentioned previously, the reduction in the rms roughness was the same for the samples; they all showed a favorable performance in both photocatalytic activity and hydrophilicity tests. However, the best efficiency was acquired for the 1/9 (SiO2/TiO2) sample (Tables 2 and 3). Therefore, it was chosen for adding the antibacterial property to this solution and the other solutions were discarded.
Copper nitrate trihydrate was added to the solution, then, the previously mentioned analyses were repeated. As pointed out by some researches [31,32,33], the presence of copper oxide leads to super-hydrophobicity. However, one has to ensure that adding copper nitrate trihydrate does not change the functionality of the surface. So, after adding copper nitrate trihydrate to the 1/9 (SiO2/TiO2) solution, the existence of crystalline structures of anatase and rutile TiO2 was evaluated again. It confirms phase search match analyses of XRD, exactly as it was for 1/9 solution without copper. According the AFM images, the rms roughness has not been changed compared to the self-clean coatings that were already obtained; it was measured <2 nm (Table 1 and Fig. 9). Moreover, one observes a crack-free, uniform, and homogeneous surface in the SEM pictures (Fig. 10), which means that the presence of copper does not have a visible effect on the surface morphology.
Photocatalytic activity and light-induced hydrophilicity of 1/9 (Cu/SiO2/TiO2) sample, with CA reduction to smaller than 15° was verified. Afterward, the viable cell count was applied on the samples. According the results of the viable cell count test shown in Table 4, the sample is capable of removing the E. coli bacteria. It was determined through viable cell count that the coating containing 0.1wt. % (Cu (NO3)3) (i.e., in Cu/SiO2/TiO2 sample) can eliminate 99% of E. coli bacteria in 1 h, and 99.99% after 24 hrs. On the contrary, for a sample devoid of copper 1/9 (SiO2/TiO2), the reduction percentage is 10% in 1 h, and 50% during 24 hrs (Fig. 11). It should be noted that this measurement was performed on a control sample with no self-cleaning and antibacterial property (see Table 4). The elimination of bacteria in the sample containing only SiO2/TiO2 is due to the presence of TiO2 in the coating. Bianchi et al. [34], have applied the method of the present work for silver and obtained a 99.99% reduction of E.coli after 24 hrs, which is the same as our results using the copper and what TOTO Ltd. obtained for its product using silver [22]. Hirai et al. [35] have obtained the same value of antibacterial effect, however, without using TiO2. It is worth mentioning that while the antibacterial property of silver has been known for many years, obtaining such a high reduction using copper is unprecedented. As shown in this study, copper, when combined with SiO2/TiO2, has an antibacterial property similar to that of silver [11, 12, 16].
In the adherence experiment, after detaching the tape, no visible peeling could be observed by naked eyes, the coating was not removed and had sustained its property. Such an adhesion makes these surfaces a viable candidate for walls, interior, and exterior surfaces. In addition, the original design of the ceramic tiles remained intact as in Fig. 12. It shows that the substrate after and before the coating had not a considerable change in color, which it is important for commercialization.
4 Conclusions
To summarize, using the sol-gel method, we prepared a SiO2/TiO2 solution with various volume ratio of Si/Ti. Solutions were applied on the ceramic tile substrates via the dip-coating technique. The crystal structures of anatase and rutile phases were observed in the layers’ structures, and roughness of the substrate surface decreased drastically by ~18 nm after applying the self-cleaning coating. After deposition, an almost uniform hydrophilic layer was formed; its WCA was reduced to about 13° after 24 hrs of weak intensity (400–800 μW/cm2) UV lamp illumination. Furthermore, the coating demonstrated the photocatalytic activity and the ability to decompose the contamination in <24 hrs in low humidity (21–37 RH). Then, the 1/9 (SiO2/TiO2) solution was chosen as the optimum one and copper nitrate trihydrate was added to the solution to obtain a self-clean and antibacterial thin-film coating on ceramic tiles. The primary objective of this study was to obtain a self-clean and antibacterial coating on ordinary ceramic tiles that can be used in various conditions (i.e., independent of weather conditions or light intensity). Therefore, the self-cleaning function of TiO2 through hydrophilicity and photocatalysis has been confirmed, especially in the presence of ultraviolet rays. When no UV light is available, or there is a very low-intensity illumination, the copper can remove 99% of E. coli bacteria in 1 h, and 99.99% in 24 hrs.
References
Benedix R, Dehn F, Quaas J, Orgass M (2000) Application of titanium dioxide photocatalysis to create self-cleaning building materials. Lacer 5:157. p
Maryani E, Nurjanah N, Hadisantoso E, and Wijayanti R. (2020)The Effect of TiO2 additives on the antibacterial properties (Escherichia coli and Staphylococcus aureus) of glaze on ceramic tiles. IOP Conference Series: Materials Science and Engineering 980:012011, p
Murugan K, Subasri R, Rao T, Gandhi AS, Murty B (2013) Synthesis, characterization and demonstration of self-cleaning TiO2 coatings on glass and glazed ceramic tiles. Prog Org Coat 76:1756. p
Gutiérrez RM-D, Villaquirán-Caicedo M, Ramírez-Benavides S, Astudillo M, Mejía D (2020) Evaluation of the antibacterial activity of a geopolymer mortar based on metakaolin supplemented with TiO2 and CuO particles using glass waste as fine aggregate. Coatings 10:157. p
Heidari S, Mohammadizadeh M, Mahjour-Shafiei M, Larijani M, Malek M (2015) Hydrogen irradiation on TiO2 nano-thin films. Appl Phys A 121:149. p
Ataei SS, Mohammadizadeh MR, Seriani N (2016) Ab initio simulation of the effects of hydrogen concentration on anatase TiO2. J Phys Chem C 120:8421. p
Sotoudeh M, Abbasnejad M, and Mohammadizadeh MR (2014) First principles study of hydrogen doping in anatase TiO2. EPJ Appl Phys 67:30401, p
Pakdel E, Daoud WA, Wang X (2013) Self-cleaning and superhydrophilic wool by TiO2/SiO2 nanocomposite. Appl Surf Sci 275:397. p
Pinho L, Rojas M, Mosquera MJ (2015) Ag–SiO2–TiO2 nanocomposite coatings with enhanced photoactivity for self-cleaning application on building materials. Appl Catal B: Environ 178:144. p
Pinho L, Mosquera MJ (2013) Photocatalytic activity of TiO2–SiO2 nanocomposites applied to buildings: influence of particle size and loading. Appl Catal B: Environ 134:205. p
Guan K (2005) Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf Coat Technol 191:155. p
Zhang M, Lei E, Zhang R, Liu Z (2019) The effect of SiO2 on TiO2-SiO2 composite film for self-cleaning application. Surf Interfac 16:194. p
Cai J-H, Huang J-W, Yu H-C, and Ji L-N (2012) Synthesis, Characterization, and Photocatalytic Activity of TiO2 Microspheres Functionalized with Porphyrin. Int J Photoenergy 2012;348292, p
De Niederhausern S, Bondi M, Bondioli F (2013) Self‐cleaning and antibacteric ceramic tile surface. Int J Appl Ceram Technol 10:949. p
da Silva AL, Dondi M, Raimondo M, Hotza D (2018) Photocatalytic ceramic tiles: Challenges and technological solutions. J Eur Ceram Soc 38:1002. p
Noyce J, Michels H, Keevil C (2007) Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol 73:2748. p
Esteban-Tejeda L, Malpartida F, Esteban-Cubillo A, Pecharromán C, Moya J (2009) Antibacterial and antifungal activity of a soda-lime glass containing copper nanoparticles. Nanotechnology 20:505701. p
Sagripanti J-L, Routson LB, Bonifacino AC, Lytle CD (1997) Mechanism of copper-mediated inactivation of herpes simplex virus. Antimicrob Agents Chemother 41:812. p
Carpenter S, Rigaud M, Barile M, Priest TJ, Perez L, and Ferguson JB (2006) The Ebers Papyrus. Bard College 3, New York
Borkow G, Gabbay J (2005) Copper as a biocidal tool. Curr Med Chem 12:2163. p
Yadav HM, Otari SV, Koli VB, Mali SS, Hong CK, Pawar SH, Delekar SD (2014) Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J Photochem Photobiol A: Chem 280:32. p
Tile Council of North America Anounces of Research Testing on Tiles with “HYDROTECT Tile Coat for Interior”Surface from TOTO Ltd. 2016; https://jp.toto.com/hydrotect/eng/about04.html
Akbari R, Mohammadizadeh M, Aminian MK, Abbasnejad M (2019) Hydrophobic Cu2O surfaces prepared by chemical bath deposition method. Appl Phys A 125:1. p
Foadi F, Kalhori H, Mohammadizadeh MR (2020) Modification of stainless steel by Cu2O coating for hydrophobic applications: a morphological study. Surf Topography: Metrol Prop 8:025014. p
Sotoudeh M, Hashemifar S, Abbasnejad M, Mohammadizadeh M (2014) Ab-initio study of hydrogen doping and oxygen vacancy at anatase TiO2 surface. AIP Adv 4:027129. p
Effting C, Folgueras MV, Güths S, Alarcon OE (2010) Microstructural characterization of ceramic floor tiles with the incorporation of wastes from ceramic tile industries. Mater Res 13:319. p
Effting C, Güths S, Alarcon OE (2007) Evaluation of the thermal comfort of ceramic floor tiles. Mater Res 10:301. p
Foadi F, Allaei SMV, Palasantzas G, Mohammadizadeh MR (2019) Roughness-dependent wetting behavior of vapor-deposited metallic thin films. Phys Rev E 100:022804. p
Akbari R, Godeau G, Mohammadizadeh M, Guittard F, Darmanin T (2019) Fabrication of superhydrophobic hierarchical surfaces by square pulse electrodeposition: copper-based layers on gold/silicon (100) substrates. ChemPlusChem 84:368. p
Akbari R, Godeau G, Mohammadizadeh M, Guittard F, Darmanin T (2019) Wetting transition from hydrophilic to superhydrophobic over dendrite copper leaves grown on steel meshes. J Bionic Eng 16:719. p
Reinosa J, Romero J, Jaquotot P, Bengochea M, Fernández J (2012) Copper based hydrophobic ceramic nanocoating. J Eur Ceram Soc 32:277. p
Tu S-H, Wu H-C, Wu C-J, Cheng S-L, Sheng Y-J, Tsao H-K (2014) Growing hydrophobicity on a smooth copper oxide thin film at room temperature and reversible wettability transition. Appl Surf Sci 316:88. p
Kwon MH, Jee WY, Chu CN (2015) Fabrication of hydrophobic surfaces using copper electrodeposition and oxidation. Int J Precis Eng Manuf 16:877. p
Bianchi CL, Cerrato G, Bresolin BM, Djellabi R, Rtimi S (2020) Digitally printed AgNPs doped TiO2 on commercial porcelain-grès tiles: synergistic effects and continuous photocatalytic antibacterial activity. Surfaces 3:11. p
Hirai, A., 2006. Process for preparing copper oxide-coated antibacterial material. U.S. Patent Application 11/129,175
Funding
Partial financial support by the research council of the University of Tehran is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moghaddasi, Z., Mohammadizadeh, M.R. Synthesis and effectiveness of Cu-infused TiO2-SiO2 based self-cleaning and antibacterial thin-films coating on ceramic tiles. J Sol-Gel Sci Technol 103, 396–404 (2022). https://doi.org/10.1007/s10971-022-05853-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05853-6