Abstract
Titanium tetraisopropoxide was hydrolyzed in tetrahydrofuran (THF), 1-butanol, or 2-butanol to prepare coating solutions for titania thin films. The compositions of the starting solutions were so simple, Ti(OC3H7i)4:H2O:solvent = 1:(0.5 or 1.0):25 in mole ratios, that they contained no chelating or peptizing agents. In spite of the absence of chelating or peptizing agents, transparent sols were obtained except for the THF solution of H2O/Ti(OC3H7i)4 = 1.0. All the transparent sols showed high stability in viscosity at least over 1 month. Titania thin films were prepared from the sols by spin- or dip-coating on Si(100) substrates via heat treatment at 700 or 900 °C. The stability of such additive-free sols was examined by comparing the thickness, refractive index, crystalline phase, crystallite size, and microstructure between films that were prepared from sols aged for 1, 5, and 30 days at room temperature. The thickness, refractive index, and crystallite size did not change noticeably with sol aging time. The films prepared from the THF-containing sols were anatase irrespective of the sol-aging time and heat treatment temperature. On the other hand, rutile phase was also observed when the films were prepared from 30-day-aged, 1-butanol-, or 2-butanol-containing sols, followed by firing at 900 °C. The films from the 2-butanol-containing sols also exhibited a change in microstructure at such a long sol aging time. These suggest that the 1-butanol- or 2-butanol-containing sols are unstable during aging while the THF-containing sol is stable as coating solution.

Highlights
-
Simple “titanium alkoxide–water–solvent” solutions were presented as titania coating solutions.
-
The solutions contain neither peptizing nor chelating agents.
-
The solvent was either tetrahydrofuran (THF), 1-butanol or 2-butanol.
-
All the solutions exhibited stability in viscosity.
-
Pot life was the longest for the THF-containing sol in terms of anatase film formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Titania (TiO2) is one of the commercial materials which is largely used as pigment in paints [1], antireflective coating materials [2] and photocatalysts [3] because of its high refractive index, nontoxicity [3, 4] and photocatalytic activity [3]. For titania thin films, there have been a lot of studies on sol–gel process where dip and spin coating are used in laboratory scale for preparing gel films, and the gel films are converted into polycrystalline anatase or rutile films by heat treatment [4, 5]. Titanium alkoxides are the most popular source materials, where alcohols are used as solvents. Hydrolysis and condensation of the alkoxides occur in these media to form polymerized species. When titanium alkoxides are hydrolyzed, chelating or peptizing agents such as acetylacetone and hydrochloric acid, respectively, are generally employed in order to suppress the formation of precipitates and to obtain homogeneous sols [6,7,8].
Recently we have found that precipitation can be avoided when 2-methoxyethanol is used as a solvent for hydrolyzing titanium isopropoxide (Ti(OC3H7i)4) even without using chelating or peptizing agents [9]. A sol obtained from a simple solution of molar compositions, Ti(OC3H7i)4:H2O:CH3OC2H4OH = 1:1:25, was demonstrated to be stable in viscosity at least for over 1 month. The stability and long pot life of the sol were evidenced in the identical characters including thickness, crystallite size, and refractive index and the similar microstructure of the films prepared from the sols aged for 1 and 30 days at room temperature. The formation of homogeneous sol and its long-period stability were thought to result from chelation of the titanium atoms by 2-methoxyethanol chelation.
Homogeneous sols that are transparent for over 1 month were also obtained from additive-free Ti(OC3H7i)4 solutions containing tetrahydrofuran (THF) or 2-butanol as a solvent in the previous work [9]. However, the examination of such sols in terms of film character as well as sol viscosity was not performed. Then in order to widen the possibility of the stable titania sols, we prepared additive-free Ti(OC3H7i)4 solutions containing THF or 2-butanol as well as 1-butanol in the present work. We examined the stability of such sols in terms of viscosity, and of thickness, refractive index, crystalline phase, crystallite size, and microstructure of the films prepared from sols aged for different periods of time.
2 Experimental
2.1 Preparation of the sols
Titanium isopropoxide (Ti(OC3H7i)4) purchased from Wako Pure Chemical Industries, Osaka, Japan, and purified water were used as the starting materials. THF(C4H8O), 1-butanol (CH3(CH2)3OH), and 2-butanol (CH3CH(OH)CH2CH3) purchased from Wako Pure Chemical Industries were used as solvents. The starting solutions of mole ratios, Ti(OC3H7i)4:H2O:solvent = 1:(0.5 or 1.0):25, were prepared by the following procedure. Titanium isopropoxide was added in 2/3 of the prescribed amount of solvent. A solution composed of water and 1/3 of the prescribed amount of solvent was prepared separately. The former solution was cooled in iced water, and the latter solution was added dropwise under magnetic stirring. The starting solutions thus prepared were kept in a sealed glass container at room temperature for various periods of time up to 30 days. The notations of the solutions, which will also be used for the films, are summarized in Table 1. 2BU-W1.0, for example, stands for the sol containing 2-butanol with a H2O/Ti(OC3H7i)4 mole ratio of 1.0.
2.2 Preparation of thin films
Gel films were deposited on Si(100) substrates (20 × 40 × 0.625 mm3) using sols aged at room temperature for 1, 5, and 30 days. The films were prepared from the THF-containing sols using dip coating at a substrate withdrawal speed of 1 cm/min, and from the 1-butanol- and 2-butanol-containing sols by spin coating at a spinning rate of 1000 rpm. The gel films were subjected to heat treatment at 700 or 900 °C for 10 min in air by placing them in an electric furnace of the prescribed temperature.
2.3 Characterization of the sols and the thin films
The viscosity of the sols was measured at room temperature using an oscillating type viscometer (VM-1G, Yamaichi Electronics, Tokyo, Japan) at a frequency of 500 Hz. The thickness and refractive index of the films were measured using a spectroscopic ellipsometer (M-2000V-Kk, J. A. Woollam Company, Nebraska, USA) with a software (CompleteEASETM, J.A. Woollam Company). The measurement was conducted at three angles of incidence of 65°, 70°, and 75° over the spectral range of 370–1000 nm, and the analysis was made based on Cauchy model.
The crystalline phase of the thin film samples was identified by X-ray diffraction (XRD) measurement using a diffractometer (RINT-Ultima III, Rigaku, Tokyo, Japan) with Cu Kα radiation operated at 40 kV and 40 mA. The measurement was carried out with grazing incidence configuration at an incidence angle of 0.5°. The crystallite size was obtained from the corrected half-height width of the diffraction peak using Scherrer’s equation. The microstructure of the films was observed by a field emission scanning electron microscope (FE-SEM) (JSM-6500F, JEOL, Tokyo, Japan). Osmium was deposited on the surface of the samples prior to the observation using an osmium coater (Neco-ST, Meiwafosis, Tokyo, Japan).
3 Results and discussion
3.1 Appearance and viscosity of the sols
The appearance of the sols is shown in Table 1. Sol THF-W0.5 was transparent and colorless at least over 1 month while Sol THF-W1.0 was opalescent at the beginning, becoming transparent in 1 day with a trace amount of white precipitates. Thus a smaller amount of water was effective in suppressing the formation of precipitates in the THF-containing sols. The 1-butanol-containing sols were colorless and transparent over 1 month. The 2-butanol-containing sols were light yellow and transparent over 1 month, where the color was lighter for 2BU-W0.5 than for 2BU-W1.0.
The results on Sol THF-W1.0 was different from that in the previous paper [9]. The white precipitates in Sol THF-W1.0 disappeared within a few days in the previous work while the precipitates remained in the present work. In both cases, THF reagents contained a stabilizing agent, and were purchased from Wako Pure Chemical Industries. However, “Wako 1st Class Grade” THF reagent was used in the previous work while “Wako Guaranteed Grade” in the present work, where the latter is higher in purity than the former. Such a difference in purity may have resulted in the difference in the formation of sols.
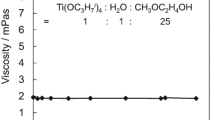
The transparent sols were kept in sealed containers at room temperature and their viscosity were measured at various periods of time. Figure 1 shows the relationship between the viscosity and the aging time. All of the sols exhibited high stability in viscosity at least over 1 month.
3.2 Stability of the sols as titania thin film precursors
3.2.1 Thickness, refractive index, and porosity
Titania gel films were deposited on Si (100) substrate, followed by heat treatment at 700 or 900 °C for 10 min. The films were prepared from the transparent sols, namely THF-W0.5, 1BU-W0.5, 1BU-W1.0, 2BU-W0.5, and 2BU-W1.0. The sols were aged at room temperature for 1, 5, and 30 days in order to examine their pot life as coating solutions. The thicknesses and the refractive index at a wavelength of 589.3 nm are shown in Table 2. No clear increase in thickness due to an increase in sol viscosity was detected, and the thickness was similar, not noticeably different between the films from the 1-, 5- and 30-day-aged sols. (Sol THF-W0.5 had the lowest viscosities among the sols prepared (Fig. 2), which allows us to expect that Films THF-W0.5 would have the smallest thickness. However, this was not the case (Table 2), which is just because solely Films THF-W0.5 were prepared by dip coating at 1 cm/min while the other films by spin coating at 1000 rpm.)
The refractive index was also similar, not significantly different between the films from the 1-, 5-, and 30-day-aged sols (Table 2). The porosity was calculated for the anatase films from their refractive index using the Lorentz–Lorenz equation [10], where the refractive index of dense anatase at 589.3 nm, 2.561 [11], was used in calculation. The porosity data thus obtained are shown in Table 2. (Description on crystalline phases will be made later.) The porosity ranged from 10 to 28%, but not significantly changed with sol aging time. Lower porosities were found for the films fired at 900 °C than those at 700 °C, which may have resulted from the progress of sintering.
3.2.2 Crystalline phases and crystallite size
The crystalline phases identified by XRD for the thin films samples are summarized in Table 3. All of the films fired at 700 °C were single phase in anatase irrespective of the type of solvent, the amount of water, and the sol aging time (Table 3 and Figs S1–S3). When fired at 900 °C, most of the films became pure anatase again, but those prepared from 30-day-aged sols contained rutile as a minor or major phase except for Sol THF-W0.5 (Table 3 and Figs 2–4). In order to examine the reproducibility of the formation of rutile phase, the sols were prepared and aged for 30 days again, and films were prepared via firing at 900 °C. As seen in the bottom two patterns in Fig. 2, Films THF-W0.5 were pure anatase both for the first and second time. As seen in the bottom two patterns in Figs 3 and 4, on the other hand, Films 1BU-W0.5, 1BU-1.0, 2BU-W0.5, and 2BU-W1.0 contained rutile for the first and/or the second time, where the reproducibility of the anatase/rutile ratio was poor. It should be noted that Films THF-W0.5 fired at 900 °C were anatase irrespective of the sol aging time, indicating that Sol THF-W0.5 is higher in stability than the 1-butanol- and 2-butanol-containing sols.
The crystallite size calculated from the width of anatase (101) peaks is shown in Table 2. The crystallite size did not show significant change with sol aging time in each film, which suggests the stability of the sols in terms of crystallite size. The larger crystallite size for the films fired at 900 °C than those at 700 °C is simply due to the grain growth at the higher temperature.
3.2.3 Microstructure
Figures 5–7 show the SEM images of Films THF-W0.5, 1BU-W0.5 and 2BU-W0.5, respectively, that were prepared from the 5- and 30-day-aged sols, followed by firing at 900 °C. As seen in Figs 5 and 6, grains 30–70 nm in size are densely arranged in Films THF-W0.5 and 1BU-W0.5. More importantly the comparison between Fig. 5a, b and between Fig. 6a, b tells us that these films did not show clear changes in microstructure with sol aging time. On the other hand, Film 2BU-W0.5 prepared from the 5-day-aged sol was composed of interconnected particles about 50 nm in size and was rather porous (Fig. 7a). When the film was prepared from the 30-day-aged sol, the microstructure changed to a relatively dense one with grains about 70 nm in size with round-shaped, delaminated parts as seen in Fig. 7b. In order to examine the reproducibility in microstructure, the 30-day-aged sol and Film 2BU-W0.5 were prepared again. Then different microstructure was observed with densely arranged grains 50–120 nm in size, abnormally grown grains larger than a few hundreds nanometers, and no round-shaped delaminated parts (Fig. 7c). The change in microstructure and the lack in its reproducibility suggest the instability of Sol 2BU-W0.5 as a coating solution.
3.2.4 Effects of the solvents on the stability of coating solutions
The previous paper [9] demonstrated that 2-methoxyethanol and 2-ethoxyethanol are the solvents that can avoid precipitation in “Ti(OC3H7i)4–H2O–solvent” solutions. The 2-methoxyethanol-containing sol was also demonstrated to have long pot life as titania coating solution. The present work suggested that 1-butanol and 2-butanol are more effective in suppressing the formation of precipitates than THF, which was evident at a H2O/Ti(OC3H7ι)4 mole ratio of 1 (Table 1). Owing to the two oxygen atoms in 2-methoxyethanol and 2-ethoxyethanol molecules were thought to act as chelating ligands, which may hinder hydrolysis and/or condensation reactions. On the other hand, a butanol molecule cannot act as a chelating agent because it has only one oxygen atom. However, butyl groups are larger in size than propoxyl groups, and hence can delay hydrolysis and/or condensation reactions via steric hindrance.
In spite of the less ability to form transparent solutions at H2O/Ti(OC3H7i)4 = 1, Sol THF-W0.5 was more stable than Sols 1BU-W0.5 and 2BU-W0.5 as coating solutions in terms of the influence of the sol aging time on the crystalline phase and microstructure of the films. THF cannot be a chelating agent, while it is characterized as an aprotic solvent. Aprotic solvents are known to have less ability to deactivate the nucleophiles because of the absence of hydrogen bonding interactions via hydrogen atoms [12]. This can explain the higher tendency to have precipitates in Sol THF-W1.0. However, how can we explain the higher stability of Sol THF-W0.5 as coating solution? Because of the aprotic nature, THF cannot undergo ligand exchange reaction with Ti(OC3H7i)4, which is contrast to the case with Sols 1BU-W0.5 and 2BU-W0.5. Propoxy and butoxy groups may be exchanged as the time passes and the coordination environment may be changed with time in Sols 1BU-W0.5 and 2BU-W0.5. Such a change in coordination environment may not occur in Sol THF-W0.5, giving rise to higher stability in polymerized structure. Although this is one possible explanation, further analytical works should be done in future.
Finally the possible contribution of titania thin films thus prepared should be clarified in future by comparing carefully the crystalline phase, refractive index, and microstructure with those prepared under the same fabrication conditions with chelating or peptizing agents.
4 Conclusions
Solutions of molar compositions, Ti(OC3H7i)4:H2O:solvent = 1:(0.5 or 1.0):25, were prepared without using any chelating or peptizing agents, followed by gel film deposition and firing at 700 and 900 °C after aging the sols at room temperature for 1, 5, and 30 days. Transparent sols were obtained except for the solution of H2O/Ti(OC3H7i)4 = 1.0 containing THF, suggesting the less ability of THF to avoid precipitation. All of the transparent sols exhibited stability in viscosity at least over 1 month, and no noticeable change with sol aging time was observed in thickness, refractive index and crystallite size of the films prepared. The films prepared from the THF-containing sols were anatase irrespective of the sol-aging time and heat treatment temperature. On the other hand, rutile phase was also formed when the films were prepared from 1-butanol- or 2-butanol-containing sols aged for 30 days, followed by firing at 900 °C. The films from the 2-butanol-containing sols also exhibited a change in microstructure at such a long sol aging time. Thus the THF-containing sol was found to be more stable as coating solution than the 1-butanol- or 2-butanol-containing sols.
References
Farrokhpay S, Morris GE, Fornasiero D, Self P (2006) Titania pigment particles dispersion in water-based paint films. J Coat Techn Res 3:275–283
Jeong SH, Kim JK, Kim BS, Shim SH, Lee BT (2004) Characterization of SiO2 and TiO2 films prepared using RF magnetron sputtering and their application to anti-reflective coating. Vacuum 76:507–515
Kako T, Umezama N, Xie K, Ye J (2013) Undoped visible-light-sensitive titania photocatalyst. J Mater Sci 48:108–114
Negishi N, Takeuchi K (2001) Preparation of TiO2 thin film photocatalysts by dip-coating using a highly viscous solvent. J Sol-Gel Sci Technol 22:23–31
Saini KK, Sharma SD, Chanderkant, Kar M, Singh D, Sharma CP (2007) Structural and optical properties of TiO2 thin films derived by sol-gel dip coating process. J Non-Cryst Solids 353:2469–2473
Pettit RB, Brinker CJ, Ashley CS (1985) Sol-gel double-layer antireflection coatings for silicon solar cells. Sol Cells 15:267–278
Yoko T, Kamiya K, Yuasa A, Sakka S (1986) Formation of H2O2 at the illuminated TiO2 film electrode prepared by the sol-gel method and its chemical states. J Electroanal Chem 209:399–404
Doeuff S, Henry M, Sanchez C, Livage J (1987) Hydrolysis of titanium alkoxides: modification of the molecular precursor by acetic acid. J Non-Cryst Solids 89:206–216
Yamazaki S, Uchiyama H, Kozuka H (2018) Additive-free alkoxide–water–alcohol solutions as precursors for crystalline titania thin films. J Sol-Gel Sci Technnol 87:537–543
Ohya Y, Saiki H, Tanaka T, Takahashi Y (1996) Microstructure of TiO2 and ZnO films fabricated by the sol-gel method. J Am Ceram Soc 79:825–830
Committee of Fain Seramikkusu Jiten (ed) Fain Seramikkusu Jiten (Fine Ceramics Dictionary), Gihodo Shuppan, Tokyo, 1987, p 317
Artaki I, Zerda TW, Jonas J (1986) Solvent effects on the condensation stages of the sol-gel process. J Non-Cryst Solids 81:381–395
Acknowledgements
TP thanks the Center of Excellence on Petrochemical and Materials Technology, Chulalongkorn University for the scholarship and financial supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Piwluang, T., Hayashido, T., Koizumi, Y. et al. Simple “titanium alkoxide–water–solvent” solutions as titania coating solutions containing no peptizing or chelating agents. J Sol-Gel Sci Technol 92, 57–65 (2019). https://doi.org/10.1007/s10971-019-05088-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05088-y