Abstract
Silica composited flower-like microspheres were synthesized by a facile sol–gel synthesis method using trivalent europium tartrate (Eu3+-TTA) as the template. Fourier transform infrared spectra (FT-IR) X-ray photoelectron spectroscopy (XPS), X-ray diffraction analyses (XRD), Energy-dispersive X-ray (EDS) spectroscopy, scanning electron microscopy (SEM), thermogravimetry-differential thermal analysis (TG-DTA) were employed to characterize the composition, structure, and morphology of the products. The microspheres were composed of nanosheets as petals. Size of the spherical shape varied with the amount of tetraethoxysilane (TEOS) addition. As the addition of TEOS decreased the diameters of microspheres increased, and it was proven that Eu3+ ions existed in the form of Si–O–Eu covalent bond. Furthermore, a sol–gel templated mechanism is proposed to interpret the formation process of flower-like microspheres shape. The hollow-liked microspheres were obtained by a following calcination process. In addition, photoluminescent emission of the products was further discussed, which indicated that Eu3+ ions exhibit characteristic luminescent emissions and their luminescent intensity after calcination is higher than that of as-synthesized sample.
Graphical abstract
The silica composited microspheres with flower-like shape were prepared successfully by a facile sol–gel synthesis method using trivalent europium tartrate (Eu3+-TTA) as a template. A sol–gel templated mechanism was proposed to interpret the formation process of flower-like shape microspheres. During this process, Eu3+-tartrate was supposed to form supramolecular template by self-assembly and coordinate with silica oligomer by means of intermolecular interactions. Furthermore, it was indicated that Eu3+ exhibits strong characteristic luminescence in this system.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rare-earth compounds arouse special attention due to their wide applications in amazing luminescent [1,2,3,4,5,6], magnetic [7, 8], catalytic [9], and other fields owing to their special electronic and optical characteristics arising from the unique f–f electronic transitions of 4f electronic shell [10]. Recently, lanthanide metal–organic materials have attracted more and more attention because of their unusual coordination characteristics and exceptional optical and magnetic properties arising from 4f electrons. Among these, tartaric acid (TTA) and its derivatives are very popular and important compounds as organic ligand for the preparation of lanthanum tartrate due to their wide variety of sources and low cost. Si et al. [11] fabricated monodispersed lanthanum tartrate micro aggregations with flower-like spherical shape in ethanol/water mixed solvents. The linear relationship between the sheet thickness and the dielectric constant of the solvent was obtained. Wang et al. [12] reported two novel coordination frameworks, [La(TTA)1.5(H2O)]•H2O and [La2(TTA)3(H2O)3]•2H2O, obtained by hydrothermal method. These polymers were predominated by the different chiralities of the tartaric acid (H2TTA).
Silica is an excellent composite reagent not only resulting from its easily chemically modified surface [13, 14], but also due to its good biocompatibility [15] and high robustness of its framework [16, 17]. Deng et al. [18] synthesized the CTAB-wrapped MWCNTs coated with uniform mesoporous silica shells via a simple single-step coating procedure. Duan et al. [19] prepared the poly (N-isopropylacrylamide) / silica composite microspheres using an inverse Pickering suspension polymerization method, and the microspheres prepared by methacryloxypropyltrimethoxysilane modified silica and dichlorodimethylsiliane modified silica showed different thermal-responsive behavior and dye release rate.
Here in this paper, the novel silica composited flower-like microspheres with different size were prepared using lanthanum tartrate as template in ethanol system by sol–gel method. In addition, it would be worth pointing out that the trivalent europium ions are composited with silica network in the form of covalent Si–O–Eu, and the luminescent property of composited microsphere was further studied.
2 Experimental
2.1 Materials and preparation
D, L-tartaric acid (TTA), absolute ethanol, ammonia, nitric acid (Beijing Chemical Reagents Co., analytical grade), tetraethoxysilane (TEOS) (Internet Aladdin Reagent Database Inc., Shanghai, gas chromatography grade), europium oxide (Goring High-Tech Material Inc., Ganzhou, 4N) and other chemicals were used as received. In the typical synthesis, 0.2 mmol TTA was dissolved in 5 ml ethanol solution. Then europium nitrate solution (1.5 M) was added into the solution, which was kept under static conditions in a thermostat water bath at 25 °C for 5 min. TEOS (0.15 ml) was added into the above solution rapidly under stirring condition. The mixture was kept stirring for 5 min, and 25 μl NH4OH (2 wt% NH3 solution) was added after that. After a vigorous stirring for 30 min, white precipate was obtained after staying for another 48 h. The emulsion was separated by centrifugation and washed with deionized water for three times, and then white gel was obtained. Afterward, the gel was transferred into a vacuum drying oven, and maintained at 55 °C for hours, followed by being thermally treated at different temperatures (450 °C, 650 °C and 750 °C) for 4 h with a heating rate of 1 °C/min. White powder was prepared finally when it was cooled to room temperature naturally.
2.2 Characterization
The phase composition and phase structure of the as-synthesized products were examined by X-ray diffraction (XRD), using a Rigaku D/max-B II X-ray diffractometer with Cu Ka radiation. Energy-dispersive X-ray (EDX) spectroscopy analysis was performed with an H JEOL JXA-840 EDX system attached to the SEM microscope. The morphologies, nanostructure and the composition analysis of the obtained Eu3+-doped silica nanowires were characterized with a field emission scanning electron microscope (FESEM) (S-4800, Hitachi). Transmission electron microscopy (TEM) images were performed at a JEM-2000EX TEM (acceleration voltage of 200 kV). Differential scanning calorimetry–thermogravimetric analyzer (DSC/TGA 1600 LF, METTLER TOLEDO, Switzerland) up to 750 °C was performed on the sample at a heating rate of 10 °C min−1 while O2 gas flow rate of 60 ml min−1. Fourier transform infrared (FT-IR) spectra were measured with a Perkin–Elmer 580B infrared spectrophotometer with the KBr pellet technique. The composition and chemical bonding in Eu3+-doped silica nanowires were investigated using a Physical Electronics Quantum 2000 X-ray photoelectron spectroscopy (XPS) instrument. The PL measurements were performed on Jobin Yvon FluoroMax-4 luminescence spectrophotometer equipped with a 150 W xenon lamp as the excitation source. All the measurements were performed at room temperature.
3 Results and discussion
3.1 Characterization of samples
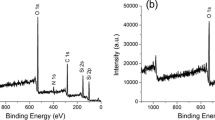
Figure 1a shows SEM image of the synthesized silica composited flower-like microspheres, it can be seen that the average diameter of microspheres were about 3 μm and their surfaces were made of many sheet-like particles. Meanwhile, EDX characterization of Eu3+-doped silica microspheres after calcination (Fig. 1b) demonstrates that the microspheres are composed of Si, O and Eu elements depending on the peaks of Si, O and Eu in the spectrum. The morphology of the pre- and post-calcinated products is also shown in Fig. 1. TEM image (Fig. 1c) before calcination shows that the sample is composed of numbers of sheet-like petals with oriented growth radiated from the spherecenter. The product after calcination (Fig. 1d) has the diameter around 3 μm approximately, which is slightly smaller than that of the precursor spheres, and exhibit hollow structure to some extent in contrast to the precursor spheres. Moreover, the TG-DTA curves (Fig. 1e) show the thermal behavior of the microsphere precursors. The small amount of weight loss before 250 °C can be attributed to the emission of H2O, corresponding to a weak endothermic peak on DTA curve. Large weight loss stage can be observed on the TG curves between 250 °C and 450 °C, together with a large exothermic peak on DTA curve. It can be attributed to the decomposition and combustion of TTA. The minor weight loss from 450 °C to 600 °C could be due to the dehydration and polycondensation of Si–OH. Interestingly, the strong endothermic peak on DTA curve located at above 600 °C results from the transformation of the microsphere core from Eu3+ intermediate to Eu2O3 [11, 20].
It would be worth pointing out that the structural parameter of silica composited flower-like microspheres can be tuned by facilely varying the addition of TEOS. For example, the microspheres have the average diameters around 4.2 μm at 0.785 ml (TEOS addition), 4.6 μm at 0.5 ml, 5.7 μm at 0.3 ml and 6.7 μm at 0.15 ml (Fig. 2), respectively. The corresponding histograms of diameter distribution by Gauss fitting are provided in Fig. 2b, d, f and h. It seems that excess TEOS prevents the microspheres from growing up continuously through hydrolytic condensation on the surface of the established spherical structure. In addition, the morphology of the microspheres was also influenced by the standing time of sol-process. The products with different shape are prepared in different standing time before adding TEOS (Fig. 3). When standing time is 2 h (Fig. 3a and b), microspheres with diameters around 3 μm are obtained. The products shown in Fig. 3c and d can still maintain the flower-like morphology when the standing time is 5 h. However, the microspheres are destroyed into flaky particles gradually if the time exceeds 10 h, as shown in Fig. 3e, f. Too long time appears to be detrimental for the cooperation between silica oligomer and trivalent europium ion.
The forming process of silica composited flower-like microspheres was characterized by SEM in Fig. 4. At the early stage of fibrous nanostructures growth, a mass of irregular particles aggregated spontaneously into the general hollow-out aggregations after NH4OH addition in ethanol system, shown in Fig. 4a. As shown in Fig. 4b, many spheres in the regular morphological form were obtained several hours later. It was noteworthy that there are a lot of folds on the rough surface of spheres (Fig. 4b inset), which might be the original hydrolytic process of TEOS. By the SEM observation in Fig. 4c and inset, it can be obviously seen that the flower-like microspheres with a good deal of sheet-like particles form during the aging process. Meanwhile, the size of the spherical structures increased gradually with the formation of the particles on the surface. So far TEOS have hydrolyzed completely and condensed into silica network structure.
3.2 Formation mechanism
To reveal the formation mechanism of silica composited flower-like microspheres, further characterizations have been done. First, elemental and chemical information of silica composited flower-like microspheres were confirmed by EELS (electron energy-loss spectroscopy). Figure 5 depicts the bright field image with all of the Si, O, and Eu mapping of the same region. These element-mapping images clearly indicate that Eu atoms are homogeneously distributed in the silica framework. These results imply that trivalent europium ions exist in the flower-like microspheres after calcination.
Second, FTIR was used to confirm the formation of silica composited flower-like microspheres (Fig. 6a). The typical vibration bands of O–Si–O in both pre- and post-calcining silica composited materials are shown in the spectrum at 1100 cm−1. The bands at 457 and 814 cm−1 are ascribed to Si–O symmetrical stretching vibration and bending vibration of silica. In addition, the band around 3370 cm−1 broadens almost disappear after thermal treatment. The reason can be ascribed that the free water molecules in the as-synthesized sample are removed. Moreover, the bands at 1610 and 1390 cm−1 are ascribed to COO− asymmetric and symmetric stretching vibration of TTA [4]. Correspondingly, the asymmetric and symmetric stretching vibration of COO− disappears after calcination. Especially, the emergence of the bands at 1516 cm−1 that corresponds to covalently bonded Si–O–Eu, which results from Eu+3 ions that occupied the oxygen site forming Si–O–Eu bonding [21, 22]. So, it can be concluded that the TTA is removed through calcination and Eu3+ ions in the shell of micosphere are in the form of Si–O–Eu bonding.
Third, we measured the XPS to characterize silica composited flower-like microspheres. Fig 6b shows the Eu 4d XPS spectrum for silica composited flower-like microspheres calcined at 450 °C. The Eu 4d3/2 and 4d5/2 peaks of the reference Eu2O3 located at 141.1 and 135.6 eV [23], respectively. For the samples, the Eu 4d double peaks are shifted higher binding energy by about 0.5 eV as compared to the Eu2O3 reference position, probably indicating the Si–O–Eu formation after calcination. The O 1 s spectrum of flower-like microspheres calcined at 450 °C is shown in Fig. 6c with their appropriate peak curve-fitting lines. Each fitting peak followed the general shape of the Gaussian function. The low energy state at 530.7 eV is assigned to O in Eu2O3. The intermediate energy state at 531.7 eV is attributed to O atoms in Eu–O–Si [24]. The high energy state at 533.1 eV is assigned to O in SiO2. This result suggests that the europium ions in the shell form Eu–O–Si bonding with silica which in the core are transferred from Eu-TTA to Eu2O3 after calcination.
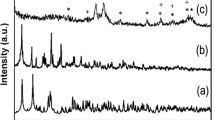
Furthermore, Fig. 6d shows the XRD pattern of samples before and after calcination treatment at different temperatures. All curves exhibit amorphous silica characteristic diffraction at 2θ = 22.5° irrespective of the condition [25]. The sharp diffraction peaks of the microspheres without calcination can be readily indexed to lanthanum tartrate. It can be noted that the crystallinity decreases as the temperature nears 450 °C. Three strong peaks can be observed when the temperature is 750 °C, which match well with Eu2O3 standard card. Combined with the aforementioned results, it is indicated that low temperature calcining process can remove impurities and result in the formation of the hollow flower-like microspheres. When the calcination temperature increases above 700 oC, excess Eu3+ ions in the core are transferred to crystalline Eu2O3.
In combination with the foregoing results, we now propose a possible mechanism of sol–gel hard template method for preparing silica composited flower-like microsphere (Scheme 1). Firstly, self-assembled Eu-TTA microsphere is fabricated with a mass of sheet-like particles by intermolecular efficient hydrogen bond in ethanol system. Therefore, the surface of microspheres has a large amount of hydroxyl groups. Secondly, silica precursors of anionic oligomeric siloxanes are adsorbed onto the self-assembled sheets through hydrogen bond between Si–OH and TTA hydroxyl during the sol–gel process. The hierarchically core–shell flower-like microsphere is obtained. Consequently the intermolecular interaction among Eu-TTA molecules in the core is destroyed during the subsequent calcination treatment, which makes the core shrink tremendously into little particles and attach on internal surface of the shell firmly. So far the adsorbed silica turn into shell structure and combine with Eu3+ by Si–O–Eu bond.
3.3 Photoluminescence properties
The photoluminescence excitation and emission spectra of silica composited flower-like microspheres obtained at different calcination temperatures under 613 emission and 393 nm excitation are shown in Fig. 7, respectively. The excitation spectra (Fig. 7a) consist of several narrow peaks, which are located at around 361, 375, 380, 394, 412, and 465 nm. The peaks are attributed to f–f transitions within the 4f [6] electron configuration [26]. All observed peaks in emission spectra (Fig. 7b) correspond to the transitions from the metastable orbital singlet state 5D0 to spin–orbital states of 7FJ (J = 0, 1, 2, 4) of Eu3+ [27], which indicates the characteristic transitions from the 5D0 to 7F0 at 578 nm, 7F1 at 587 nm, 592 nm, and 597 nm, 7F2 at 613 nm, 7F3 at 650 nm, and 7F4 at 700 nm. The intensity of emission band around 613 nm enhances gradually as the calcination temperature increases to 650 °C. Moreover, the spectral shapes after calcination are clearly distinct from the Eu3+ in as-synthesized microsphere, meaning the relaxation process from different electron levels of the Eu3+ relying on the silica [28]. The luminescence of the Eu3+ intensity significantly depends on the site environment of Eu3+ [29]. The presence of hydroxyl groups (as-synthesized sample) can lower the luminescence efficiency of the Eu3+ through a non-radiative phonon quenching [30]. Therefore, the luminescence intensities of the calcined microspheres are all higher than as-synthesized sample. However, the strength decreases at 750 °C slightly, which is probably due to the partial loss of the Eu3+ in the core of the microsphere during crystal transition by high-temperature calcination.
4 Conclusion
In conclusion, the silica composited flower-like microspheres were prepared successfully by a facile sol–gel synthesis method using trivalent europium tartrate (Eu3+-TTA) as a template. The morphology of microspheres was micron-sized flower-like spherical structure composed of nano-sized sheet-like particle, with increasing diameter as the content of TEOS decreasing. Based on the determination of FT-IR and XPS analysis, it was confirmed that Eu3+ ions are combined with silica framework in the form of Si–O–Eu through covalent bond. Moreover, a sol–gel templated mechanism was proposed to interpret the formation process of flower-like microspheres shape. During this process, Eu3+-tartrate was supposed to form supramolecular template by self-assembly and coordinate with silica oligomer by means of intermolecular interactions. The hollow-liked microspheres were obtained by a following calcination process. In addition, photoluminescent excitation and emission of europium ions in the samples were studied by changing calcination temperatures. It was indicated that Eu3+ exhibits strong characteristic luminescence in this system and the calcined microspheres have high luminescence intensities depending on the site environment of Eu3+. Such materials can be used as catalysts and luminescent materials.
References
Liu X, Lin C, Lin J (2007) White light emission from Eu3+ in CaIn2O4 host lattices. Appl Phys Lett 90:081904
Sohn K-S, Shin N (2002) Energy transfer between Tb3+ ions in Y4−xTbx Al2O9 phosphor. Electrochem Solid-State Lett 5:H21–H23
Song Y, Xu X, Zou H, Sheng Y, You H (2012) MSi2O2−δN2+ 2/3δ:Eu (M=Sr, Ba) phosphors for field emission displays. J Alloy Compd 513:86–90
Yue MB, Xue T, Jiao WQ, Wang YM, He M-Y (2011) CTAB-directed synthesis of mesoporous γ-alumina promoted by hydroxy carboxylate: the interplay of tartrate and CTAB. Solid State Sci 13:409–416
Miura K, Suzuki T, Hanaizumi O (2015) Photoluminescence properties of europium and cerium co-doped tantalum-oxide thin films prepared using co-sputtering method. J Mater Sci Chem Eng 03:30–34
Chen L, Lei X, Chen G, Zhong R, Lan S, Liang X, Lu J (2015) Synthesis, characterization and photophysical properties of phenanthroline derivatives and their europium complexes. J South-Cent Univ Natl 18:1315–1322
Varma C, Yafet Y (1976) Magnetic susceptibility of mixed-valence rare-earth compounds. Phys Rev B 13:2950
Luneau D, Rey P (2005) Magnetism of metal-nitroxide compounds involving bis-chelating imidazole and benzimidazole substituted nitronyl nitroxide free radicals. Coord Chem Rev 249:2591–2611
Gao Z, Shi Y (2010) Suppressed formation of CO2 and H2O in the oxidative coupling of methane over La2O3/MgO catalyst by surface modification. J Nat Gas Chem 19:173–178
Willbold M (2016) Europium. Living Reference Work Entry, Encyclopedia of Geochemistry, Part of the series Encyclopedia of Earth Sciences Series, pp 1–3
Si L, Yue L, Jin D (2011) Solvothermal synthesis of flower‐like lanthanum tartrate and lanthanum oxide microspheres in ethanol–water mixed system. Cryst Res Technol 46:1149–1154
Wang Y, Liu G-X, Chen Y-C, Wang K-B, Meng S-G (2010) Two novel lanthanum–tartrate complexes with distinctive new topologies: hydrothermal synthesis and crystal structures. Inorg Chim Acta 363:2668–2672
And ELL, Wachs IE (2015) In situ spectroscopic investigation of the molecular and electronic structures of SiO2 supported surface metal oxides. J Phys Chem C 111:14410–14425
Soga K (2015) Highly isospecific, immobilized zirconocene catalysts supported on chemically modified SiO2. Macromol Symp 89:249–258
Tang F, Li L, Chen D (2012) Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 24:1504–1534
Li L-L, Fang C-J, Sun H, Yan C-H (2008) Hierarchical self-assembly of pH-responsive nanocomposites with molecular-scale and mesoscale periodicities. Chem Mater 20:5977–5986
Buso D, Nairn KM, Gimona M, Hill AJ, Falcaro P (2016) Fast synthesis of MOF-5 microcrystals using Sol−Gel SiO2 nanoparticles. Chem Mater 23:929–934
Deng X, Qin P, Luo M, Shao E, Zhao H, Yang X, Wang Y, Shen H, Jiao Z, Wu M (2013) Mesoporous silica coating on carbon nanotubes: layer-by-layer method. Langmuir 29:6815–6822
Duan L, Chen M, Zhou S, Wu L (2009) Synthesis and characterization of poly (N-isopropylacrylamide)/silica composite microspheres via inverse Pickering suspension polymerization. Langmuir 25:3467–3472
Firdous A, Quasim I, Ahmad MM, Kotru PN (2009) Studies on gel-grown pure and strontium-modified lanthanum tartrate crystals. J Cryst Growth 311:3855–3862
Haranath D, Gandhi N, Sahai S, Husain M, Shanker V (2010) Highly emissive and low refractive index layers from doped silica nanospheres for solar cell applications. Chem Phys Lett 496:100–103
Hunag W-H, Shieh J-M, Lien Y-C, Jhou K-J, Tu C-H, Wang C, Shen C-H, Hsieh W-H, Kuo H-C, Pan F-M (2012). Novel solar down-conversion luminescent and switchable interface polarization material by europium doped Si–O polar structures, In Quantum Electronics and Laser Science Conference, Optical Society of America, p JTh4J. 5
Uwamino Y, Ishizuka T, Yamatera H (1984) X-ray photoelectron spectroscopy of rare-earth compounds. J Electron Spectrosc Relat Phenom 34:67–78
Her J-L, Lin C-W, Chang K-Y, Pan T-M (2012). Label-free detection of creatinine using a disposable poly-N-isopropylacrylamide as an encapsulating creatinine deiminase based Eu 2 Ti 2 O 7 electrolyte-insulator-semiconductors. Int J Electrochem Sci 7
Shui M, Song Y, Ren Y, Wang X (2010) Structural analysis of amorphous silica prepared by water glass-based precursors and its thermal, spectral characterization. Phys B: Condens Matter 405:1316–1320
Chen W, Sammynaiken R, Huang Y (2000) Photoluminescence and photostimulated luminescence of Tb 3+ and Eu 3+ in zeolite-Y. J Appl Phys 88:1424–1431
Wan N, Xu J, Lin T, Zhang X, Xu L (2008). Energy transfer and enhanced luminescence in metal oxide nanoparticle and rare earth codoped silica. Appl Phys Lett 92
Tagaya M, Ikoma T, Yoshioka T, Motozuka S, Xu Z, Minami F, Tanaka J (2011) Synthesis and luminescence properties of Eu (III)-doped nanoporous silica spheres. J Colloid Interface Sci 363:456–464
Nassar EJ, Ciuffi KJ, Ribeiro SJL, Messaddeq Y (2003) Europium incorporated in silica matrix obtained by sol-gel: luminescent materials. Mater Res 6:557–562
Berry A, King T (1989) Characterisation of doped sol–gel derived silica hosts for use in tunable glass lasers. J Phys D Appl Phys 22:1419
Acknowledgements
This present work was financially supported by the scientific research project of Inner Mongolia University for the Nationalities (NMDYB1765) and the Opening Research Funds Projects of Key Laboratory of Inner Mongolia Autonomous Region of Chemistry of Natural Product and Functional Molecular Synthesis, Inner Mongolia University for the Nationalities (MDK2016012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Gao, F., Sheng, Y., Song, Y. et al. Sol–gel synthesis of silica composited flower-like microspheres using trivalent europium tartrate as a template. J Sol-Gel Sci Technol 85, 470–479 (2018). https://doi.org/10.1007/s10971-017-4551-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4551-4