Abstract
The carbon nanotubes/vanadium oxide composites have been prepared through a facile hydrothermal method. Morphology features of the samples are investigated by field-emission scanning electron microscope. Raman and X-ray diffraction patterns confirm the formation of phase structure. Thermogravimetric analysis was used to quantify the content of carbon nanotube in the composites. When used as cathode materials for lithium-ion batteries, the composites exhibit improved electrochemical performance compared to electrode materials free of carbon nanotubes. V2O5/carbon nanotubes can deliver a good capacity of 196.8 mA h g−1 at a current density of 100 mA g−1 after 50 cycles. However, the electrode materials free of carbon nanotubes (V2O5) can reach a capacity of 171 mA h g−1 at the same conditions. Moreover, V2O5/carbon nanotubes present a higher capacity than that of V2O5 under the same rate conditions. The improved electrochemical performance can be attributed to the fact that the carbon nanotubes in the V2O5/carbon nanotube composites can effectively facilitate ionic diffusion by raising the electrical conductivity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The development of high-power applications, such as electric vehicle and hybrid electric vehicle, has promoted extensive research efforts in rechargeable lithium-ion batteries (LIBs) with good electrochemical performances [1, 2]. It is well known that the capacity and the cycle stability are important factors for LIBs’ practical applications [3, 4]. The electrode materials play a major role in (such as LiMn2O4 [5], LiCoO2 [2], and LiFePO4 [6]) determining electrochemical performance of LIBs, especially the cathode material. Among these electrode materials, vanadium pentoxide (V2O5) is one of the most promising candidates for cathode materials in LIBs owing to its unique intercalation structure, low cost and abundance. Moreover, the maximum amount of lithium ions (up to 3 Li+ per V2O5) can be intercalated in a potential window between 4.0-1.5 V vs. Li, and a higher theoretical capacities of ~440 mA h g−1 than those of commercially available cathodes, such as LiCoO2, can be achieved [7, 8]. Therefore, V2O5 as a cathode material may take the place of commercial cathode materials to meet the demand of high capacities and high energy densities for LIBs in the future.
Nevertheless, the capacity retention and rate capacity of V2O5 materials are vital elements hindering their practical applications, because of its poor electrical conductivity, low lithium-ion diffusion coefficient, and irreversible structural changes upon deep charge and discharge [9, 10]. In order to overcome these drawbacks, synthesizing nanostructured electrode materials is a facile and effective method; the reason is that nanostructured materials possess a large surface area and short paths, can effectively facilitate ionic diffusion and can alleviate the structure changes upon cycling [11–13]. Thus, a great many nanostructured V2O5 materials have been synthesized to improve the electrochemical performance, such as nanowires [14, 15], nanosheets [16, 17], and hierarchical structures [18, 19]. Meanwhile, the lithium storage process involves both ion diffusion and electron transport in the electrode materials, thus nanostructured electrode materials in combination with conductive materials, such as metal cations and carbonaceous materials, can further improve the electrochemical performance. For instance, an Mn-doped V2O5 sheet network can deliver a larger average capacity of 165 mA h g−1 compared to 99 mA h g−1 for V2O5 at a current density of 1 A g−1 [20]; the V2O5/rGO (46 wt%) electrode exhibits an excellent cyclability at a high current density of 5700 mA g−1 and delivers a discharge capacity of 93 mA h g−1 after 200 cycles [21]; the V2O5/CNT hybrid displays high specific capacity of 850 mA h g−1 as compared to 350 mA h g−1 for classical V2O5 powder electrode [22].
Carbon nanotubes (CNTs), one-dimensional carbonaceous tubular materials, have been extensively used as an ideal conductive material in energy storage applications in view of its large surface area, good electrical, and thermal properties [23, 24]. Besides, they are also usually used as an attractive electrode material for LIBs and capacitors [25, 26]. Therefore, preparing vanadium oxides and CNT composites is an effective and desirable method for obtaining electrode materials with good electrochemical performance. In this work, we prepared the CNTs/vanadium oxide composites through a facile hydrothermal method. The composites as cathode materials for lithium-ion batteries exhibit improved electrochemical performance compared to electrode materials free of CNTs. CNTs/vanadium oxide composites (V2O5-60CNT) can deliver a high initial capacity of 305 mA h g−1, and a good capacity of 196.8 mA h g−1 can be reached at a current density of 100 mA g−1 after 50 cycles. However, the electrode materials free of CNTs (V2O5) can reach an initial capacity of 263 mA h g−1, and a capacity of 171 mA h g−1 can be delivered at a current density of 100 mA g−1 after 50 cycles. Moreover, V2O5/CNTs present a higher capacity than V2O5 under the same rate conditions. The improved electrochemical performance can be attributed to the fact that the CNTs in the V2O5/CNT composites can effectively facilitate ionic diffusion by raising the electrical conductivity.
2 Experimental section
2.1 Synthesis of functional CNTs
The functional CNTs were prepared according to a previous reported method [27]. Briefly, 0.3 g of pristine CNTs was loaded on a porous SiO2 griddle of glass steamer and then placed into 100 mL Teflon-vessel, at the bottom of which 3 mL HNO3 was added previously. Subsequently, the vessel was transferred into a sealed Teflon container and treated in an electrical oven at 200 °C for 3 h. Finally, the products were washed with distilled water and ethanol, and dried for one night at 60 °C.
2.2 Synthesis and characterization of V2O5/CNT composites
A functional V2O5 sol was prepared firstly via a sol–gel method according to our previous work [28, 29]. Briefly, raw V2O5 powder, benzyl alcohol, and isopropanol were mixed at a certain molar ratio, and then the suspension was heated at high temperature under condensate reflux and concentrated to 1/3 volume through heating reflux. Thus, a light yellow functional V2O5 sol composed of vanadium oxide (VOx) oligomers was obtained, in which the V2O5 content was ~52 mg mL−1.The V2O5/CNT composites were prepared by a facile hydrothermal method. Typically, the as-prepared functional CNTs (30 and 60 mg) were dispersed into 20 mL of deionized water and ultrasonic treatment for 45 min. Subsequently, 2 mL of acetic acid was added. After stirring for 10 min, 10 mL of the as-prepared V2O5 sol was added by drop to the above solutions. After stirring for 20 min, the resultant solutions were transferred into a sealed Teflon container and treated in an electrical oven at 200 °C for 3 h. The precipitate obtained after hydrothermal treatment was filtered and washed with pure ethanol and deionized water, and dried for one night at 60 °C; the as-prepared precursors were annealed in air at 350 °C for 1 h with a heating rate of 10 °C min−1, and the obtained products were denoted as V2O5-30CNT and V2O5-60CNT, respectively. For comparison, the vanadium oxides free of CNTs were also prepared under the similar procedure and denoted as V2O5.
Morphological features of the samples were studied using a field-emission scanning electron microscope (FE-SEM, S-4800). Structural properties of the samples were examined using Bruker X-ray diffractometer with Cu-Kα radiation (λ = 1.5406 Å) between 10–60°. Raman spectra (Jobin-Yvon HR800) were recorded from 100 to 2000 cm−1 using a 514 nm argon ion laser. The functional groups on the carbon nanotubes were analyzed by X-ray photoelectron spectroscopy (XPS) The thermogravimetry analysis (TGA) and differential thermogravimetry analysis were carried out on a SDT Q600 over the temperature range from 50 to 700 °C using a heating rate of 10 °C min−1 under air atmosphere.
2.3 Electrochemical measurements
The coin cells were assembled to investigate the electrochemical properties. The working electrodes (cathodes) were prepared by mixing vanadium oxide materials, carbon black as a conducting agent and poly (vinylidene fluoride) as a binder at a weight ratio of 8:1:1 in N-methylpyrrolidone solvent, which were uniformly pasted on aluminum foils. The coated electrodes were dried in a vacuum at 120 °C for 8 h and then cut into disks (diameter 12 mm).The cells were assembled in an argon-filled glove box with water and oxygen contents less than 1 p.p.m. The cells were assembled based on the configuration of vanadium oxide materials/electrolyte/Li with a liquid electrolyte (1 M LiPF6 in ethylene carbonate/dimethyl carbonate (volume ratio 1:1)). A microporous membrane (Celgard 2500) was used as a separator and lithium metal as a counter electrode. The cells were then aged for 24 h before measurement. Charge–discharge and cyclic voltammetry (CV) tests were performed at a scanning rate of 0.1 mV s−1 by using a LAND cell-testing system and CHI660C (Chenghua, Shanghai) electrochemical workstation within the potential range of 2–4 V vs. Li. Electrochemical impedance spectroscopy (EIS) measurements were performed at frequencies between 100 kHz and 0.01 Hz with the amplitude of the AC signal was 5 mV. The loaded materials on aluminum foils are ~1.0 mg. All tests were performed at the room temperature, and specific capacity was calculated based on the mass of active materials.
3 Results and discussion
Figure 1a and b show the C1s XPS spectra of the pristine CNTs and functional CNTs, respectively. It is clear that the peak areas of three peaks present at 286.2 eV (hydroxyl, C–OH), 287.5 eV (carbonyl, C=O), and 289.9 eV (carboxyl, COOH) for the functional CNTs are bigger than those of the pristine CNTs [30, 31], besides the same peaks at 284.5 eV (carbon sp2, C=C) and 285.1 eV (carbon sp3, C–C) [30, 32]. The corresponding binding energy of functional groups from curve fitting of C1s XPS spectra for pristine CNTs and functional CNTs as shown in Table 1. It suggests that the surfaces of functional CNTs possess a large number of oxygen-containing functional groups after oxidation treatment. Since oxygen-containing functional groups have a tendency for adhesion of metal ions, the functional CNTs are more promising to immobile foreign materials compared to the pristine CNTs [22, 30]. Meanwhile, the CNTs after oxide treatment have a porous structure marked by red arrows as shown in Fig. 1c, which is in agreement with previous reported results [27, 30]. Such porous structure can effectively facilitate ion transport and increase the electrode/electrolyte contact area, which is of importance for high-rate LIBs [30].
The morphology and structure of the samples were characterized by field emission scanning electron microscopy (FESEM). Figure 2a shows the prolate flake morphology of as-prepared vanadium oxides, which due to V2O5 sol have the tendency to preferential growth along the (110) crystal plane. The morphology of prolate flake has been changed after calcination at 350 °C, and shrinks to form randomly distributed particles, as shown in Fig. 2b. The results for vanadium oxides free of CNTs obtained after hydrothermal treatment are similar to our previous work [28]. For comparison, the as-prepared samples display rod-like fibres structure due to the addition of CNTs with oxygen-containing functional groups as shown in Fig. 2c and e. After calcination, the rod-like fibres can be changed and form rod-like particles (Fig. 2d and f).
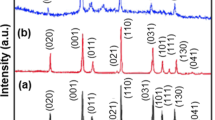
Figure 3a shows the wide-angle X-ray diffraction (XRD) patterns of the calcined samples. As shown, the diffraction peaks for all the samples clearly indicate the formation of pure phase structure without any impurity traces and all of peaks can be indexed to the orthorhombic phase V2O5 (JCPDS no.41-1426, space group: Pmmm (59), a = 1.1516 nm, b = 0.3565 nm, c = 0.4372 nm). The Raman patterns of the calcined samples were presented in Fig. 3b. The Raman patterns also show typical peaks of crystalline V2O5 at 140, 194, 282, 406, 518, 688, and 993 cm−1 for all the samples. The peaks at 140 and 194 cm−1 were assigned to the skeleton bending vibrations of the V–O–V bonds [33].The peaks at 282 and 406 cm−1 were assigned to the bending vibrations of the V=O bonds [34, 35]. The peaks at 518 and 688 cm−1 were ascribed to stretching vibrations of triply coordinated oxygen bonds, stretching vibrations of doubly coordinated oxygen, respectively. The peak at 993 cm−1 corresponds to the stretching vibrations of V=O bonds [36]. It should be noted that the characteristic peaks of the D and G bands of CNTs at 1350 and 1580 cm−1 were not detected in the V2O5/CNT composites, which may be attributed to low content of CNT in the composites [36]. To quantify the content of CNT in the composites, TGA was carried out in air from 50 to 700 °C at a rate 10 °C min−1 and presented in Fig. 3c. As can be seen from the TG curves, when the temperature beyond 650 °C, the curves of weight loss trend to flat, indicating the weight loss rarely changes. Therefore, the mass fraction at 650 °C was considered as the final remaining mass in the composites, the content of CNTs was calculated according to the formula W = 0.994(1−C M) + 0.2151CM [36], where W stands for the final weight of the composites in TG curves, C M is the mass fraction of CNT in the composites, 0.994 and 0.2151 are the final weight of pure V2O5 free of CNTs and pure CNTs, respectively. The contents of CNTs in the V2O5-30CNT and V2O5-60CNT are about 6.86 and 8.72%, respectively.
To investigate the electrochemical performance of composites, several electrochemical measurements were carried out. Figure 4 shows the CV curves of the samples at a scan rate of 0.1 mV s−1 between 2–4 V. For V2O5 electrode, there are three main cathodic peaks at a potential of 3.38, 3.18, and 2.28 V for the second cycle, corresponding to the phase transformations from α-V2O5 to ε-Li0.5V2O5, δ-LiV2O5, and γ-Li2V2O5, respectively [14, 37]. Three obvious anodic peaks at a potential of 2.54, 3.23, and 3.43 V are observed in the following anodic scan, indicating the lithium-ion extraction process in the cathode materials [38]. The following third and fifth CV curves also display similar cathodic and anodic peaks. However, the current intensities for the third and fifth CV curves are smaller than those of the second cycle, which indicates the capacity decreases with the increasing cycle. The similar cathodic and anodic characteristic peaks are observed in the V2O5-30CNT and V2O5-60CNT. It is worth noting that the current intensities for the composites are higher than those of V2O5 electrode at the same cycling number, suggesting that addition of CNTs can benefit to enhance the capacity during electrochemical cycle. Figure 5 presents the charge/discharge profiles of the samples in the second, third, and fifth cycles at a current density of 100 mA g−1. In agreement with the CV results described above, the charge/discharge curves for all the samples display multiple redox plateaus between 2–4 V, indicating vanadium oxide multi-step phase transition from α-V2O5 to ε-Li0.5V2O5 and finally to the γ-Li2V2O5.The second discharge capacities of the V2O5, V2O5-30CNT, and V2O5-60CNT are 262.8, 295 and 305.2 mA h g−1, respectively. Moreover, the capacity performance of the three materials follows the order of V2O5-60CNT > V2O5-30CNT > V2O5 at the same cycling number.
Figure 6a shows the cycling performance of the samples at a current density of 100 mA g−1 between 2–4 V. A discharge capacity of 196.8 mA h g−1 can be delivered for V2O5-60CNT after 50 cycles at a current density of 100 mA g−1, which corresponds to 64.5% of the initial discharge capacity. The discharge capacities of V2O5 and V2O5-30CNT can be reached at 171 and 167.4 mA h g−1, corresponding to the capacity retention of 65 and 56.7%, respectively. It can be seen that V2O5-60CNT has higher capacity than those of V2O5 and V2O5-30CNT, which can be attributed to addition of appropriate amount of CNTs. The rate capability of the samples was evaluated at various current densities within the potential window of 2–4 V, as shown in Fig. 6b. Under the same rate conditions, V2O5/CNTs present a higher capacity than V2O5. V2O5, V2O5-30CNT, and V2O5-60CNT can deliver discharge capacities of 55, 69, and 69.5 mA h g−1 at a rate of 10 C (1000 mA g−1), respectively. After the high-rate cycling, a capacity of 236 mA h g−1 can be obtained for V2O5-60CNT electrode when the current density is returned to 1 C (100 mA g−1). V2O5-30CNT and V2O5 can deliver capacities of 195 and 156 mA h g−1 under the same conditions, respectively. Therefore, the V2O5-60CNT electrode exhibits good rate capability and capacity performance. The influence of CNTs on the rate capabilities is further verified by comparing EIS results of the samples (Fig. 6c). The plots display a depressed semicircle in the high-frequency region and a sloped line in the low-frequency region, corresponding to the charge transfer resistance (Rct) at the electrode/electrolyte interface and the Warburg impedance associated with lithium-ions diffusion in the cathode materials, respectively [39]. The smaller the diameter of the semicircle is, the smaller the Rct will be. A lower Rct induces Li+ ion and electron to transfer more quickly, resulting in the enhancements in electrode reaction kinetics. What we can see from the plots is that the Rct of V2O5,V2O5-30CNT, and V2O5-60CNT materials follows the order of V2O5-60CNT ˂ V2O5-30CNT ˂ V2O5 after cycling. The reduced Rct at the electrolyte/electrode interface results from the addition of CNTs. The improved electrochemical performance of the V2O5-60CNT can be attributed to the fact that addition of CNTs can raise the conductivity and benefit the Li+ ion intercalation and deintercalation process.
4 Conclusion
The V2O5/CNT composites were synthesized by a facile hydrothermal method. V2O5-60CNT can deliver a good capacity of 196.8 mA h g−1 at a current density of 100 mA g−1 after 50 cycles. The electrode materials free of CNTs (V2O5) can reach a capacity of 171 mA h g−1 at the same conditions. Moreover, V2O5/CNTs present a higher capacity than that of V2O5 under the same rate conditions. The improved electrochemical performance can be attributed to the fact that the CNTs in the V2O5/CNT composites can effectively facilitate ionic diffusion by raising the electrical conductivity.
References
Goriparti S, Miele E, De Angelis F, Di Fabrizio E, Proietti Zaccaria R, Capiglia C (2014) Review on recent progress of nanostructured anode materials for Li-ion batteries. J Power Sources 257:421–443
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195(4):939–954
Tang Y, Zhang Y, Deng J, Qi D, Leow WR, Wei J, Yin S, Dong Z, Yazami R, Chen Z, Chen X (2014) Unravelling the correlation between the aspect ratio of nanotubular structures and their electrochemical performance to achieve high-rate and long-life lithium-ion batteries. Angew Chem Int Ed 53(49):13488–13492
Qie L, Chen W-M, Wang Z-H, Shao Q-G, Li X, Yuan L-X, Hu X-L, Zhang W-X, Huang Y-H (2012) Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv Mater 24(15):2047–2050
Kim DK, Muralidharan P, Lee H-W, Ruffo R, Yang Y, Chan CK, Peng H, Huggins RA, Cui Y (2008) Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett 8(11):3948–3952
Wang Y, Wang Y, Hosono E, Wang K, Zhou H (2008) The design of a LiFePO4/Carbon nanocomposite with a core–shell structure and its synthesis by an in situ polymerization restriction method. Angew Chem Int Ed 47(39):7461–7465
Wang S, Li S, Sun Y, Feng X, Chen C (2011) Three-dimensional porous V2O5 cathode with ultra high rate capability. Energy Environ Sci 4(8):2854–2857
Pandey GP, Liu T, Brown E, Yang Y, Li Y, Sun XS, Fang Y, Li J (2016) Mesoporous hybrids of reduced graphene oxide and vanadium pentoxide for enhanced performance in lithium-ion batteries and electrochemical capacitors. ACS Appl Mater Interfaces 8(14):9200–9210
Muster J, Kim GT, Krstić V, Park JG, Park YW, Roth S, Burghard M (2000) Electrical transport through individual vanadium pentoxide nanowires. Adv Mater 12(6):420–424
Wang Y, Takahashi K, Lee KH, Cao GZ (2006) Nanostructured vanadium oxide electrodes for enhanced lithium-ion intercalation. Adv Funct Mater 16(9):1133–1144
Singhal A, Skandan G, Amatucci G, Badway F, Ye N, Manthiram A, Ye H, Xu JJ (2004) Nanostructured electrodes for next generation rechargeable electrochemical devices. J Power Sources 129(1):38–44
Pan AQ, Wu HB, Zhang L, Lou XW (2013) Uniform V2O5 nanosheet-assembled hollow microflowers with excellent lithium storage properties. Energy Environ Sci 6(5):1476–1479
Wang S, Lu Z, Wang D, Li C, Chen C, Yin Y (2011) Porous monodisperse V2O5 microspheres as cathode materials for lithium-ion batteries. J Mater Chem 21(17):6365–6369
Mai L, Xu L, Han C, Xu X, Luo Y, Zhao S, Zhao Y (2010) Electrospun ultralong hierarchical vanadium oxide nanowires with high performance for lithium ion batteries. Nano Lett 10(11):4750–4755
Zhang L, Zhao K, Xu W, Meng J, He L, An Q, Xu X, Luo Y, Zhao T, Mai L (2014) Mesoporous VO2 nanowires with excellent cycling stability and enhanced rate capability for lithium batteries. RSC Adv 4(63):33332–33337
An Q, Wei Q, Mai L, Fei J, Xu X, Zhao Y, Yan M, Zhang P, Huang S (2013) Supercritically exfoliated ultrathin vanadium pentoxide nanosheets with high rate capability for lithium batteries. Phys Chem Chem Phys 15(39):16828–16833
Liang S, Hu Y, Nie Z, Huang H, Chen T, Pan A, Cao G (2015) Template-free synthesis of ultra-large V2O5 nanosheets with exceptional small thickness for high-performance lithium-ion batteries. Nano Energy 13:58–66
Li G, Qiu Y, Hou Y, Li H, Zhou L, Deng H, Zhang Y (2015) Synthesis of V2O5 hierarchical structures for long cycle-life lithium-ion storage. J Mater Chem A 3(3):1103–1109
Tang Y, Rui X, Zhang Y, Lim TM, Dong Z, Hng HH, Chen X, Yan Q, Chen Z (2013) Vanadium pentoxide cathode materials for high-performance lithium-ion batteries enabled by a hierarchical nanoflower structure via an electrochemical process. J Mater Chem A 1(1):82–88
Xu Y, Dunwell M, Fei L, Fu E, Lin Q, Patterson B, Yuan B, Deng S, Andersen P, Luo H, Zou G (2014) Two-dimensional V2O5 sheet network as electrode for lithium-ion batteries. ACS Appl Mater Interfaces 6(22):20408–20413
Rui X, Zhu J, Sim D, Xu C, Zeng Y, Hng HH, Lim TM, Yan Q (2011) Reduced graphene oxide supported highly porous V2O5 spheres as a high-power cathode material for lithium ion batteries. Nanoscale 3(11):4752–4758
Sathiya M, Prakash A, Ramesha K, Tarascon JM, Shukla A (2011) V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J Am Chem Soc 133(40):16291–16299
Likodimos V, Steriotis TA, Papageorgiou SK, Romanos GE, Marques RRN, Rocha RP, Faria JL, Pereira MFR, Figueiredo JL, Silva AMT, Falaras P (2014) Controlled surface functionalization of multiwall carbon nanotubes by HNO3 hydrothermal oxidation. Carbon 69:311–326
Ding S, Chen JS, Lou XW (2011) One-Dimensional hierarchical structures composed of novel metal oxide nanosheets on a carbon nanotube backbone and their lithium-storage properties. Adv Funct Mater 21(21):4120–4125
Pham DT, Lee TH, Luong DH, Yao F, Ghosh A, Le VT, Kim TH, Li B, Chang J, Lee YH (2015) Carbon nanotube-bridged graphene 3D building blocks for ultrafast compact supercapacitors. ACS Nano 9(2):2018–2027
Annamalai KP, Gao J, Liu L, Mei J, Lau W, Tao Y (2015) Nanoporous graphene/single wall carbon nanohorn heterostructures with enhanced capacitance. J Mater Chem A 3(22):11740–11744
Ming J, Wu Y, Yu Y, Zhao F (2011) Steaming multiwalled carbon nanotubesvia acid vapour for controllable nanoengineering and the fabrication of carbon nanoflutes. Chem Commun 47(18):5223–5225
Liang X, Gao G, Wu G, Yang H (2016) Synthesis and characterization of novel hierarchical starfish-like vanadium oxide and their electrochemical performance. Electrochim Acta 188:625–635
Wu Y, Gao G, Wu G (2015) Self-assembled three-dimensional hierarchical porous V2O5/graphene hybrid aerogels for supercapacitors with high energy density and long cycle life. J Mater Chem A 3(5):1828–1832
Wang L, Zhuo L, Cheng H, Zhang C, Zhao F (2015) Porous carbon nanotubes decorated with nanosized cobalt ferrite as anode materials for high-performance lithium-ion batteries. J Power Sources 283(0):289–299
Becerril HA, Mao J, Liu Z, Stoltenberg RM, Bao Z, Chen Y (2008) Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2(3):463–470
Zhuo L, Wu Y, Ming J, Wang L, Yu Y, Zhang X, Zhao F (2013) Facile synthesis of a Co3O4-carbon nanotube composite and its superior performance as an anode material for Li-ion batteries. J Mater Chem A 1(4):1141–1147
Fang W-C (2008) Synthesis and electrochemical characterization of vanadium Oxide/Carbon nanotube composites for supercapacitors. J Phys Chem C 112(30):11552–11555
Kim T, Shin J, You T-S, Lee H, Kim J (2015) Thermally controlled V2O5 nanoparticles as cathode materials for lithium-ion batteries with enhanced rate capability. Electrochim Acta 164(0):227–234
Wei Q, Jiang Z, Tan S, Li Q, Huang L, Yan M, Zhou L, An Q, Mai L (2015) Lattice breathing inhibited layered vanadium oxide ultrathin nanobelts for enhanced sodium storage. ACS Appl Mater Interfaces 7(33):18211–18217
Wu Y, Gao G, Yang H, Bi W, Liang X, Zhang Y, Zhang G, Wu G (2015) Controlled synthesis of V2O5/MWCNT core/shell hybrid aerogels through a mixed growth and self-assembly methodology for supercapacitors with high capacitance and ultralong cycle life. J Mater Chem A 3(30):15692–15699
Pan A, Zhang J-G, Nie Z, Cao G, Arey BW, Li G, Liang S-q, Liu J (2010) Facile synthesized nanorod structured vanadium pentoxide for high-rate lithium batteries. J Mater Chem 20(41):9193–9199
Zhou X, Wu G, Wu J, Yang H, Wang J, Gao G (2014) Carbon black anchored vanadium oxide nanobelts and their post-sintering counterpart (V2O5 nanobelts) as high performance cathode materials for lithium ion batteries. Phys Chem Chem Phys 16(9):3973–3982
Chen L, Gu X, Jiang X, Wang N, Yue J, Xu H, Yang J, Qian Y (2014) Hierarchical vanadium pentoxide microflowers with excellent long-term cyclability at high rates for lithium ion batteries. J Power Sources 272(0):991–996
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (U1503292, 51472182) and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Liang, X., Gao, G., Liu, Y. et al. Carbon nanotubes/vanadium oxide composites as cathode materials for lithium-ion batteries. J Sol-Gel Sci Technol 82, 224–232 (2017). https://doi.org/10.1007/s10971-016-4293-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4293-8