Abstract
CaAlSi mixed metal oxide nanoparticles with formula Ca2Al2SiO7 and CaAl2O4 (with molar ratio of 70:30) was prepared by sol–gel method and used as a basic heterogeneous catalyst for transesterification of soybean oil with methanol to methyl esters (biodiesel). The catalyst was characterized using X-ray diffraction, scanning electron microscope, Fouier Transform Infrared, and Termal Gravimetric Analysis techniques. Effect of important reaction parameters such as methanol to oil molar ratio, reaction time, and amount of catalyst were examined. It was found that reaction of methanol and soybean oil with the molar ratio of 30 in the presence of 6 % catalyst (based on soybean oil weight) affords biodiesel almost quantitatively at 60 °C within 6 h. The catalyst can be easily recovered and reused for four cycles without significant loosing activity.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Energy is a basic requisite for economic development in countries. As a result, energy importance has been increasing along with the growth of human population and industrialization. Common sources of energy are petroleum, natural gas, and coal obtained from fossil fuels. Seriously fossil energy resources reducing and increasing global environmental concerns increasing petroleum such as plant biomass [1–6]. Compared to other fuels among many possible sources, biodiesel as a clean renewable fuel has recently been recognized as the best candidate for a diesel fuel substitution because it can be used in any compression ignition engine without the need for modification. Transesterification is a method for production of fatty acid methyl esters (FAME) used as biodiesel, as nontoxic, biodegradable and renewable fuel, essentially free of sulfur and aromatics with proper viscosity, high flash point, and high octane number makes it a cleaner burning fuel than petroleum. It is also considered as the best alternative fuel readily used in diesel engines [7–11]. Vegetable oils and animal fats cannot be used for fuel application due to undesirable properties such as high viscosity, poor volatility undergo atomization and polymerization during combustion leading to fuel line filter chamber clogging. Therefore, the increasing demand in the world for a clean fuel has encouraged the scientists to search for alternative renewable energy [11]. One of these alternative fuels is biodiesel. In biodiesel synthesis, methanol is mostly used as alcohol because of its low cost and ability to produce alkyl esters with lower viscosity.
Transesterification has traditionally been catalyzed by acids, bases, or carried out in the presence of enzyme catalysts [12–17]. Commercial production of biodiesel employs homogeneous alkali catalyst such as KOH or NaOH [18]. The use of homogeneous alkali catalyst has some difficulties such as separation of catalyst, formation of soap, and formation of emulsifier between FAME and glycerol [19, 20]. In order to minimize these problems, attempts have been made to use heterogenized catalyst systems in biodiesel production. Utilization of solid catalysts offers several advantages such as easy catalyst separation, mild reaction conditions, and no toxicity. Therefore, many efforts have been devoted toward design of heterogeneous catalytic systems for transesterification of vegetable oils [21–31]. Examples include impregnation alkaline metal salts on microporous or mesoporous materials such as zeolite [29–31] or MCM-41 [32], layered silicate such as montmorilonite [33], alkaline metal oxides [34, 35], and inorganic mixed oxides [36–45].
In this presentation, preparation of a new nanoparticles with Ca mixed oxides along with its usage as heterogeneous catalyst for transesterification of soybean oil with methanol to methyl esters (biodiesel) production is described.
2 Materials and methods
Soybean oil was obtained from sigma. Its properties are given in Table S1. Si(OC2H5)4, Ca(NO3)2.4H2O, Al(NO3)3.9H2O, citric acid, polyethylene glycol 20000 (PEG), methanol, ethanol, n-hexane, ammunia (25 %), and methyl heptadecanoate (>99 wt.%) as the standard of Gas Chromatography (GC) were purchased from Merck Chemical Company (Darmstadt, Germany) and used without further purification.
Fouier Transform Infrared (FTIR) spectra of the samples (5 mg) were collected on a Bruker (Tensor 27) instrument using KBr (100 mg) pellet in the range of 4000–400 cm−1 under the atmospheric condition. The chemical analysis was carried out with atomic absorption Chermo double beam instrument. The X-ray diffraction (XRD) patterns of samples were recorded on a Philips PW1800 diffractometer using monochromatic Nickelfilter with Cu Kα radiation (l = 1.5405 Å). The X-ray generator was run at 40 kV and 30 mA and the diffractograms were recorded in the 2θ range of 4°–90°. The phases were identified using the powder diffraction file (PDF) database (JCPDS, International Center for Diffraction Data). The adsorption–desorption isotherms and other physical properties such as surface area, pore volume, and so on were determined by Brunauer-Emmett-Teller (BET), BELSORP Mini from MicroTrac Bel Corp. Products were analyzed by GC and Gas Chromatography-Mass (GC-Mass) using Agilent 6890 series with a Flame Ionization Detector (FID) detector, HP-5, 5 % phenylmethylsiloxane capillary and helium as carrier gas, and Agilent 5973 mass selective detector, HP-5 MS 6989 network GC system. The scanning electron microscope (SEM) was performed on KYKY-EM3200 operating at an accelerated voltage of 25 kV.
2.1 Preparation of CaAlSi mixed oxide nanoparticles catalyst
The sol–gel method was used for the preparation of CaAlSi mixed oxide designates as CASO nanoparticles. In a typical procedure, stoichiometric amounts of calcium nitrate tetrahydrate (2.36 g), aluminum nitrate nonahydrate (2.47 g), and tetraethoxysilane (2.06 g) were dissolved in 10 mL of water/ethanol (v/v 2:1) under vigorous stirring. Then, certain amount of citric acid solution in water (citric acid/M2+ = 2:1 molar ratio) and PEG (0.1 g/mL) were added into the above solution, while the solution pH was maintained at 8–9 by using ammonia solution (25 %). The resultant mixture was stirred for 3 h and heated at 90 °C in a water bath until homogeneous gel is formed. After drying, it was calcined at 900 °C for 5 h to afford the final sample as a white solid.
2.2 Transesterification of soybean oil, general procedure
Catalytic transesterification of soybean oil with methanol having average molecular weight of 881 g/mol calculated from the saponification value (S.V. = 190 mg KOH/g) [27] was carried out in a 50 mL high pressure autoclave. Soybean oil (5 ml), methanol (with the methanol/oil molar ratio of 6:1 upto 30:1), and catalyst (CASO nanoparticles, 1–6 wt.%) were loaded in the autoclave. The mixture was heated at 60 °C for 1–8 h while stirring at 300 rpm. The solid was separated by means of centrifuge and washed with CH2Cl2 and methanol. After evaporation of solvent under reduced pressure at 60 °C, the two layers containing methyl esters, soybean oil, mono and diglycerides in the upper and glycerol in the lower phases were separated by a decanter. The FAME content was determined by GC using the European regulated procedure EN14103 [44]. The separated catalyst was reused in fresh reactions.
3 Results and discussion
3.1 XRD studies
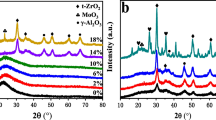
The XRD patterns of the CaAlSi mixed oxide nanoparticles are shown in Fig. 1. As can be seen, it contains Ca2Al2SiO7 (JCPDS PDF No. 35-0755) coexisting with CaAl2O4 (JCPDS PDF No. 34-0440) as the major and minor phases, respectively. The XRD pattern of the Ca2Al2SiO7 shows the d values at 3.70, 3.060, 2.8440, 2.4300, 2.3940, 2.0398, 1.754, and 1.3738 corresponding to 111, 201, 211, 310, 221, 212, 312, and 521 reflections, and the d values of CaAl2O4 appears at 2.97, 2.52, and 2.41 corresponding to 220, 303, and 313 planes.
3.2 FTIR analysis
In order to obtain more information about CaAlSi mixed oxide nanoparticles, FTIR spectrum of the sample was recorded in order to study the vibration modes displayed by different species on the catalyst surface (Figure S1). A broad peak appeared in the spectrum at 3448 and 1628 cm−1 is due to the stretching and bending vibration of the –OH. The bands observed at 1135 and 963 cm−1 are attributed to the antisymmetric and symmetric vibrations of TO4 (T: Si, Al). Whereas the bands displayed at 800–650 cm−1 are due to the T–O–T′ stretching and bending vibrations [36], those resonated at 542 cm−1 may be assigned to the Ca–O stretching [36].
3.3 SEM and elemental analysis
The SEM of the CaAlSi mixed oxide nanoparticles reveals a uniform morphology of spherical shape with 48–54 nm particle size (Fig. 2). Moreover, elemental EDS analysis of CaAlSi mixed oxide nanoparticles (Fig. 3) shows the presence Ca, Al, Si, and O. The composition of the prepared CaAlSi mixed oxide nanoparticles obtained by atomic absorption determined the Ca, Al, and Si % as 25.32, 9.45, and 19.09 %, respectively [45].
3.4 TGA/DTA studies
The TGA and Differential Termal Analysis (DTA) curves of CaAlSi mixed oxide nanoparticles are shown in Fig. 4. As indicated in the TGA curve, constant mass is achieved at above 700 °C after losing 62.6 % of the original mass. The first major weight loss of 36 % occurring at 210 °C is an exothermic peak due to the dehydroxylation and loosing of organic compounds. The other exothermic weight loose should be due to the decomposition of the remaining organic compounds.
3.5 Adsorption–desorption isotherms studies
Adsorption–desorption isotherms of nitrogen measured on CaAlSi mixed oxide nanoparticles results are shown in Fig. 5. Some physical properties data obtained such as V m, as(BET), total pore volume, and mean pore diameter are 12.011 cm3 (STP)g1, 52.276 m2 g−1, 0.3197 cm3 g−1, and 24.464 nm, respectively (Table S2).
3.6 Transesterification reaction parameters
Biodiesel is composed of methyl or ethyl esters of fatty acids produced through transesterification or alcoholysis of different lipid sources with methanol or ethanol in the presence of base, acid, or enzyme catalysts (Scheme 1). Transesterification is necessary to improve the properties of the biofuel because vegetable oils and animal fats have high kinematic viscosity and low volatility, which makes them inappropriate to be directly used as fuel in modern engines.
3.7 Optimization of biodiesel yield
In order to optimize the biodiesel yield, the effect of the amount of catalyst, reaction time, and within the range of 1–6 wt.% (based on the soybean oil weight) and methanol/oil ratios on the transesterification reactions were investigated. Initially, the effect of the amount of catalyst using methanol with the molar ratio of 30:1 under reflux condition within 6 h was studied (Fig. 6). As seen, there is a direct relation between the oil conversion and the amount of catalyst. Significantly, transesterification reaction proceeded with 82 % oil conversion together with the maximum formation of biodiesel in the presence of 6 % catalyst.
On the other hand, it was found that whereas 82 % oil conversion occurs within 6 h, reaction proceeds almost quantitatively by increasing the reaction time to the 8 h (Fig. 7).
The stoichiometry of transesterification reaction requires 3 mol of methanol per 1 mol of triglyceride to obtain biodiesel and glycerol with the ratio of 3:1. Elevation of the ratio by introducing an excess amount of methanol shifts the equilibrium toward the product side (Fig. 8). As indicated, maximum of biodiesel yield is obtained by using the molar ratio of 30:1 in the presence of 6 % catalyst within 6 h. Rate acceleration results by decreasing the soybean oil viscosity coefficient via methanol dilution and promotion of mass transfer. However, utilization of methanol in high excess is not favorable due to higher energy consumption (Fig. 8).
3.8 Catalyst recycling studies and stability
In order to ascertain whether the prepared CaAlSi mixed oxide nanoparticles behaves as solid catalyst in a truly heterogeneous manner, the nanoparticles were recovered by separation after the completion of the first run by centrifugation followed by using it again for successive batch reaction at 65 °C under optimum condition. A slight decrease in catalytic activity from 95 to 94 %, 92, and 91 % in the second, third, and fourth runs, respectively Fig. 9, was found. The similarity of FTIR and XRD patterns of CaAlSi mixed oxide nanoparticles observed before and after each reaction run confirmed the heterogeneity of the reaction catalyst. On the other hand, neither significant amount of Ca, Al, or Si nor catalytic activity was observed in the filtrate under reaction conditions.
The fuel properties of the prepared biodiesel at the optimal process conditions presented in Table 1 were found to comply with ASTM and EN standards. As seen, the produced biodiesel using CaAlSi mixed oxide nanoparticles as catalyst meets the ASTM and EN limits.
3.9 Reaction mechanism
Although the precise mechanism is not known, a mechanistic postulate as shown in Scheme 2 may be invoked to rationalize the formation of biodiesel. Upon treatment of the O2− on the surface of CaAlSi mixed oxide nanoparticles as catalyst with methanol, H+ abstraction occurs followed by the formation of I1 as shown in Scheme 2. In the next step, the triglyceride ester S undergoes attacks of I1 perhaps via methoxide anion, affording the tetrahedral intermediate T1 and CaAlSi mixed oxide nanoparticles (CASOH+). The subsequent acid–base reaction of T1 with CASOH+ regenerates CASO with concomitant formation of tetrahedral intermediate T2. Rapid conversion of T2 affords T3 and finally biodiesel as the reaction product.
4 Conclusion
In this study, CaAlSi mixed oxide nanoparticles (CASO) were prepared by sol–gel method followed by calcination upto 900 °C. The CaAlSi mixed oxide nanoparticles (CASO) were characterized by chemical analysis, XRD, SEM, EDX, TGA, and FTIR techniques. It was found that CASO successfully catalyze transesterification of soybean oil with methanol to biodiesel in 95 % yield within 8 h. The heterogeneity character and reusability of the CaAlSi mixed oxide nanoparticles were also investigated.
References
Sharma YC, Singh B, Upadhyay SN (2008) Advancements in development and characterization of biodiesel: a review. Fuel 87:2355–2373
Marchetti JM, Miguel VU, Errazu AF (2007) Possible methods for biodiesel production. Renew Sustain Energ Rev 11:1300–1311
Sharma YC, Singh B, Korstad J (2011) Latest developments on application of heterogenous basic catalysts for an efficient and ecofriendly synthesis of biodiesel: a review. Fuel 90:1309–1324
Lin L, Cunshan Z, Vittayapadung S, Xiangqian S, Mingdong D (2011) Opportunities and challenges for biodiesel fuel. Appl Energ 88:1020–1031
Leung DYC, Wu X, Leung MKH (2010) A review on biodiesel production using catalyzed transesterification. Appl Energ 87:1083–1095
Yusuf NNAN, Kamarudin SK, Yaakub Z (2011) Overview on the current trends in biodiesel production. Energ Convers Manage 52:2741–2751
Lee HV, Taufiq-Yap YH, Hussein MZ, Yunus R (2013) Transesterification of jatropha oil with methanol over Mg-Zn mixed metal oxide catalysts. Energy 49:12–18
Dossin TF, Reyniers MF, Berger RJ, Marin GB (2006) Simulation of heterogeneously MgO catalyzed transesterification for fine-chemical and biodiesel industrial production. Appl Catal B: Environ 67:136–148
Asri NP, Machmudah S, Budikarjono K, Roesyadi A, Goto M (2013) Palm oil transesterification in sub- and supercritical methanol with heterogeneous base catalyst. Chem Eng Process 72:63–67
Rashtizadeh E, Farzaneh F, Talebpour Z (2014) Synthesis and characterization of Sr3Al2O6 nanocomposite as catalyst for biodiesel production. Bioresour Technol 154:32–37
Lee AF, Bennett JA, Manayil JC, Wilson K (2014) Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem Soc Rev 43:7887–7916
Liu Y, Lotero E, Goodwin Jr JG, Mo X (2007) Transesterification of poultry fat with methanol using Mg-Al hydrotalcite derived catalysts. Appl Catal A: Gen 331:138–148
Lotero E, Liu Y, Lopez DE, Suwannakarn K, Bruce DA, Goodwin Jr JG (2005) Synthesis of biodiesel via acid catalyst. Ind Eng Chem Res 44:5353–5363
Hayyan A, Alam MZ, Mirghani MES, Kabbashi NA, Hakimi NINM, Siran YM, Tahiruddin S (2011) Reduction of high content of free fatty acid in sludge palm oil via acid catalyst for biodiesel production. Fuel Process Technol 92:920–924
Wang X, Liu X, Zhao C, Ding Y, Xu P (2011) Biodiesel production in packed-bed reactors using lipase–nanoparticle biocomposite. Bioresour Technol 102:6352–6355
Marchetti JM, Errazu AF (2008) Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides. Biomass Bioenerg 32:892–895
Demirbas A (2008) Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energ Convers Manage 49:125–130
Kazemian H, Turowec B, Siddiquee MN, Rohani S (2013) Biodiesel production using cesium modified mesoporous ordered silica as heterogeneous basecatalyst. Fuel 103:719–724
Meng YL, Wang BY, Li SF, Tian SJ, Zhang MH (2013) Effect of calcinationtemperature on the activity of solid Ca/Al composite oxide-based alkalinecatalyst for biodiesel production. Bioresour Technol 128:305–309
Gomes JFP, Puna JFB, Goncalves LM, Bordado JCM (2011) Study on the use of Mg-Al hydrotalcites as solid heterogeneous catalysts for biodiesel production. Energy 36:6770–6778
Lam MK, Lee KT, Mohamed AR (2010) Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol Adv 28:500–518
Serio MD, Tesser R, Pengmei L, Santacesaria E (2008) Heterogeneous catalysts for biodiesel production. Energ Fuel 22:207–217
Helwani Z, Othman MR, Aziz N, Kim J, Fernando WJN (2009) Solid heterogeneous catalysts for transesterification of triglycerides with methanol: a review. Appl Catal A: Gen 363:1–10
Zabeti M, Daud WMAW, Aroua MK (2009) Activity of solid catalysts for biodiesel production: a review. Fuel Process Technol 90:770–777
Kawashima A, Matsubara K, Honda K (2009) Acceleration of catalytic activity of calcium oxide for biodiesel production. Bioresour Technol 100:696–700
Bournay L, Casanave D, Delfort B, Hillion G, Chodorge JA (2005) New heterogeneous process for biodiesel production: a way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal Today 106:190–192
Kawashima A, Matsubara K, Honda K (2008) Development of heterogeneous base catalysts for biodiesel production. Bioresour Technol 99:3439–3443
Li E, Rudolph V (2008) Transesterification of vegetable oil to biodiesel over MgO-functionalized mesoporous catalysts. Energ Fuel 22:145–149
Brito A, Borges ME, Otero N (2007) Zeolite Y as a heterogeneous catalyst in biodiesel fuel production from used vegetable oil. Energ Fuel 21:3280–3283
Nozue Y, Amako Y, Kawano R, Mizukane T, Nakano T (2012) Insulating state and metallic phase transition of heavily sodium-doped low-silica X (LSX) zeolites. J Phys Chem Solids 73:1538–1541
Sara S, Vieira S, Magriotis ZM, Santos NAV, Saczk AA, Carla E, Hori CE, Arroyo PA (2013) Biodiesel production by free fatty acid esterification using lanthanum (La3+) and HZSM-5 based catalysts. Bioresour Technol 133:248–255
Lin MCY, Cheng HH (2012) Application of mesoporous catalysts over palm-oil biodiesel for adjusting fuel properties. Energ Convers Manage 53:128–134
Rashtizadeh E, Farzaneh F, Ghandi M (2010) A comparative study of KOH loaded on double aluminosilicate layers, microporous and mesoporous materials ascatalyst for biodiesel production via transesterification of soybean oil. Fuel 89:3393–3398
Kouzu M, Hidaka JS (2012) Transesterification of vegetable oil into biodiesel catalyzed by CaO: a reviw. Fuel 93:1–12
Mootabad Hi, Salamatinia B, Bhatia S, Abdullah AZ (2010) Ultrasonic assisted biodiesel production process from palm oil using alkaline earth metal oxides as the heterogeneous catalysts.. Fuel 89:1818–1825
Feyzia M, Shahbazi E (2015) Catalytic performance and characterization of Cs–Ca/SiO2–TiO2 nanocatalysts for biodiesel. J Mol Catal A: Chem 404:131–138
Ngamcharussrivichai C, Totarat P, Bunyakiat K (2008) Ca and Zn mixed oxide as a heterogeneous base catalyst for transesterification of palm kernel oil. Appl Catal A: Gen 341:77–85
Lukic I, Kesic Z, Svetolik Maksimovic S, Zdujic M, Liu H, Krstic J, Skala D (2013) Kinetics of sunflower and used vegetable oil methanolysis catalyzed by CaO_ZnO. Fuel 113:367–378
Refaat AA (2011) Biodiesel production using solid metal oxide catalysts. Int J Environ Sci Tech 8(1):203–221
Meng YL, Tian SJ, Li SF, Wang BY, Zhang MH (2013) Transesterification of rapeseed oil for biodiesel production in trickle-bed reactors packed with heterogeneous Ca/Al composite oxide-based alkaline catalyst. Bioresour Technol 136:730–734
Tantirungrotechai J, Thepwatee S, Yoosuk B (2013) Biodiesel synthesis ove Sr/MgO solid base catalyst. Fuel 106:279–284
Su M, Yang R, Li M (2013) Biodiesel production from hempseed oil using alkaline earth metal oxides supporting copper oxide as bi-functional catalysts for transesterification and selective hydrogenation. Fuel 103:398–407
Mierczynski P, Chalupka KA, Maniukiewicz W, Kubicki J, Szynkowska MI, Maniecki TP (2015) SrAl2O4 spinel phase as active phase of transesterification of rapeseed oil. Appl Catal B: Environ 164:176–183
Granados ML, Zafra Poves MD, Alonso DM, Mariscal R, Galisteo FC, Moreno-Tost R (2007) Biodiese from sunflower oil by using activated calcium oxide. Appl Catal B Environ 73:317–326
Rashtizadeh E, Farzaneh F (2013) Transesterification of soybean oil catalyzed by Sr–Ti mixed oxides nanocomposite. J Taiwan Inst Chem Engin 44:917–923
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Farzaneh, F., Dashtipour, B. & Rashtizadeh, E. Transesterification of soybean oil for biodiesel production over CaAlSi mixed oxide nanoparticles. J Sol-Gel Sci Technol 81, 859–866 (2017). https://doi.org/10.1007/s10971-016-4253-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4253-3