Abstract
Hf0.5Zr0.5O2 thin films were prepared on silicon substrates by sol–gel method. The crystallization temperature, thickness, density, surface morphology, crystalline structure, and chemical bonding features of the films were investigated using TGA, DSC, XRR, AFM, GIXRD, and XPS techniques. The results showed that the crystallization temperature was 496 °C, the film surfaces were smooth and flat, and no pores and micro-cracks were discernable. The density increased significantly from 5.1 to 8.0 g/cm3 after annealing at 700 °C. The crystalline structure depends strongly on the film thickness. The tetragonal phase could be stabilized in Hf0.5Zr0.5O2 films thinner than 12.9 nm. An increase in the thickness led to a gradual appearance of the monoclinic phase, which ultimately became the dominant phase for films thicker than 46.1 nm. The results could be explained by the surface energy effect. Measured at 1 MV/cm, the leakage current density was about 3.5 × 10−6 A/cm2, further indicating high quality of the thin films derived from aqueous solution precursor.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The transition metal oxides HfO2- and ZrO2-based high-permittivity (high-k) dielectric materials have been intensively studied and practically implemented in microelectronics [1, 2]. Pure HfO2 transforms from monoclinic into tetragonal and cubic structures as temperature increases at ambient pressure. The application of HfO2 with monoclinic phase has been limited since its dielectric constant is significantly lower than those of the cubic or tetragonal phases. Therefore, making the high-permittivity phase to be stable at room temperature would be highly desirable. Efforts have been made to improve the properties of HfO2 by adding different elements such as N, Si, Al, and La [3–6]. Addition of these elements helps to increase the crystallization temperature of HfO2 and stability of the tetragonal or cubic phases, but sacrifices the k value due to the low permittivity of the dopants. ZrO2 and HfO2 have very similar crystal chemistry, and they are completely miscible. It has been shown that adding ZrO2 into HfO2 could stabilize the atomic layer deposition (ALD) process at high temperatures without lowering the dielectric constant, which was attributed to an increase in the tetragonal phase fraction in the crystalline films [7].
Pure and doped HfO2 thin films can be prepared by various advanced vapor methods: atomic layer deposition [8, 9], chemical vapor deposition [10–12], and physical vapor deposition [13, 14]. In contrast to these techniques requiring sophisticated vacuum system, deposition of thin films by sol–gel method can enable the ease of composition control, high purity, large area coverage, as well as low cost. These advantages are especially preferred in fundamental study of material properties. Recently, Jiang et al. [15] reported a unique solution approach to depositing high-quality pure HfO2 thin films using inorganic aqueous precursors. In comparison with conventional sol–gel routes using organic precursors [16–18], the coating can simply be operated in atmosphere condition, and especially, the very small size of sol particles (no larger than 2 nm) allow controllable growth of ultra-thin nano-films. In this paper, we extended this approach to preparing pure ZrO2 and Hf0.5Zr0.5O2 thin films, and the thickness-dependent phase evolution and dielectric properties have been investigated in detail.
2 Experimental
2.1 Precursor and film preparation
HfOCl2·8H2O (0.0048 mol) and ZrOCl2·8H2O (0.0048 mol) were dissolved in H2O (40 mL) to ensure the Hf4+:Zr4+ molar ratio of 1:1, followed by the addition of ammonia (0.0268 mol) to precipitate. The precipitate acquired was centrifuged and then washed with deionized water to remove Cl− and ammonia. Finally, H2O2 (0.2 mol) and HNO3 (0.0112 mol) were added into the precipitate and stirred to obtain a clear precursor solution. Hf0.5Zr0.5O2 films were prepared by spin-coating the solution on 2 × 2 cm2 p-type (100) silicon substrates with a low resistivity of 10−2–10−3 Ω cm. All substrates received a modified RCA cleaning process with the native oxide layer being removed as the last step using diluted HF solution. After drying, the substrate surface was treated in air plasma for 10 min to make the contact angle of the solution of about 3° [19]. The solution was spin-coated at 3000 rpm for 30 s, followed by an immediate hot-plate polymerization at 150 °C for 30 s. Before the next coating, a 3-min air plasma treatment was carried out to improve the wettability of the film surface. This procedure was repeated until the desired thickness was obtained. Crystallization of the films was performed by 30-s rapid thermal annealing at 700 °C in a N2 atmosphere.
2.2 Structural and chemical characterization
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) of the precursor were performed separately using Mettler-Toledo TGA/SDTA851e and Mettler-Toledo DSC822e in the temperature range of 25–630 °C under flowing argon. The heating rate was set as 10 K/min.
The surface morphology of the thin films was characterized by tapping mode atomic force microscopy (AFM, Bruker Innova) in area of 3 × 3 μm2.
Grazing incidence X-ray diffraction (GIXRD) and X-ray reflectivity (XRR) data of Hf0.5Zr0.5O2 thin films were measured on Bruker D8 Discover diffractometer with Cu K-alpha radiation (40 kV, 40 mA). For GIXRD, the incident beam was conditioned by using a 0.2-mm divergence slit. The exit beam was conditioned with a Soller slit. The incident angle was set to 0.5°. For XRR, the incident beam was conditioned by using a 0.2-mm divergence slit and the exit beam was conditioned with a 0.1-mm detector slit, respectively. The data were collected from 0.2° to 5° (2θ), with a step size of 0.002° and 0.5 s/step. Simulations and fitting of the XRR spectra were conducted by using DIFFRACplus LEPTOS analytical software to reveal the thickness, surface roughness, and density of the films.
X-ray photoelectron spectroscopy (XPS) measurements were carried out using Thermo ESCALAB 250 X-ray photoelectron spectroscopy with an Al Ka X-ray source to probe the atomic ratio of hafnium and zirconium as well as the bonding features. XPS PEAKs 4.1 software was used to decompose and fit spectrum peaks.
For electrical characterization, TiN dots (100 μm radius) were deposited onto the crystallized films by DC reactive magnetron sputtering. The thickness of the TiN electrode was ~100 nm, and the resistivity was measured as about 75 μΩ cm. The polarization–electric field (P–E) and leakage current density–electric field (J–E) measurements were performed using the Multiferroic 100 V Test System (Radiant Technologies, USA) at room temperature. The TiN dots were electrically stressed in measurements, and the low-resistivity Si substrate was grounded.
3 Results and discussion
3.1 TGA and DSC measurements
TGA and DSC data were recorded to gain insights into the decomposition chemistry of the Hf0.5Zr0.5O2 precursor. To improve the resolution of the analysis, the precursor solution was preheated at 80 °C to volatilize most of the water. As seen from Fig. 1, several endotherm and exotherm peaks can be observed in the DSC curve, and each corresponds to a rapidest weight loss rate recorded by TGA. Referring to the decomposition chemistry analysis for pure HfO2 aqueous precursor prepared using the same method [15], we attribute the first endotherm peak at 110 °C to a combined loss of the water and decomposition of the peroxo-groups from the solution. The second endotherm peak at 297 °C connects with nitrate decomposition. The exotherm peak at 496 °C should be attributed to the decomposition of tightly bounded hydroxyl species accompanied by crystallization.
3.2 AFM characterization
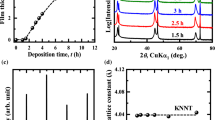
Figure 2 displays the AFM images for as-deposited and 700 °C annealed Hf0.5Zr0.5O2 thin films grown on Si substrate. In general, the film surfaces are smooth and flat, and no pores and micro-cracks are discernable. The root-mean-square (RMS) surface roughness is only about 0.13 nm for as-deposited film. Annealing process leads to an increase in surface roughness, which are 0.40, 0.48, and 0.70 nm for films with thickness of 12.9, 29.5, and 46.1 nm. Additionally very smooth surface morphologies of all samples were also confirmed by scanning electron microscopy (data not shown here), and no noticeable grain and defects have been observed in SEM images of different magnification.
3.3 XRR studies
Figure 3 shows the XRR patterns for Hf0.5Zr0.5O2 films with one coating cycle before and after annealing at 700 °C. By fitting the measured curves, their thickness, density, and surface roughness data were obtained and compared in Table 1. Annealing causes shrinkage and densification of the as-deposited thin films, resulting in a reduction in the thickness from 21.4 to 12.2 nm and an increase in the density from 5.1 to 8.0 g/cm3. In addition, the observation of increasing surface roughness after annealing is consistent with the results from AFM measurement.
3.4 GIXRD determinations
GIXRD patterns of 700 °C annealed HfO2, ZrO2, and Hf0.5Zr0.5O2 thin films with various thicknesses are shown in Fig. 4. The 29.5-nm-thick ZrO2 and 14.7-nm-thick HfO2 films were crystallized into pure tetragonal (t-) and pure monoclinic (m-) phases, respectively. An addition of ZrO2 into HfO2 helps to stabilize the t-phase; however, the t-phase stability at room temperature depends strongly on the film thickness. The 46.1-nm-thick Hf0.5Zr0.5O2 film was crystallized mainly into m-phase, indicated by the m-(−111) and weak m-(111) peaks. When the thickness is reduced to 29.5 nm, the appearance of the t-(011) peak and weakening of the m-(−111) peak imply the film is composed by a mixture of the tetragonal and monoclinic phases. For the 12.9-nm-thick Hf0.5Zr0.5O2 film, only the t-(011) peak at 30.5° exists, indicating that the film is crystallized mainly into t-phase. In Hf0.5Zr0.5O2 films prepared by ALD, the monoclinic phase fraction was determined to be about 10 % [19].
Our result of film thickness-dependent monoclinic to tetragonal phase transition is in accordance with previous studies for HfO2, ZrO2, and HfO2–ZrO2 solid solution nano-films [7, 20–23], and the underlying mechanism should be attributed to the surface energy effect suggested firstly by Garvie [24]. For thin films with a large value of the molar surface, the thickness-dependent monoclinic to tetragonal phase transition temperature \(\Delta T_{m \to t} (t)\) can be derived as:
where T bulk, m→t is monoclinic to tetragonal phase transition temperature of the bulk, α is a positive constant, t is the film thickness, \(\Delta \gamma_{m \to t}\) is the change of surface energy, and b is the molar density. Parameters for ZrO2: T bulk, m→t = 1430 K; HfO2: T bulk, m→t = 2066 K [25].
Garvie [24] stated that the tetragonal ZrO2 has a lower surface free energy than monoclinic ZrO2, so \(\Delta \gamma_{m \to t}\) is negative. b is positive. Thus, the monoclinic to tetragonal phase transition temperature \(\Delta T_{m \to t} (t)\) decreases with a reduction in film thickness t. At a critical thickness, the tetragonal phase can be stabilized even at room temperature. Theoretical calculations give the critical thickness of approximate 15 nm for ZrO2 and less than 2 nm for HfO2 [26]. Note that ZrO2 and HfO2 have very similar crystal chemistry and are miscible completely. Therefore, the aforementioned surface energy effect discussion is also applicable for HfO2–ZrO2 solid solution thin films, and an intermediate critical thickness is expected. For our Hf0.5Zr0.5O2 films, the critical thickness for t-phase stabilized at room temperature is around 12.9 nm, agreeing well with the expectation. It is worthy to mention that the crystal polymorph of the films is influenced strongly by many factors such as composition homogeneity, crystallization dynamics, defect type and density. They are acting either simultaneously or individually under different conditions. Thus, different film deposition methods and even the same preparation technique using different tools may cause inevitable variations in experimental critical thickness. For instance, the critical thickness of tetragonal Hf0.5Zr0.5O2 films formed by ALD is less than 10 nm [7, 20, 21].
3.5 XPS analysis
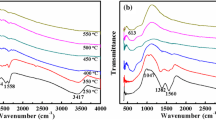
Figure 5 shows low-resolution XPS spectrum of sol–gel-derived Hf0.5Zr0.5O2 thin films annealed at 700 °C and high-resolution XPS spectra of Hf 4f, Zr 3d, and O 1s regions. For all the thin films analyzed, the binding energies were calibrated by setting carbon peak at 284.6 eV. In Fig. 5a, only peaks corresponding to Hf, Zr, and O elements are observed, but no other impurities (e.g., chloride ions) are involved. As shown in Fig. 5b, the Hf 4f spectrum consists of two components, i.e., the 4f 7/2 part at 17.7 eV and the 4f 5/2 part at 19.4 eV, which are in good agreement with the results of pure HfO2 thin films derived also by sol–gel method [27]. Similarly, Fig. 5c shows that Zr 3d spectrum consists of two components, i.e., the 3d 5/2 part at 182.1 eV and the 4f 3/2 part at 184.4 eV, which agree well with those reported by Shimizu [28], whose films were derived by sol–gel as well. The O 1s spectrum is located at 530.6 eV (Fig. 5d). Accordingly, it can be concluded that the XPS analyses confirm the formation of Hf–O and Zr–O bonds in our films. In addition, the Hf and Zr contents were determined as 15.7 and 19.4 at.%, respectively, which is very close to the designed composition of Hf0.5Zr0.5O2.
3.6 Electrical characterization
Figure 6 shows the electrical characterization results of HfO2, ZrO2, and Hf0.5Zr0.5O2 thin films. Note that an application of the same 4 and 3 V potentials across the films with different thicknesses results in different electric fields. From the average gradient of polarization–electric field (P–E) curves in Fig. 6a, the relative dielectric permittivity (ε r ) can be calculated for three film samples, and the results are found to relate closely to their polymorphs. The monoclinic HfO2 film has the lowest ε r of 13.6, while the tetragonal ZrO2 film has the highest ε r of 42.1. For Hf0.5Zr0.5O2, the ε r is determined as 27.8, which is comparable to the same composition film prepared by ALD [7]. The intermediate permittivity of Hf0.5Zr0.5O2 implies the existence of a small fraction of monoclinic phase in addition to the main tetragonal phase. Our experimental results agree well with theoretical prediction for HfO2 that the dielectric constant increases with increasing crystal structure symmetry [29]. The reason of small hysteresis observed in P–E curves is still unclear, and perhaps there exist mobile ions and re-orientable vacancy defect dipoles in the film.
As shown in Fig. 6b, the leakage current densities of HfO2, Hf0.5Zr0.5O2, and ZrO2 films are 2.4, 3.5, and 2.2 × 10−6 A/cm2 measured at 1 MV/cm. The leakage current density is lower than HfO2 thin films spin-coated using metal–organic reagent [30, 31], indicating high quality of our thin films derived from aqueous solution precursor.
4 Conclusions
In this work, stoichiometric Hf0.5Zr0.5O2 thin films have been prepared on highly doped Si (100) substrates using inorganic aqueous precursor, followed by deposition of TiN electrode dots on top to measure the electrical properties. Rather smooth and dense Hf0.5Zr0.5O2 thin films can be obtained after annealing at 700 °C. The XPS analyses confirm the formation of Hf–O and Zr–O bonds as well as atomic ratio of Hf and Zr approximating 1:1. The addition of ZrO2 helps to stabilize the tetragonal phase in HfO2, and the stability depends strongly on film thickness. When the film thickness is below 12.9 nm, the Hf0.5Zr0.5O2 films are crystallized mainly into tetragonal phase with a relative dielectric permittivity of 27.8 and leakage current density 3.5 × 10−6 A/cm2 measured at 1 MV/cm.
References
Robertson J (2004) High dielectric constant oxides. Eur Phys J Appl Phys 28:265–291
Choi JH, Mao Y, Chang JP (2011) Development of hafnium based high-k materials—a review. Mater Sci Eng R 72:97–136
Akbar MS, Cho HJ, Choi R, Kang CS, Kang CY, Choi CH, Rhee SJ, Kim YH, Lee JC (2004) Optimized NH3 annealing process for high-quality HfSiON gate oxide. IEEE Electron Device Lett 25:465–467
Tomida K, Kita K, Toriumi A (2006) Dielectric constant enhancement due to Si incorporation into HfO2. Appl Phys Lett 89:2902
Park PK, Kang SW (2006) Enhancement of dielectric constant in HfO2 thin films by the addition of Al2O3. Appl Phys Lett 89:2905
Mereu B, Dimoulas A, Vellianitis G, Apostolopoulos G, Scholz R, Alexe M (2005) Interface trap density in amorphous La2Hf2O7/SiO2 high-k gate stacks on Si. Appl Phys A 80:253–257
Müller J, Böscke TS, Schröder U, Reinicke M, Oberbeck L, Zhou D, Weinreich W, Kücher P, Lemberger M, Frey L (2009) Improved manufacturability of ZrO2 MIM capacitors by process stabilizing HfO2 addition. Microelectron Eng 86:1818–1821
Aarik J, Aidla A, Kiisler AA, Uustare T, Sammelselg V (1999) Influence of substrate temperature on atomic layer growth and properties of HfO2 thin films. Thin Solid Films 340:110–116
Aarik J, Aidla A, Mandar H, Uustare T, Kukli K, Schuisky M (2001) Phase transformations in hafnium dioxide thin films grown by atomic layer deposition at high temperatures. Appl Surf Sci 173:15–21
Balog M, Schieber M, Patai S, Michman M (1972) Thin films of metal oxides on silicon by chemical vapor deposition with organometallic compounds. I. J Cryst Growth 17:298–301
Williams PA, Jones AC, Tobin NL, Chalker PR, Taylor S, Marshall PA, Critchlow GW (2003) Growth of hafnium dioxide thin films by liquid-injection MOCVD using alkylamide and hydroxylamide precursors. Chem Vap Depos 9:309–314
Jones AC, Chalker PR (2003) Some recent developments in the chemical vapour deposition of electroceramic oxides. J Phys D Appl Phys 36:R80
Pereira L, Barquinha P, Fortunato E, Martins R (2005) Influence of the oxygen/argon ratio on the properties of sputtered hafnium oxide. Mater Sci Eng B118:210–213
Hu H, Zhu C, Lu YF, Wu YH, Liew T, Li MF, Cho BJ, Choi WK, Yakovlev N (2003) Physical and electrical characterization of HfO2 metal–insulator–metal capacitors for Si analog circuit applications. J Appl Phys 94:551–557
Jiang K, Anderson JT, Hoshino K, Li D, Wager JF, Keszler DA (2011) Low-energy path to dense HfO2 thin films with aqueous precursor. Chem Mater 23:945–952
Zaharescu M, Teodorescu VS, Gartner M, Blanchin MG, Barau A, Anasescu M (2008) Correlation between the method of preparation and the properties of the sol–gel HfO2 thin films. J Non-Cryst Solids 354:409–415
Wang KJ, Cheong KY (2008) Investigation of sol–gel derived HfO2 on 4H-SiC. Appl Surf Sci 254:1981–1985
Shimizu H, Nemoto D, Ikeda M, Nishide T (2010) Characteristics of sol–gel-derived and crystallized HfO2 thin films dependent on sol solution. Jpn J Appl Phys 49:121502
Müller J, Böscke TS, Schröder U, Mueller S, Bräuhaus D, Böttger U, Mikolajick T (2012) Ferroelectricity in simple binary ZrO2 and HfO2. Nano Lett 12:4318–4323
Park MH, Kim HJ, Kim YJ, Lee W, Moon T, Hwang CS (2013) Evolution of phases and ferroelectric properties of thin Hf0.5Zr0.5O2 films according to the thickness and annealing temperature. Appl Phys Lett 102:242905
Triyoso DH, Hegde RI, Schaeffer JK, Roan D, Tobin PJ, Samavedam SB, White JBE (2006) Impact of Zr addition on properties of atomic layer deposited HfO2. Appl Phys Lett 88:2901
Shandalov M, Mclntyre PC (2009) Size-dependent polymorphism in HfO2 nanotubes and nanoscale thin films. J Appl Phys 106:084322
Böscke TS, Hung PY, Kirsch PD, Quevedo-Lopez MA, Ramírez-Bon R (2009) Increasing permittivity in HfZrO thin films by surface manipulation. Appl Phys Lett 95:052904
Garvie RC (1965) The occurence of metastable tetragonal zirconia as a crystalline size effect. J Phys Chem 69:1238–1243
Wang C, Zinkevich M, Aldinger F (2006) The zirconia–hafnia system: DTA measurements and thermodynamic. J Am Ceram Soc 89:3751–3758
Navrotsky A (2005) Thermochemical insights into refractory ceramic materials based on oxides with large tetravalent cations. J Mater Chem 15:1883–1890
Brezesinski T, Smarsly B, Iimura KI, Grosso D, Boissière C, Amenitsch H, Sanchez C (2005) Self-assembly and crystallization behavior of mesoporous, crystalline HfO2 thin films: a model system for the generation of mesostructured Transition–Metal Oxides. Small 1:889–898
Shimizu H, Nishide T (2012) Characterization of sol–gel-derived and crystallized HfO2, ZrO2, ZrO2–Y2O3 thin films on Si (001) wafers with high dielectric constant. In: Mastai Y (ed) Advances in crystallization processes, chap. 13. InTech, Rijeka
Zhao Xinyuan, Vanderbilt David (2002) First-principles study of structural, vibrational, and lattice dielectric properties of hafnium oxide. Phys Rev B 65:233106
Wang ZJ, Kumagai T, Kokawa H, Tsuaur J, Ichiki M, Maeda R (2005) Crystalline phases, microstructures and electrical properties of hafnium oxide films deposited by sol–gel method. J Cryst Growth 281:452–457
Yu JJ, Fang Q, Zhang JY, Wang ZM, Boyd IW (2003) Hafnium oxide layers derived by photo-assisted sol–gel processing. Appl Surf Sci 208:676–681
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. NSFC 51272034) and the Open Research Fund of State Key Laboratory of Electronic Thin Films and Integrated Devices (UESTC) (No. KFJJ201101). The authors are responsible for the content of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, Y., Zhou, D., Guo, C. et al. Thickness-dependent phase evolution and dielectric property of Hf0.5Zr0.5O2 thin films prepared with aqueous precursor. J Sol-Gel Sci Technol 77, 430–436 (2016). https://doi.org/10.1007/s10971-015-3871-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3871-5