Abstract
In this study, the antibacterial property of silver-incorporated mesoporous bioactive glass microspheres (Ag-MBGMs) was investigated. The samples belonging to the 80SiO2·(15 − x)CaO·5P2O5·xAg2O system where 0 ≤ x ≤ 5 mol% were successfully prepared for bone regeneration and drug carrier applications. The obtained samples were evaluated by X-ray diffraction (XRD), transmission electron microscope (TEM), scanning electron microscope, Fourier transform infrared spectroscopy, and N2 adsorption/desorption measurements. All Ag-MBGMs had spherical particles with particle size 6–10 µm. TEM images showed mesopore structure. The textural properties showed a decrease in surface area and pore volume with an increase in silver content. XRD patterns exhibited the presence of pseudowollastonite and hydroxyapatite phase for silver-incorporating MBGMs, which might increase its bioactivity and bone bonding ability. Finally, all Ag-MBMGs had an antibacterial effect against both Escherichia coli and Staphylococcus aureus. Therefore, Ag-MBGMs may provide more potential for use as injectable, anticancer and drug-loading biomaterials for bone tissue engineering applications.
Graphical Abstract
In this study, the mesoporous bioactive glass microspheres (MBGMs) belonging to the system 80SiO2·(15 − x)CaO·5P2O5·xAg2O (x = 1, 3, and 5) were successfully prepared by the sol–gel process with surfactant-assisted mesoporous template. Effects of silver addition in MBGMs system were investigated for the first time such as particle shape and size, textural analysis, phase composition, in vitro bioactivity, and antibacterial effect. All prepared MBGMs have uniform microspherical particle (2–5 µm) with hierarchically structure. The textural analysis revealed that all prepared MBGMs had high porosity and relatively high surface area. The incorporation of apatite, pseudowollastonite, and metallic silver crystals were found for adding silver content more than 1 mol%. All prepared MBGMs can induce the crystalline hydroxyl carbonate apatite layer on the surface after soaking in simulated body fluid solution for 7 days. All silver-containing MBGMs had a very good antibacterial effect against Staphylococcus aureus and Escherichia coli. In addition, silver-incorporated MBGMs exhibited anticancer property against HepG2 cells.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mesoporous bioactive glass microspheres (MBGMs) have gained increasing attention from biological material researchers due to their important features of excellent bioactivity, biocompatibility both in vitro and in vivo, high specific surface area, well-ordered mesoporous structure, and tunable pore diameter and volume. Moreover, their spherical particles facilitate procedures of new bone tissue formation and optimize drug release and dispersion in the liquid as compared to irregular-shaped particles [1–6]. For example, Miao et al. [5] succeeded in synthesizing MBGMs by the combination of sol–gel and emulsion methods. Zhao et al. [6] have synthesized MBGMs with various P2O5 contents using co-templates of nonionic block copolymer EO20–PO70–EO20 (P123) and cationic surfactant cetyltrimethylammonium bromide (CTAB, C19H42BrN).
One major complication in the implant of biomaterials is post-operation infection because colonies of bacteria are easily formed on the surface of biomaterials after implantation. Although the implant of biomaterials is often treated with antibiotics, there are some disadvantages of the surgical treatment using antibiotics such as allergy, microbial flora depletion, and bacterial resistance [7]. In order to reduce the disadvantages of using antibiotics, many researchers have been interested on improving antimicrobial properties of biomaterials.
Several metal ions have been studied in antimicrobial bioactive glasses, such as Ag+, Cu2+, and Zn2+. Nevertheless, silver ion as an antibacterial agent was well verified. The important feature of the silver ion is its antimicrobial property, which is particularly necessary for the microorganism growth inhibition [8–13]. Moreover, several studies revealed that silver particle had an anticancer property [14–16].
There are interesting in antibacterial property of the bioactive glasses for use as bone reconstruction. Although, there were a few studies about the effect of silver-containing MBGMs, and their ability to induce cytotoxic responses on tumor cells has not been extensively studied. Moreover, it possibly opens up new opportunities in implanting, for drug delivery, and as multifunctional coating materials in orthopedic devices. Kawashita et al. [17] have previously shown a silica glass doped with silver and aluminum ions prepared using the sol–gel method. They prepared the silica glass microspheres with the composition of Si/Al/Ag = 1/0.01/0.01 from TEOS, EtOH, AgNO3, and 2-methoxyethanol. In this work, they are the first investigated silver-incorporated mesoporous bioactive glass microspheres in the system 80SiO2·(15 − x)CaO·5P2O5·xAg2O with x = 0, 1, 3, and 5 mol% and were prepared via sol–gel technique from TEOS, Ca(NO3)2·4H2O, AgNO3, EtOH, and HCl. The mixed surfactant of CTAB/P123 system was assisted in the sol–gel method for the design of surfactant-templated bioactive glasses for controlling their morphology and structure. The silver-incorporated MBGMs were characterized by X-ray diffraction, FT-IR, and scanning electron microscope, and their in vitro bioactivity and antimicrobial properties as compared to those of silver-free MBGMs were investigated. The Escherichia coli (gram negative bacteria) and Staphylococcus aureus (gram positive bacteria) were chosen for the preliminary investigation because they were major bacterium and found in high concentration at biomaterial-related infection sites. Moreover, the motivation of this study was to examine the ability to induce cytotoxic responses on HepG2 (human liver cancer cell line).
2 Materials and methods
2.1 Materials
Tetraethyl orthosilicate (TEOS, 98 %, Acros), triethyl phosphate (TEP, 99 %, Acros), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, Ajax), silver nitrate (AgNO3), absolute ethanol (EtOH, Carlo Erba), and hydrochloric acid (HCl, 37 %, Merck) were all analytical grade. Non-ionic surfactant Pluronic P123 (Triblock copolymer EO20–PO70–EO20, Mw = 5650, 98 %) and cetyltrimethyl-ammonium bromide (CTAB, Mw = 364.5, 99 %) were supplied by Sigma-Aldrich. CTAB and P123 were used as cotemplates for microspheres synthesis as reported in Zhao et al. [6] work.
2.2 Sol–gel synthesis of silver-doped mesoporous bioactive glass microspheres
Mesoporous bioactive glass microspheres (MBGMs) with silver belonging to the system 80SiO2·(15 − x)CaO·5P2O5·xAg2O with x = 0, 1, 3, and 5 mol% are denoted as MBGMs, 1Ag-MBGMs, 3Ag-MBGMs, and 5Ag-MBGMs, respectively. MBGMs and silver-incorporated MBGMs were prepared by the sol–gel technique, using P123 and CTAB as cosurfactants [6]. Initially, 15 ml absolute ethanol (EtOH), 18 ml deionized water, and 36 ml of 2 M HCl (as hydrolysis catalyst) were blended. P123 and CTAB were added into the blended solution in weight ratio 6:1. Afterward, TEOS, TEP, Ca(NO3)2·4H2O, and AgNO3 were added under vigorous stirring, respectively. After magnetic stirring at room temperature for 60 min, the clear sol was obtained. The obtained sol was heated at 80 °C for 5 h and followed by heating at 130 °C for 12 h, respectively. The precipitated powder was filtered and washed with absolute ethanol for three times. The ordered mesoporous bioactive glass was obtained through drying in a fume hood overnight and then calcining at 550 °C for 5 h (2 °C/min).
2.3 Characterization of mesoporous bioactive glass microspheres
Powder X-ray diffraction (XRD) patterns were recorded with a German Bruker D2 X-ray diffractometer with Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 10 mA for phase composition identification. Mesoporous architecture of MBGMs was observed by transmission electron microscope (TEM, FEI Tecnai G2 20) operated at 200 kV. Microspheres shape and size were analyzed by scanning electron microscope (SEM, JEOL-6010LV) at an acceleration voltage of 10–20 kV. All samples were secured to the stub using the carbon tape and were sputter-coated with gold. Fourier transform infrared (FTIR) spectra were recorded on a Fourier transform infrared spectrometer (Spectrum GX, Perkin Elmer) in a wave number range of 400–4000 cm−1 with a resolution of 4 cm−1. The specific surface area and mean pore size were investigated by N2 adsorption–desorption isotherms at −196 °C on an automatic surface area analyzer (BELSORP MINI, BEL, Japan) under continuous adsorption conditions. Prior to the N2 adsorption/desorption measurements, the samples were degassed under vacuum at 150 °C for 6 h. The Brunauer–Emmett–Teller (BET) method was utilized to calculate the specific surface areas. The mesopore size distribution was determined from the desorption branch of the isotherm by means of the Barret–Joyner–Halenda (BJH) method. Microspheres size distribution was also determined using laser diffraction technique using particle size distribution analyzer (Horiba, Japan).
2.4 In vitro bioactivity assessment of silver-doped MBGMs
Assessment of in vitro bioactivity was carried out in a simulated body fluid (SBF) solution proposed by Kokubo [18]. The SBF solution has a composition and ionic concentration similar to those of the inorganic part of human plasma. SBF was prepared by dissolving NaCl, KCl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2, and Na2SO4 in distilled water and buffering at pH 7.4 with tris(hydroxymethyl)aminomethane (HOCH2)3CNH2 and 1 M HCl. For this purpose, each type of MBGMs was immersed in 50 ml SBF solution in a polyethylene container covered with a tight lid at a solid/liquid ratio of 1 mg/ml at 37 °C in static stage for 7 days. Afterward, the powders were recovered by filtration, rinsed with ethanol, and dried in air at room temperature. The immersed SBF solutions after filtration were analyzed by inductive coupled plasma (ICP) spectroscopy for determining the ions concentrations. Scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectroscopy were used to determine the change on the morphology and chemical composition of the bioactive glass microspheres.
2.5 Antibacterial tests of silver-doped MBGMs
The antimicrobial activity of the silver-doped MBGMs was investigated using antimicrobial disk susceptibility tests (NCCLS M2-A9: Performance standards for antimicrobial disk susceptibility tests). Iso-Sensitest agar plates were incubated with standardized culture of S. aureus (ATCC 6538) or E. coli (ATCC 25922). Silver-doped MBGMs disks of 13 mm diameter and 1 mm thickness were in contact on plate and kept in air at 37 °C overnight. The diameters of any inhibition zones formed around the disks were measured.
2.6 Cell culture
The human hepatocellular carcinoma cell line HepG2 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) containing 10 % fetal bovine serum (FBS) (Gibco, USA) and 1 % penicillin/streptomycin antibiotic solution. Cells were incubated at 37 °C with 5 % CO2 and 95 % air atmosphere.
2.7 Investigation of HepG2 cell activity using the MTT method
MBGMs and Ag-MBGMs were sterilized using autoclave at 121 °C for 15 min and dispersed in the medium with different concentrations (2, 5, 10, 15 and 20 mg/ml). The suspension of MBGMs and Ag-MBGMs were incubated at 37 °C with 5 % CO2 and 95 % air atmosphere for 24 h and then were centrifuged for 10 min with 4000 rpm. The upper liquid was extracted. The cells were seeded in 96-well culture plates at a density of 2 × 104 cells/well and allowed to attach for 24 h. The extracts with different concentrations were added to each well. The culture without extract was used as the control condition. Each condition had six replicate wells. After 24 h of the addition of extracts, cell viability was evaluated using MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay. In brief, the supernatant was removed from the well, and the wells were washed with phosphate-buffered saline (PBS) three times. A total of 150 μl of fresh media and 20 µl of MTT working solution (5 mg/ml in PBS) were added into each well followed by incubation for 4 h at 37 °C. After incubation, the supernatant was removed, and 150 μl of dimethyl sulfoxide (DMSO) was added into each well to dissolve the blue formazan product formed. The absorbance was investigated at 595 nm using a 96-well microplate reader.
3 Results and discussion
3.1 XRD and FTIR analysis of the synthesized silver-doped MBGMs
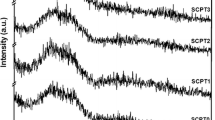
The X-ray diffraction patterns of samples after calcination at 550 °C are shown in Fig. 1. XRD patterns of silver-free MBGMs exhibited a broad diffraction peak at ~20°–30°, indicating that the main phase of MBGMs is low-crystalline silicate. However, XRD patterns of the silver-doped MBGMs (x = 1 and 3 mol%) presented their glass nature with pseudowollastonite (JCPDS no. 74-0874) and hydroxyapatite (JCPDS no. 09-0432). With 5 mol% silver incorporated to MBGMs, the XRD pattern shows the well-defined crystallization of pseudowollastonite, hydroxyapatite, and metallic silver (JCPDS no. 87-0720) as dispersed phase silver. It is worth nothing that the pseudowollastonite phase was formed at lower temperature than that obtained using the quenching techniques [19]. It should be possible due to the higher reaction of the powders synthesized by sol–gel method. Additionally, the crystallization of apatite and pseudowollastonite in silver-doped MBGMs may support biomineralization in osteoblast cell cultures and allow a strong bond between surfaces of the silver-doped MBGMs to bone [20]. The results showed that the doping with Ag2O may favor the nucleation of pseudowollastonite and hydroxyapatite. Moreover, reports in the literature also showed that a high amount of Ag-doping in calcium phosphate glasses could lead to formation of metallic silver within the glass matrix [21].
FTIR spectra of the MBGMs and silver-doped MBGMs are shown in Fig. 2. The band at 1633 cm−1 was found in all samples which attributed to the bending of O–H in water because of their large specific area and high surface-adsorbed water and hydroxyl groups. The existence of characteristic absorption bands of Si–O–Si bonds at 1062, 802, and 450 cm−1 appears in all samples. The shoulders at 962 and 1300 cm−1 are corresponded to the Si–O stretching vibration of one non-bridging oxygen (Si–O–NBO). Both the Ca2+ and Ag+ ions are known to act as network modifier, and it is possible that the Ag+ ions may acts like a stronger network modifier than the Ca2+ ions. With increase in the silver content in glass system, the broadening of infrared absorption bands of Si–O–Si stretching vibration is more observed. The presence of pseudowollastonite, hydroxyapatite, and metallic silver in XRD patterns of all silver-doped MBGMs support that both the network modify role of Ag+ ions and metallic silver formation disturb the Si–O–Si glass network connectivity. The vibration bands at approximately 550 and 600 cm−1 are attributed to the P–O-bending vibrations of PO4 3− tetrahedra characteristic of crystalline apatite-like phase. It is indicated that the addition of silver in MBGMs system allows the precipitation of pseudowollastonite, hydroxyapatite, and metallic silver.
3.2 Particle size and morphology of the synthesized silver-doped MBGMs
Figure 3 shows the TEM images of all four samples. Mesopores with pore diameter approximately in range of 4–5 nm can be clearly observed. The incorporation of CTAB and P123 in glass system plays an important role in ordering of mesopore in bioactive glass structures [6]. Micelles of CTAB and P123 were created through self-organized of CTAB and P123 molecules under suitable synthesis conditions.
After calcination at 550 °C for 5 h, all samples exhibited perfectly dispersed spherical morphology with uniform size distribution (average size approximated 7–10 µm), as shown in Fig. 4.
Zhu et al. [22] reported both dexamethasone (DEX) and bovine serum albumin (BSA) as the model chemotherapeutic drug and protein could be loaded in the mesoporous bioactive glass/hydroxyapatite composites with high loading capacities. Zhu and coworkers [23] reported that the mesoporous bioactive glass scaffold can load over twofold higher amount of gentamicin as the model drug than the bioactive glass scaffold. The particle size distribution of the MBGMs with different compositions analyzed by a particle size distribution analyzer is shown in Fig. 5. The diameter of the particles ranges from 2 to 30 µm.
The N2 adsorption/desorption isotherms and the corresponding pore size distribution of samples are plotted in Fig. 6a, b, respectively. The isotherms curve corresponds to the type IV isotherm of the Brunaure–Deming–Deming–Teller theory (BDDT) classification, implying that each of these samples contains mesopores. The mesoporous diameters tend to increase with the increase in Ag2O content. Silver is known to act as a glass network modifier. For 80SiO2·(15 − x)CaO·5P2O5·xAg2O system, Ag ions were substituted by Ca ions in the glass structure. The suitable reason was the increase in Ag2O content, which caused the formation of silver nanoparticles or silver clusters, and may disturb the formation of hierarchical structures of glass [24]. Saravanapavan et al. [25] suggested that this effect was caused by silver ions acting as glass network modifier leading to a lower network connectivity of glass and effectively disturbing the glass network.
Furthermore, type H1 hysteresis loops in a mesoporous range are characteristic of cylindrical pores. As calculated from the linear part of the BET plot, all samples had relatively high surface area (350–560 m2/g) depending on the silver doping concentration as summarized in Table 1. The total volumes of single-point adsorption at P/P 0 = 0.990 were between 0.4 and 0.6 cm2/g for all samples. As can be seen from Table 1, surface area for Ag-doping MBGMs decreased, which is a result of reduction in pore volume while the mean pore diameter has less changed.
The pore size and pore size distribution derived from the desorption branch of the nitrogen sorption isotherm through BJH model are displayed in Fig. 6b. All MBGMs samples reveal a relative narrow pore size distribution, and the average pore diameters of all samples are between 4.3 and 5.3 nm which can be used as biological protein molecules or drug carrier.
3.3 In vitro bioactivity assessment of silver-doped MBGMs
Figure 7 shows the XRD patterns of the MBGMs with four different chemical compositions. After 7 days of soaking in SBF solution, XRD patterns of all silver-doped MBGMs exhibit several new diffraction peaks located at 26° and 32° which suggest the (002) and (211) reflections of the apatite phase (JCPDS no. 09-0432), respectively. Moreover, silver-doped MBGMs also demonstrate silver chloride phase (JCPDS no. 85-1355) which were raised at the 2θ peak values of 27.8°, 32.3°, 46.2°, 54.8°, 57.5°, and 67.5°. Besides, Gargiulo et al. [26] reported that the possible reason of the present of AgCl phase after soaking Ag-MBGMs in SBF solution was the releasing of Ag+ ions which immediately react with the chlorides in the SBF solution. Therefore, the highly crystalline AgCl (insoluble salt) was found on the surface of the glass particles. However, the XRD characteristic peaks of pseudowollastonite (JCPDS no. 74-0874), which are nearly close to these peaks of crystalline silver chloride at 2θ of 27.8°, 32.3°, and 46.2° (JCPDS no. 85-1355), are difficult to observe in the XRD pattern. The metallic silver phase decreased after immersion in SBF due to Ag ions release and well-crystalline AgCl formation. It has been pointed out that the silver ions present at the glass surface can favor formation of silver phosphate nanoclusters via binding to the phosphate ions available in SBF, and these nanoclusters can serve as nucleation sites for CaP precipitate [24].
The silver-free MBGMs show only one broad peak, which is similar to that of the sample before soaking, corresponding to no apatite formation on the silver-free MBGMs surface as compared to the silver-doped MBGMs at the same condition.
FTIR spectra (Fig. 8) further confirm the formation of an apatite-like layer on the surface of all samples after immersion in SBF solution for 7 days, where phosphate absorption bands at about 1043, 963, 603, and 566 cm−1 and carbonate absorption bands at about 1490 cm−1 were detected. It proves that substances formed on the MBGM surface are carbonate apatite, similar to biological apatite in bone.
From the element concentrations analyzed by ICP spectroscopy in Fig. 9, the Ag ion release studied in SBF demonstrated a rapid silver release during the first 24 h of immersion followed by a slow release trend reaching the concentration of 1, 1.3, and 1.8 ppm in SBF for 1, 3, and 5 mol% Ag-doped MBGMs, respectively. The more content of Ag-doping showed more Ag ions release in SBF for long periods. Indeed, slow release of Ag ions is required to occur after an initial fast ion release in order to treat effectively possible bacterial infections. However, in this study, the Ag ions release for long over time so that silver chloride or silver phosphate may not precipitate. The Ca and P concentrations in SBF decreased with the immersion time as shown in Fig. 9b, c. This can be resulted from the apatite-like phase precipitation. Moreover, 5Ag-MBGMS show higher Si concentration released in SBF in comparison with other Ag-doped MGBMs (Fig. 9d). It indicates that MBGMs with high Ag-doping have the high degree of glass degradation.
Figure 10 shows the pH variation during immersion of MBGMs and Ag-MBGMs in SBF. The pH values increased from 7.40 to almost 7.6–7.75 after immersion for 1 day. Moreover, Ag-doped MBGMs showed similar pH values in range of 7.70–7.75 which was higher than the pH value of Ag-free MBGMs. This condition was suitable for human body, and it favored apatite formation.
SEM images of the MBGMs with different chemical compositions before and after immersion in SBF solution for 7 days is shown in Fig. 11.
The surface of MBGMs becomes concavo–convex, and some tiny pieces or particles can be found on the spherical surface after immersion for 7 days, while that of the silver-doped MBGMs were fully covered by silver chloride and apatite precipitates, and they were irregularly distributed on the surface. Nezafati et al. [27] reported that Ag-doping in bioactive glass affected the morphology of HA crystals formed on the glass surface in SBF. It was noted that the incorporation of silver resulting pseudowollastonite and hydroxyapatite formation in the MBGMs indicated their good bioactivity in SBF solution. The bioactivity of wollastonite was higher than other biocompatible glass and glass–ceramics because of silicon ions present in the wollastonite, which played an important role in bone regeneration. Moreover, it had the ability to bond with bone easier than hydroxyapatite or tricalcium phosphate [28].
3.4 Antibacterial tests
Antibacterial assay revealed that silver-doped MBGMs had an antibacterial effect against both S. aureus and E. coli. However, silver-free MBGMs had no antibacterial activity. The inhibition zones of the growth of both bacteria around the MBGMs disks in the agar plate are shown in Figs. 12, 13.
The size of inhibition zones established around all silver-doped glass disks were more than 0.5 mm. The size of inhibition zones around all MBGMs disks slightly increased with the increase in Ag content as summarized in Table 2.
According to the previous report [29], they found that the antibacterial property of the silver-doped bioactive glass on S. aureus and E. coli was attributed to the dissolution of Ag+ ions from the glass matrix. Moreover, the crystallization of AgCl found in XRD pattern can be dissolved after immersion of Ag-MBGMs in the culture medium. As a result, Ag-MBGMs have antibacterial property [30].
3.5 MTT assay
The in vitro cytotoxicity of MBGMs and Ag-MBGMs were evaluated against human liver cancer HepG2 cells. The number of cells decreased with the increasing amounts of silver in MBGMs. It was found that 10 mg/ml of 1Ag-MBGMs, 5 mg/ml of 3Ag-MBGMs, and 2 mg/ml of 5Ag-MBGMs were well enough to induce 50 % of cell mortality (Fig. 14).
In fact, silver nanoparticles may induce reactive oxygen species and cause damage to cellular components leading to cell death [31]. Nevertheless, this is the first report on cytotoxic effects of silver-incorporated MBGMs against human liver cancer HepG2 cells. Nevertheless, a recent study had revealed that ionic Ag can induce a significantly higher cytotoxicity on human mesenchymal stem cells and red blood cells as compared to AgCl [32, 33]. Indeed, more specific investigations are necessary in order to understand the potential cytotoxic effect of AgCl precipitation associated with bioactive glass surface.
4 Conclusions
The low temperature sol–gel process has been successfully used to prepare a new composition of silver-incorporated mesoporous bioactive glasses microsphere with different silver contents (1, 3, and 5 mol%). These glasses have uniform microspheres with 2–5 µm in diameter and a hierarchical structure with mesopore size range (4.3–5.3 nm.). Textural analysis of all glass samples exhibited high porosity and had relatively high surface area. X-ray diffraction results revealed the incorporation of apatite, pseudowollastonite, and metallic silver crystals by adding silver content of more than 1 mol% in the bioactive glass microsphere system. The bioactivity of all MBGMs were tested by soaking in SBF solution for 7 days, and the in vitro bioactivity test indicated that MBGMs induced the deposition of a crystalline HCA layer on their surface, and in silver-doped, MBGMs also exhibited AgCl phase. Silver-doped MBGMs showed a very good antibacterial effect against S. aureus and E. coli. Moreover, Ag-MBGMs exhibited anticancer property against HepG2 cells, which suggest the use of these materials for medical application and drug delivery.
References
Xia W, Chang J (2006) Well-ordered mesoporous bioactive glasses (MBG): a promising bioactive drug delivery system. J Control Release 110(3):522–530. doi:10.1016/j.jconrel.2005.11.002
Zhu Y, Wu C, Ramaswamy Y, Kockrick E, Simon P, Kaskel S, Zreiqat H (2008) Preparation, characterization and in vitro bioactivity of mesoporous bioactive glasses (MBGs) scaffolds for bone tissue engineering. Microporous Mesoporous Mater 112(1–3):494–503. doi:10.1016/j.micromeso.2007.10.029
Hu Q, Chen X, Zhao N, Li Y (2013) Facile synthesis and in vitro bioactivity of monodispersed mesoporous bioactive glass sub-micron spheres. Mater Lett 106:452–455. doi:10.1016/j.matlet.2013.04.075
Lei B, Chen X, Wang Y, Zhao N (2009) Synthesis and in vitro bioactivity of novel mesoporous hollow bioactive glass microspheres. Mater Lett 63(20):1719–1721. doi:10.1016/j.matlet.2009.04.041
Miao G, Chen X, Dong H, Fang L, Mao C, Li Y, Li Z, Hu Q (2013) Investigation of emulsified, acid and acid-alkali catalyzed mesoporous bioactive glass microspheres for bone regeneration and drug delivery. Mater Sci Eng C 33(7):4236–4243. doi:10.1016/j.msec.2013.06.022
Zhao S, Li Y, Li D (2010) Synthesis and in vitro bioactivity of CaO–SiO2–P2O5 mesoporous microspheres. Microporous Mesoporous Mater 135(1–3):67–73. doi:10.1016/j.micromeso.2010.06.012
Gristina AG (1987) Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237(4822):1588–1595
El-Kady AM, Ali AF, Rizk RA, Ahmed MM (2012) Synthesis, characterization and microbiological response of silver doped bioactive glass nanoparticles. Ceram Int 38(1):177–188. doi:10.1016/j.ceramint.2011.05.158
Kamitakahara M, Ohtsuki C, Inada H, Tanihara M, Miyazaki T (2006) Effect of ZnO addition on bioactive CaO–SiO2–P2O5–CaF2 glass–ceramics containing apatite and wollastonite. Acta Biomaterialia 2(4):467–471. doi:10.1016/j.actbio.2006.03.001
Palza H, Escobar B, Bejarano J, Bravo D, Diaz-Dosque M, Perez J (2013) Designing antimicrobial bioactive glass materials with embedded metal ions synthesized by the sol–gel method. Mater Sci Eng C 33(7):3795–3801. doi:10.1016/j.msec.2013.05.012
Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, Xiao Y (2013) Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 34(2):422–433. doi:10.1016/j.biomaterials.2012.09.066
Diba M, Boccaccini AR (2014) 9—Silver-containing bioactive glasses for tissue engineering applications. In: Baltzer N, Copponnex T (eds) Precious metals for biomedical applications. Woodhead Publishing, Cambridge, p 177–211. doi:10.1533/9780857099051.2.177
Vernè E, Nunzio SD, Bosetti M, Appendino P, Vitale Brovarone C, Maina G, Cannas M (2005) Surface characterization of silver-doped bioactive glass. Biomaterials 26(25):5111–5119. doi:10.1016/j.biomaterials.2005.01.038
Lankoff A, Sandberg WJ, Wegierek-Ciuk A, Lisowska H, Refsnes M, Sartowska B, Schwarze PE, Meczynska-Wielgosz S, Wojewodzka M, Kruszewski M (2012) The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol Lett 208(3):197–213. doi:10.1016/j.toxlet.2011.11.006
Prasad RY, McGee JK, Killius MG, Suarez DA, Blackman CF, DeMarini DM, Simmons SO (2013) Investigating oxidative stress and inflammatory responses elicited by silver nanoparticles using high-throughput reporter genes in HepG2 cells: effect of size, surface coating, and intracellular uptake. Toxicol In Vitro 27(6):2013–2021. doi:10.1016/j.tiv.2013.07.005
Thati B, Noble A, Creaven BS, Walsh M, McCann M, Devereux M, Kavanagh K, Egan DA (2009) Role of cell cycle events and apoptosis in mediating the anti-cancer activity of a silver(I) complex of 4-hydroxy-3-nitro-coumarin-bis(phenanthroline) in human malignant cancer cells. Eur J Pharmacol 602(2–3):203–214
Kawashita M, Toda S, Kim HM, Kokubo T, Masuda N (2003) Preparation of antibacterial silver-doped silica glass microspheres. J Biomed Mater Res A. 66(2):266–274
Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27(15):2907–2915. doi:10.1016/j.biomaterials.2006.01.017
Ma J, Chen CZ, Wang DG, Meng XG, Shi JZ (2010) Influence of the sintering temperature on the structural feature and bioactivity of sol–gel derived SiO2–CaO–P2O5 bioglass. Ceram Int 36(6):1911–1916. doi:10.1016/j.ceramint.2010.03.017
Sautier JM, Kokubo T, Ohtsuki T, Nefussi JR, Boulekbache H, Oboeuf M, Loty S, Loty C, Forest N (1994) Bioactive glass-ceramic containing crystalline apatite and wollastonite initiates biomineralization in bone cell cultures. Calcif Tissue Int 55(6):458–466. doi:10.1007/bf00298560
Simon V, Albon C, Simon S (2008) Silver release from hydroxyapatite self-assembling calcium–phosphate glasses. J Non-Cryst Solids 354(15–16):1751–1755. doi:10.1016/j.jnoncrysol.2007.08.063
Zhu M, Zhang J, Tao C, He X, Zhu Y (2014) Design of mesoporous bioactive glass/hydroxyapatite composites for controllable co-delivery of chemotherapeutic drugs and proteins. Mater Lett 115:194–197. doi:10.1016/j.matlet.2013.10.058
Zhu Y, Kaskel S (2009) Comparison of the in vitro bioactivity and drug release property of mesoporous bioactive glasses (MBGs) and bioactive glasses (BGs) scaffolds. Microporous Mesoporous Mater 118(1–3):176–182. doi:10.1016/j.micromeso.2008.08.046
Vulpoi A, Baia L, Simon S, Simon V (2012) Silver effect on the structure of SiO2–CaO–P2O5 ternary system. Mater Sci Eng C 32(2):178–183. doi:10.1016/j.msec.2011.10.015
Saravanapavan P, Patel MH, Hench LL (2003) Effect of composition and texture on controlled rate of release of an antibacterial agent from bioactive gel-glasses. Bioceramics 15(240–242):233–236. doi:10.4028/www.scientific.net/KEM.240-242.233
Gargiulo N, Cusano AM, Causa F, Caputo D, Netti PA (2013) Silver-containing mesoporous bioactive glass with improved antibacterial properties. J Mater Sci Mater Med 24(9):2129–2135
Nezafati N, Moztarzadeh F, Hesaraki S (2012) Surface reactivity and in vitro biological evaluation of sol gel derived silver/calcium silicophosphate bioactive glass. Biotechnol Bioprocess Eng 17(4):746–754. doi:10.1007/s12257-012-0046-x
Anjaneyulu U, Sasikumar S (2014) Bioactive nanocrystalline wollastonite synthesized by sol–gel combustion method by using eggshell waste as calcium source. Bull Mater Sci 37(2):207–212. doi:10.1007/s12034-014-0646-5
Bellantone M, Williams HD, Hench LL (2002) Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrob Agents Chemother 46(6):1940–1945. doi:10.1128/aac.46.6.1940-1945.2002
Valappil SP, Pickup DM, Carroll DL, Hope CK, Pratten J, Newport RJ, Smith ME, Wilson M, Knowles JC (2007) Effect of silver content on the structure and antibacterial activity of silver-doped phosphate-based glasses. Antimicrob Agents Chemother 51(12):4453–4461
Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6(8):1794–1807
Bellantone M, Coleman NJ, Hench LL (2000) Bacteriostatic action of a novel four-component bioactive glass. J Biomed Mater Res 51(3):484–490. doi:10.1002/1097-4636(20000905)51:3<484:aid-jbm24>3.0.co;2-4
Zhang S, Du C, Wang Z, Han X, Zhang K, Liu L (2013) Reduced cytotoxicity of silver ions to mammalian cells at high concentration due to the formation of silver chloride. Toxicol In Vitro 27(2):739–744. doi:10.1016/j.tiv.2012.12.003
Acknowledgments
This work has been financed by The Royal Golden Jubilee Ph.D. program supported from Thailand Research Fund and Suranaree University of Technology. The authors would like to thank Dr. Ratchadaporn Oonsivilai and Miss Thasaneewan Srisan for their helps in MTT assay test.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phetnin, R., Rattanachan, S.T. Preparation and antibacterial property on silver incorporated mesoporous bioactive glass microspheres. J Sol-Gel Sci Technol 75, 279–290 (2015). https://doi.org/10.1007/s10971-015-3697-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3697-1