Abstract
The predominant mineral, montmorillonite, in smectite-rich clays, the most promising buffer & backfill, controls their sorption characteristics towards radionuclides. The role of sulphate on Am(III) sorption by Na-montmorillonite has been investigated. The influence of pH, ionic strength & \({\text{[SO}}_{{4}}^{{2 - }} {]}\) on Am(III) sorption by Na-montmorillonite has been studied. The contribution of Am(III) hydroxo complexes in aqueous speciation is ~ 50% at pH 7.5 while that of Eu(III) hydroxo complexes is ~ 10%. In contrast to Eu(III), Am(III) sorption could be modeled by either ≡SOAmOH+ or ≡SOAmCO3. Log K for Am(III) ion exchange & ≡SOAmCO3 is less than that for similar Eu(III) species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep geological disposal of high level nuclear waste (HLW) has been accepted worldwide for its long-term confinement, containment and seclusion from biosphere. To achieve the indented purpose, deep geological repositories are designed using multi natural and engineered barriers. Smectite rich clay minerals are one of the most promising engineered barriers proposed to be used as buffer and backfill in the repositories [1]. The sorption of radionuclides on these buffers and backfill plays a key role in retarding their migration after breach from waste form and canisters [2, 3]. Montmorillonite, a 2:1 phyllosilicate, is the major component of the smectite rich clay minerals. The high retention capacity for radionuclides, low hydraulic conductivity coupled with high swelling potential make them the preferred choice as backfill/buffer material in deep geological repositories [4, 5]. One of the controlling factors for radionuclide migration from repositories will be their sorption onto the clay minerals [6]. The realistic performance assessment of the repositories requires a thorough understanding of the interaction between radionuclides and clay minerals.
One of the key factors which affect the radionuclide sorption on clay minerals is the anions present in groundwater [7,8,9,10]. Radionuclide sorption may either decrease or enhance in presence of anions depending upon the intrinsic properties of anions, clay minerals and radionuclides. In some cases, anions form strong complexes with radionuclides in aqueous medium resulting in decreased sorption of radionuclides. In contrast, radionuclides complexes with anions and/or sorption of anions followed by that of radionuclides may result in formation of radionuclide and/or ligand bridged complexes thereby enhancing the sorption of radionuclides. Numerous literature reports entailing the role of anions in influencing the sorption of heavy metals on clay minerals exists [7,8,9]. Nevertheless, the effect of anions on actinides and lanthanides sorption by clay minerals has not been studied comprehensively [10, 11]. The presence of phosphate showed no observable effect on U(VI) sorption by montmorillonite, however, the formation of U(VI)-phosphate surface complex was revealed by spectroscopic measurements [12]. Decreased U(VI) sorption on bentonite in presence of sulphate ions was attributed to competitive sorption of uranyl and sulphate ions on bentonite and complex formation between uranyl and sulphate in aqueous phase [13]. Similarly, EXAFS measurements showed that the carbonate surface complexes of U(VI) were formed while investigating U(VI) sorption on Na-montmorillonite [10]. The complexing anions have been found to influence the sorption of trivalent lanthanides/actinides as well [14,15,16]. The decrease in Eu(III)/Am(III) sorption on montmorillonite with increase in carbonate concentration has been attributed to the surface species comprising of metal ions, hydroxide and/or carbonate ions [14]. The ternary surface complexes in the aforementioned system have been confirmed by spectroscopic measurements [11]. Phosphate was found to enhance Eu(III) sorption on sodium bentonite in the pH range of 4–7 due to the formation of ligand bridged Eu(III) surface complexes [15]. With increase in phosphate concentration and decrease in Na-montmorillonite suspension strength, the change from Eu(III) surface complexation to Eu(III) surface precipitation has been affirmed using ATR-FTIR spectroscopy [16].
Sulphate is one of the most common polyatomic anions present in ground water. Natural sources such as sulfate mineral dissolution, atmospheric deposition, sulfide mineral oxidation coupled with anthropogenic sources such as coal mines, metallurgical refineries, milling production sites are responsible for presence of sulphate in groundwater in the range 1–38 mmol/L [17, 18]. Therefore, it is imperative to understand the role of sulphate on the sorption of radionuclides. The surface complexes pertaining to americium sulphate and americium carbonate have been employed to model Am(III) sorption on volcanic rocks [19]. Am(III) sorption on bentonite was found to decrease in presence of sulphate. The sulphate bearing Am(III) surface species was used to explain the Am(III) sorption profile on bentonite [20]. In our previous work, the concentration dependent influence of sulphate on Eu(III) sorption by Na-montmorillonite was observed [21]. However, an extensive study delineating mechanistic role of sulphate ions in influencing Am(III) sorption on clay minerals is absent in literature.
In the present work, well characterized montmorillonite K10 was used as a sorbent for Am(III). Long half-life radioisotopes of americium are (241Am, 243Am) present in HLW. The role of sulphate in influencing the sorption of Am(III) on montmorillonite was investigated as a function of time, pH and ionic strength. Subsequently, Am(III) sorption profiles on Na-montmorillonite has been successfully modeled using surface complexation modeling (SCM). The results obtained in the present studies were compared with Eu(III)-Na-montmorillonite system to demonstrate the dissimilarities, if any, in both the systems.
Experimental
Chemicals and materials
Montmorillonite K-10 clay (Sigma-Aldrich (CAS No: 1318–93-0)) was used for sorption studies. The clay was purified and converted to homoionic Na form by employing the procedure, details of which are furnished elsewhere [21]. Analytical reagent (AR) grade Na2SO4 (SD Fine chemicals, Mumbai) and NaCl (SD Fine chemicals, Mumbai) were used to maintain sulphate concentration and ionic strength respectively. The solutions and suspensions for the entire study were prepared with deionized water (18 MΩ. cm).The sorption of Am(III) was studied using radionuclide 241Am, with Am(III) stock available in our laboratory. The gamma activity of Am(III) was determined using 3" × 3" well type NaI(Tl) detector, having almost 100% detection efficiency for 60 keV, coupled to 4096 channel analyzer. Each sample was counted for adequate time to get 10,000 counts to reduce the statistical error in counting.
Batch sorption experiments
Na-montmorillonite suspensions with solid to solution ratio of 1 g L−1 were prepared in NaCl, in 50 mL polypropylene tubes. The suspensions were left undisturbed overnight after which the metal ion/sulphate were added depending upon the experimental set. Lab India pico model pH meter was used for adjusting the pH of the suspensions. The simultaneous addition of 241Am and sulphate was done for the sorption experiments that were carried out in presence of sulphate. All the experiments were performed at room temperature (25 ± 2 °C) in atmospheric conditions.
Kinetic studies of Am(III) sorption on Na-montmorillonite in absence and presence of sulphate were performed to determine the time required to attain the sorption equilibrium. The studies were carried out at pH 4.5 and 0.1 M NaCl with 241Am and sulphate concentration as 6.7 × 10−9 M and 5 × 10−3 M respectively. Percentage sorption at appropriate time intervals was determined by centrifugation of clay suspensions at 16,000 rpm for 45 min to separate the phases (solid and aqueous) followed by radiometric quantification of initial concentration of 241Am employed in the sorption experiment (A0) and 241Am concentration in the supernatant (A) using NaI(Tl) detector. Hence, % sorption for kinetic data sets were calculated using the equation:
The influence of pH on Am(III) (6.7 × 10−9 M) sorption by Na-montmorillonite was studied in the pH range 3–8 at constant ionic strength using 0.1 M NaCl. For the pH variation in presence of sulphate, the concentration of sulphate was kept 5 × 10−3 M. The effect of ionic strength on Am(III) (6.7 × 10−9 M) sorption by Na-montmorillonite was investigated in the range 0.1–2 M NaCl at two pH values (3 and 6) in absence and presence (5 × 10−3 M) of sulphate. Batch sorption experiments for pH and ionic strength variation were carried out by following same procedure as adopted for kinetics experiments. Table 1 gives the details of the Am(III) and sulphate concentrations along with the other parameters for the experiments pertaining to Am(III) sorption on Na-montnorillonite.
Surface complexation modeling
Bradbury and Baeyens developed ‘‘2-site surface complexation and cation exchange (2SPNE/CE)’’ model for modeling metal ion sorption on clay minerals [22]. Am(III) sorption on Na-montmorillonite has been modeled using the similar approach. For modeling the sorption profiles of Am(III), two different kinds of sites namely, ion exchange sites (X) and amphoteric sites (≡SOH) have been taken into consideration. The pH dependent protonation/deprotonation of amphoteric sites present at the edges of the clay platelets controls the formation of inner sphere metal complexes on clay minerals. The Am(III) sorption profiles were fitted using FITEQL 4.0 software [23].
The site types, capacity and protolysis constants of montmorillonite employed for modeling sorption profiles are given in Table 2 [22]. The ion exchange sites govern the charge distribution of the clay mineral in the entire pH range while contribution from amphoteric sites is relatively small (~ 5% of total sites), hence no explicit electrostatic term has been taken into consideration while modeling Am(III) sorption on Na-montmorillonite.
Results and discussion
Characterization of Na- montmorillonite clay
The detailed characterization of Na-montmorillonite has been reported in our previous study [21]. The cation exchange capacity, surface area, pore volume, constituent minerals elemental and mineralogical composition of Na-montmorillonite has been summarized in Fig. 1 [21].
Characterization Summary of Na-montmorillonite. Data taken from [21]
Aqueous speciation of Am(III)
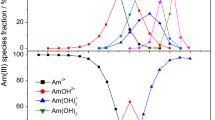
The aqueous speciation of Am(III) was generated using VISIUAL MINTEQ 3.1 [24]. The log K for Am(III)-complexes are listed in Table 3. The speciation diagram for Am(III) ([Am(III)] = 6.7 × 10−9 M) shows Am3+ is the predominant species in the pH range 2.0–6.5 followed by the dominance of different Am-hydroxo and Am-carbonate complexes (Fig. 2a). While, in the presence of 5 × 10−3 M sulphate, \({\text{AmSO}}_{{4}}^{ + }\) is the most dominant species (~ 58%) followed by Am3+ (~ 35%), in the pH range of 2.0–6.5 (Fig. 2b).
To understand the differences, if any, in the aqueous speciation of Am(III) and Eu(III), the speciation of Eu(III) has been generated, at same concentration as that of Am(III), using log K of Eu(III)-complexes [21]. In the case of europium (Fig. 3a), the dominant species is \({\text{EuCO}}_{{3}}^{ + }\) (~ 50%) with little contribution of EuOH2+(~ 8%) in the pH range 6.5 to 8 whereas for americium, AmOH2+ and \({\text{AmCO}}_{{3}}^{ + }\) are the major species (~ 40% each) in the similar pH range. The contribution of hydroxo species is higher for Am(III) compared to Eu(III). In presence of sulphate, for pH < 6.5, the predominant species are similar; however, their proportion varies for both the metal ions (Figs. 2b and 3b). Hence, the Am(III) aqueous speciation differs from Eu(III) aqueous speciation in terms of percentage distribution of species.
The aqueous speciation of Am(III) as function of ionic strength has been generated at pH 3 and 6, in absence and presence of sulphate (Fig. 4). In absence of sulphate at pH 3 (Fig. 4a), Am3+ is dominant species upto I = 1 M after which AmCl2+ dominates Am(III) speciation. The proportion of Am3+ decreases with increasing ionic strength due to the increase in the formation of AmCl2+. In contrast in presence of sulphate, Am3+, \({\text{AmSO}}_{{4}}^{ + }\), AmCl2+ are the species defining Am(III) speciation in the entire ionic strength range at pH 3, with their relative proportions changing with ionic strength (Fig. 4b). At pH 6, Am3+, AmCl2+ and \({\text{AmSO}}_{{4}}^{ + }\) exhibit a similar trend in Am(III) speciation as in pH 3 along with minor contribution from hydroxo and carbonato species of Am(III) (Fig. 4c, d).
Sorption of Am(III) by Na-montmorillonite in presence and absence of sulphate
Kinetics of sorption
The Fig. 5 shows the effect of sulphate on kinetics of Am(III) sorption by Na-montmorillonite, at pH 4.5. Am(III) sorption attains equilibrium (~ 75%) within 16 h, in absence of sulphate. However, lower equilibrium sorption (~ 60%) is attained in presence of sulphate. The presence of sulphate affected the Eu(III) sorption on Na-montmorillonite in similar manner as observed in present study [21]. For complete equilibrium sorption, equilibration time of 24 h and 48 h were fixed for binary and ternary systems.
Influence of pH
The pH dependent sorption profiles of Am(III) on Na-montmorillonite in presence and absence of sulphate are shown in Fig. 6. The sorption profile of Am(III) can be divided into three distinct pH regions depending upon the sorption.
-
i.
Lower pH (< 4.0) region: Am(III) sorption increases slowly with pH.
-
ii.
Intermediate pH range 4.0–6.0: sorption increases rapidly.
-
iii.
Above pH 6.0: sorption of Am(III) remains nearly constant with pH.
The observed trend is in accordance with the sorption profile of Am sorption in literature [20, 25, 26]. In presence of sulphate (5 × 10−3 M), the Am(III) sorption on Na-montmorillonite decreased significantly in the pH range 3.0–6.0, which signifies that presence of sulphate affects Am(III) sorption by Na-montmorillonite. \({\text{UO}}_{{2}}^{{2 + }}\) and Eu(III) sorption on montmorillonite also decreased in similar manner in presence of sulphate [13, 21]. At pH > 6.0, Am-hydroxo and Am-carbonate complexes are expected to govern Am(III) speciation, therefore Am(III) sorption onto Na-montmorillonite remains unaffected by presence of sulphate.
For understanding the role of sulphate in modifying sorption of Am(III) on Na-montmorillonite, two aspects should be taken into account i.e. sorption of sulphate on Na-montmorillonite and aqueous speciation of Am(III) in presence and absence of sulphate. Sulphate sorption on Na-montmorillonite was found to be negligible [21]. Hence, sulphate can be considered as complexing inorganic anion present in aqueous phase which modifies Am(III) sorption on Na-montmorillonite in the pH range 3.0–6.0. In the presence of sulphate, formation of singly charged Am(III)-sulphate complex results in less electrostatic attraction between Am(III) and clay surface resulting in decreased Am(III) sorption at lower pH values. Similar decrease in sorption of Am(III) by smectite rich natural clay has been observed in presence of sulphate [20].
Influence of ionic strength
The Fig. 7 represents the sorption of Am(III) by Na-montmorillonite in presence and absence of sulphate under varying ionic strength at pH 3.0 and 6.0. It is evident that at low pH (3.0), the sorption of Am(III) decreases with ionic strength, while at higher pH (6.0), it is independent of ionic strength. This can be explained in term of surface chemistry of Na-montmorillonite clay. There exist two types of sites, namely, ion exchange sites and edge sites [22]. At low pH (pH = 3), the decrease in sorption of Am(III) by Na-montmorillonite with increasing ionic strength indicates ion exchange mechanism for sorption. In contrast, lower Am(III) sorption at pH = 3 in presence of sulphate is attributed to \({\text{AmSO}}_{{4}}^{ + }\) (at I = 0.1 M NaCl: Fig. 7a), one of the dominant species in speciation, which experiences lesser electrostatic attraction towards clay surface as compared to Am3+. The ion sites are saturated with increase in ionic strength of background electrolyte resulting in decrease in sorption of Am(III) on clay surface. However at higher pH (6.0) the ionic strength invariant Am(III) sorption was observed, indicating surface complexation mechanism of sorption involving inner sphere surface complexes. Am(III) aqueous speciation is modified by presence of sulphate which in turn alters Am(III) surface speciation, thereby decreasing Am(III) sorption in presence of sulphate.
Modeling of Am(III) sorption profiles on Na-montmorillonite
The basis of selecting Am(III) surface species to optimize Am(III) sorption profiles is that the predominant aqueous Am(III) complexes undergo interaction with the clay minerals to form surface complexes. The aforementioned approach has its genesis from the Linear Free Energy Relation (LEFR) wherein a linear correlation exists between the stability constant of aqueous complexes and surface complexes [27, 28]. Surface Complexation Modeling (SCM) assumes the surface complexation is analogous to aqueous complexation. Hence, mass action and mass balance conditions applicable to aqueous complexes can be extended to surface complexes.
Na-montmorillonite-Am(III) system
The aqueous speciation of Am(III) exhibits Am3+, AmOH2+ and AmCO3+ as the predominant Am(III) species in the pH range chosen for sorption studies (Fig. 2a, b). As mentioned earlier, Na-montmorillonite possesses both ion exchange and amphoteric sites. The ionic strength variation studies reveal that in the lower pH range ion exchange is dominant mode of sorption. Hence, the ion exchange reaction for Am(III), given in Eq. (2), is taken into consideration while modeling the Am(III) sorption profile.
In addition, the monodentate Am(III) surface species pertaining to Am3+, AmOH2+ and AmCO3+ were considered in the optimization of Am(III) sorption profile. The choice of monodentate Am(III) surface species was based on spectroscopic studies of trivalent lanthanides on Na-montmorillonite [15]. The simultaneous consideration of monodentate mononuclear americium carbonato surface complex (≡SOAmCO3) and monodentate mononuclear americium hydroxo surface complex (≡SOAmOH+) along with ion exchange species (≡X3Am), monodentate mononuclear americium surface complex (≡SOAm2+) did not yield satisfactory fit. However, the sorption profile of Am(III) could be successfully achieved by considering ≡X3Am, ≡SOAm2+ and either ≡SOAmOH+ or ≡SOAmCO3 (Fig. 8a, b). The Am(III) surface complexation reactions are listed in Eqs. (3, 4 and 5).
The stability constants (log K) for the Am(III) surface species along with Eu(III) surface species on Na-montmorillonite are tabulated in Table 2. The log K for ion exchange for Am(III) is one order of magnitude less than that for Eu(III), even though hydrated radius of Am(III) (4.60 Aº [29]) and Eu(III) (4.50 Aº [30]). The observed difference in log K for the ion exchange reaction of Am(III) and Eu(III) can be attributed to slightly higher hydration energy of Eu(III) compared to Am(III). The log K for Am(III) surface species are slightly less than that for corresponding Eu(III) species, except the carbonate bearing surface species. The lesser value of log K for ≡SOAmCO3 in comparison with ≡SOEuCO3 is the manifestation of the differences in Am(III) and Eu(III) aqueous speciation.
Na-montmorillonite-Am(III)-sulphate systems
The modeling of Am(III) sorption on Na-montmorillonite in Na-montmorillonite-Am(III) -sulphate system was performed using the similar approach used for Na-montmorillonite -Am(III) system. It is worth mentioning that log K of Am(III) surface species obtained for Na-montmorillonite-Am(III) system were fixed while modeling Am(III) sorption on Na-montmorillonite-Am(III)-sulphate system. Primarily, the Am(III) surface complexes that fit the Am(III) sorption profile in Na-montmorillonite-Am(III) system were taken into consideration for fitting sorption profile of Am(III) in Na-montmorillonite-Am(III)-sulphate systems. However, fitting of Am(III) sorption profile in the lower pH range of 3–5 could not be achieved. AmSO4+ is one of the major contributors to the Am(III) aqueous speciation in presence of sulphate (Fig. 2b). In addition, sulphate bearing Am(III) surface complex ≡SOAmSO4 (Eq. (6)) has been employed to model Am(III) sorption on volcanic tuff/smectite rich natural clay in presence of sulphate [19,20,21].
The consideration of surface complex ≡SOAmSO4 along with the aforementioned species could not reproduce the Am(III) sorption profile in low pH range. Since ion exchange phenomenon is dominant at low pH for Na-montmorillonite, sorption of Am(III) via ion exchange of \({\text{AmSO}}_{{4}}^{ + }\) on Na-montmorillonite was considered (Eq. (7)).
The sorption profile of Am(III) on Na-montmorillonite-Am(III)-sulphate system could be successfully reproduced by considering ion exchange and surface complexation reaction of \({\text{AmSO}}_{{4}}^{ + }\) in addition to the surface species employed for fitting Am(III) sorption on Na-montmorillonite-Am(III). The log K of sulphate bearing Am(III) surface species are provided in Table 2. The modeled and experimental data considering ≡SOAmOH+ (Fig. 9a) and ≡SOAmCO3 (Fig. 9b) are shown in Fig. 9.
It can be seen from Table 2 that log K for sulphate bearing Am(III) surface species is nearly same as that of sulphate bearing Eu(III) species. Thus, it can be seen though the surface speciation trend of Am(III) on Na-montmorillonite resembles that of Eu(III) when Am(III)/Eu(III) carbonate species is considered to model the sorption profiles. Am(III) and Eu(III) surface speciation on Na-montmorillonite differs mainly with regard to surface hydroxo complexes. The subtle differences in surface speciation of Am(III) and Eu(III) is governed by their chemical properties.
Conclusions
Am(III) sorption on Na-montmorillonite increases with pH upto pH ≤ 6.0 and saturates thereafter in absence and presence of sulphate. In presence of sulphate, Am(III) sorption is found to be less in the pH range 3–6.0 owing to negligible sorption of sulphate on Na-montmorillonite and formation of Am(III) sulphate complexes in aqueous phase. The ionic strength variation studies revealed the dominance of ion exchange at lower pH (3–6) and surface complexation at higher pH. The aqueous speciation diagram of Am(III) and Eu(III) exhibited the difference in proportion of Am(III)/Eu(III) hydroxo complexes. The modeling of sorption profiles of Am(III) on Na-montmorillonite followed by its comparison with Eu(III) sorption profiles revealed significant differences in log K value of ion exchange reaction of Am(III) and Eu(III). Moreover, Am(III) sorption profile could be explained by considering either ≡SOAmOH+ or ≡SOAmCO3. Although, Eu(III) is an appropriate analogue to investigate Am(III) sorption behaviour, the aforementioned differences should be taken into account while extrapolating Eu(III) sorption behaviour to Am(III) systems.
References
Schwyn B, Wersin P, Ruedi J, Schneider J, Altmann S, Missana T, Noseck U (2012) FUNMIG integrated project results and conclusions from a safety case perspective. Appl Geochem 27:501–515
Missana T, Alonso U, Garcia-Gutierrez M, Mingarro M (2008) Role of bentonite colloids on europium and plutonium migration in a granite fracture. Appl Geochem 23:1484–1497
Lutzenkirchen J (2012) Summary of studies on (ad)sorption as a ‘“well-established”’ process within FUNMIG activities. Appl Geochem 27:427–443
Bradbury MH, Baeyens B (2005) Modelling the sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on montmorillonite: linear free energy relationships and estimates of surface binding constants for some selected heavy metals and actinides. Geochim Cosmochim Acta 69:875–892
Rabung Th, Pierret MC, Bauer A, Geckeis H, Bradbury MH, Baeyens B (2005) Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 1: batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim Cosmochim Acta 69:5393–5402
Wang X, Sun Y, Alsaedi A, Hayat T, Wang X (2015) Interaction mechanism of Eu(III) with MX-80 bentonite studied by batch, TRLFS and kinetic desorption techniques. Chem Eng J 264:570–576
Adebowale KO, Unuabonah IE, Olu-Owolabi BI (2005) Adsorption of some heavy metal ions on sulfate- and phosphate-modified kaolin. Appl Clay Sci 29:145–148
Zhu J, Cozzolino V, Fernandez M, Torres Sánchez RM, Pigna M, Huang Q, Violante A (2011) Sorption of Cu on a Fe-deformed montmorillonite complex: effect of pH, ionic strength, competitor heavy metal, and inorganic and organic ligands. Appl Clay Sci 52:339–344
Olu-Owolabi BI, Unuabonah EI (2010) Kinetics and thermodynamics of the removal of Zn2+ and Cu2+ from aqueous solution by sulphate and phosphate modified bentonite clay. J Hazad Mat 184:731–738
Fernandes MM, Baeyens B, Dahn R, Scheinost AC, Bradbury MH (2012) U(VI) sorption on montmorillonite in the absence and presence of carbonate: a macroscopic and microscopic study. Geochim Cosmochim Acta 93:262–277
Fernandes MM, Scheinost AC, Baeyens B (2016) Sorption of trivalent lanthanides and actinides onto montmorillonite: macroscopic, thermodynamic and structural evidence for ternary hydroxo and carbonato surface complexes on multiple sorption sites. Water Res 99:74–82
Troyera LD, Maillot F, Wang Z, Mehta VS, Giammar DE, Catalano JG (2016) Effect of phosphate on U (VI) sorption to montmorillonite: ternary complexation and precipitation barrier. Geochim Cosmochim Acta 175:86–99
Bachmaf S, Planer-Friedrich B, Merkel BJ (2008) Effect of sulphate, carbonate, and phosphate on the uranium(VI) sorption behavior onto bentonite. Radiochim Acta 96:359–366
Fernandes MM, Baeyens B, Bradbury MH (2008) The influence of carbonate complexation on lanthanide/actinide sorption on montmorillonite. Radiochim Acta 96:691–697
Chen Z, Jin Q, Guo Z, Montavon G, Wu W (2014) Surface complexation modeling of Eu(III) and phosphate on Na-bentonite: binary and ternary adsorption systems. Chem Eng J 256:61–68
Patel MA, Kar AS, Kumar S, Tomar BS (2017) Effect of phosphate on sorption of Eu(III) by montmorillonite. J Radioanal Nucl Chem 313:537–545
Liu X, Simunek J, Li L, He J (2013) Identification of sulfate sources in groundwater using isotope analysis and modeling of flood irrigation with waters of different quality in the Jinghuiqu district of China. Environ Earth Sci 69:1589–1600
Samborska K, Halas S, Bottrell SH (2013) Sources and impact of sulphate on groundwaters of Triassic carbonate aquifers, Upper Silesia. Pol J Hydrol 486:136–150
Ding M, Kelkar S, Meijer A (2014) Surface complexation modeling of americium sorption onto volcanic tuff. J Env Radioactiv 136:181–187
Kumar S, Pente AS, Bajpai RK, Kaushik CP, Tomar BS (2013) Americium sorption on smectite-rich natural clay from granitic groundwater. Appl Geochem 35:28–34
Patel MA, Kar AS, Kumar S, Das MK, Raut VV, Tomar BS (2019) Effect of sulfate on sorption of Eu(III) by Na-montmorillonite. Radiochim Acta 107(2):115–128
Bradbury MH, Baeyens B (2002) Sorption of Eu on Na- and Ca-montmorillonite: experimental investigations and modelling with cation exchange and surface complexation. Geochim Cosmochim Acta 66:2325–2334
Herbelin AL, Westall JC (1999) FITEQL—a computer program for determination of chemical equilibrium constant from experimental data. Department of chemistry. Oregon State University, Oregon, pp. 97331
Gustafsson JP (2014) Visual MINTEQ ver. 3.1.
Bradbury MH, Baeyens B (2006) Modelling sorption data for the actinides Am(III), Np(V) and Pa(V) on montmorillonite. Radiochim Acta 94:619–625
Coppin F, Berger G, Bauer A, Castet S, Loubet M (2002) Sorption of lanthanides on smectite and kaolinite. Chem Geol 182:57–68
Bradbury MH, Baeyens B (2009) Sorption modelling on illite Part II: actinide sorption and linear free energy relationships. Geochim Cosmochim Acta 73:1004–1013
Dzombak DA, Morel FM (1990) Surface complexation modeling: hydrous ferric hydroxide. Wiley-Interscience, New York
Choppin GR, Jensen MP (2006) Actinides in solution: complexation and kinetics In: Morss LR, Edelstein NK and Fuger J (eds) In: The chemistry of actinide and transactinide elements. Springer, Dordrecht
Kielland J (1937) Individual activity coefficients of ions in aqueous solutions. J Am Chem Soc 59:1675–1678
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kar, A.S., Patel, M.A. & Tomar, B.S. Retention of Am(III) by montmorillonite: effect of sulphate. J Radioanal Nucl Chem 332, 3069–3077 (2023). https://doi.org/10.1007/s10967-023-09014-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09014-z