Abstract
The desorption of uranium from a U(VI)-contaminated C-S-H matrix has been investigated as a function of the EDTA concentration in solution under N2- and ambient atmosphere. The Kd values evaluated from the experimental data indicate that U(VI) is retarded in the solid phase due to sorption of EDTA through interaction with U(VI) at the C-S–H surface. On the other hand, under ambient conditions the formation of stable U(VI)-carbonato species results in higher U(VI) concentration in solutions compared to corresponding systems under N2-atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Before final storage in underground repositories low- and intermediate-level radioactive waste is usually solidified in cementitious matrices [1]. The main binding component of a cementitious matrix is calcium silicate hydrate (C-S-H). C-S-H has a layered structure, which consists of Ca–O sheets linked on each side to silicate chains [2]. The immobilization of uranium by the C-S-H matrix occurs via sorption of U(VI) species (e.g. UO2(OH)3−, UO2(OH)42−)) on the solid surface and incorporation of the radionuclide in the C-S-H phase. The effective incorporation of lanthanide and actinide ions by substituting Ca ions in the interlayers and the Ca–O layer of the C-S-H phase is ascribed to its high recrystallization rate [3].
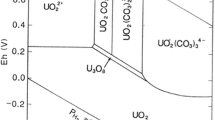
Uranium is used as primary fuel nuclear power reactors and therefore is an important component of nuclear waste. Under the redox conditions prevailing in the alkaline cementitious environment, uranium is expected to exist predominantly in its hexavalent oxidation state (U(VI)) and predominantly in the form of the tri- or tetra-hydroxo uranyl complex (UO2(OH)3−, UO2(OH)42−) [4, 5]. According to the Eh–pH diagram calculated using the Geochemist’s Workbench Ⓡ [5], the tri-hydroxo uranyl complex (UO2(OH)3−) is the predominant species in the pH range between 8 and 12, whereas the tetra-hydroxo uranyl complex (UO2(OH)42−) dominates for pH > 12. The geochemical behavior of uranium in the near field of the nuclear waste repositories is of particular interest, because of the amounts the element and its isotopes are present in the spent fuel, as well as because the U(VI) chemistry is expected to be similar to other hexavalent actinides (e.g. Pu(VI)) [6]. The interaction of U(VI) with C-S-H has been subject of several studies, which investigated its sorption by the cementitious phase and applied sophisticated spectroscopies to better understand and describe the interaction between U(VI) and C-S-H [4, 7,8,9,10,11,12,13]. Investigations have been carried out also on ternary U(VI)-C-S-H organic ligand systems [14], including EDTA [15]. These studies indicate the strong impact of the organic ligand on the sorption of U(VI) by C-S-H. Nevertheless, there are no systematic studies on the effect of EDTA on the desorption of U(VI) from C-S-H matrices, which have been previously contaminated with uranium during preparation.
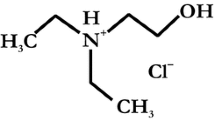
EDTA (ethylene diamine tetraacetic acid) is a chelating agent that is found at increased levels in the environment and in radioactive wastes because it has been used as a decontamination agent in nuclear facilities and other industrial processes [16]. EDTA is a hexadentate ligand that forms very stable complexes with polyvalent metal ions, including U(VI) and therefore governs their stability in the aqueous solutions [17] and affects their leaching from cementitious and other mineral matrices [18, 19]. Generally, investigations on the (de)sorption of uranium by C-S-H matrices are fundamental regarding the safety and performance assessment of radioactive waste disposal facilities. This is because cementitious matrices are the main components of the engineered barrier, which encapsulate and isolate uranium and other (radio)toxic elements from the biosphere [1, 7,8,9,10,11].
In this study, the impact of EDTA on the desorption of U(VI) from a cementitious C-S-H matrix has been investigated by contacting a U(VI)-contaminated C-S-H phase with EDTA solutions of varying concentrations. The experiments were performed under N2- and ambient atmosphere to study the effect of carbonate on the uranium desorption. In addition, the related solid phases have been characterized by XRD and FTIR measurements.
Experimental
In all experiments, analytical grade reagents and de-ionized water were used. The 232U-tracer solution, which was added for the uranium quantification, was obtained from National Physical Laboratory (NPL, Teddington, UK). The EDTA solutions of varying concentrations (0.0001, 0.001, 0.01 and 0.1 M) have been prepared by dissolution of disodium EDTA dihydrate (C10H14N2O8 2Na·2H2O, Sigma-Aldrich, Darmstadt, Germany) in de-ionized water. The U(VI) stock solution was prepared by dissolution of UO2(NO3)2·6H2O (Merck) in de-ionized water.
Solid phase preparation and characterisation
Solid calcium-silicate-hydrate (C-S-H) has been synthesized according to Maddalena et al. [20] at a C:S ratio of 1.27 and a U(VI)/Ca(II) ratio of 1/10000. The solid product has been characterized by FTIR spectroscopy (FTIR-ATP 8900, IR Prestige-2, Shimadzu,) and X-Ray diffraction (Shimadzu XRD-6000 Series). The preparation of C-S-H was carried out by mixing 12.02 g Ludox (50%, Sigma-Aldrich, Darmstadt, Germany) with 8.52 g CaO (Sigma-Aldrich, Darmstadt, Germany) in 35 mL aqueous solution containing U(VI), under N2. The product was cast in cubes and left for one month under water-vapour saturated N2-atmosphere. Following, the C-S-H cubes have kept overnight in dried-aceton to remove excess water and finally dried under vacuum at 70 °C for 24 h.
Desorption studies
The desorption investigations were performed in batch type experiments using 0.2 g C-S-H/U in 20 mL aqueous solution of EDTA (0, 0.0001, 0.001 and 0.01 M), at a U(VI)/Ca(II) ratio of 1/10000 and pH 11. Τhe experiments were performed under ambient conditions (e.g. 23 ± 2 °C, 0.03% CO2) and N2 atmosphere to investigate the effect of carbonate on the desorption of U(VI) from the studied system. After 30 days contact time, aliquots of the solution have been taken, filtrated by means of membrane filters (pore size: 450 nm) and the uranium concentration in solution was analysed by alpha-spectroscopy (Alpha Analyst Integrated Alpha Spectrometer, Canberra) after electrodeposition on stain-less steel discs as described elsewhere [21]. Prior electro-deposition the solution was traced with U-232 (50 mBq) to evaluate the uranium yield.
The partition coefficient, Kd (L/kg), is here defined as the ratio of the amount (in moles) of the radionuclide sorbed per mass of dry C-S-H (Cads in mol/kg) to the equilibrium concentration of the radionuclide in solution (Caq in mol/L). Because of the relative low amount of uranium added in the system used we can assume that the surface binding sites are in great excess with respect to the radionuclide concentration in solution. Therefore, we expect a linear relation between sorbed and non-sorbed species.
Results and discussion
Solid phase characterisation
The FTIR spectra of the C-S-H solid precipitated in the absence (C-S-H) and in the presence of U(VI) are shown in Fig. 1. In both spectra, the broad peak at 3450 cm−1 is ascribed to the O–H streching vibration, whereas the band at 1636 cm−1 corresponds to the bending vibration of the coordinated water. In addition, the strong peak at 1430 cm−1 is related to the bending mode of the Ca-OH vibration and the peak at 964 cm−1 could be associated with the antisymmetric stretching vibration of Si–O–Si and the stretching vibration of O–Si–O [22].
The spectra in Fig. 1 almost identical indicating that two solids formed under different conditions are similar. This was expected because the amount of U(VI) added to the precursor solution was relatively low with a U(VI)/Ca(II) ratio equal to 1:10,000. In addition, the x-ray diffractograms of the two solids have been obtained and compared.
The diffractograms of the two C-S-H solids are shown in Fig. 2. and are characteristic C-S-H diffractograms with a disordered layered structure similar to tobermorite [22]. However, a careful observation of the peak position in the 2θ range between 35 and 50 degrees, reveals a small shift of the peaks to higher 2θ values in the diffractogram of the U(VI)-contaminated C-S-H sample. This is an indication that U(VI) has been incorporated in the C-S-H phase forming a solid solution [23].
Effet of EDTA on the U(VI) desorption from C-S-H
In a previous study we investigated the effect of EDTA on the sorption of U(VI) by a similar C-S-H solid [15]. In the previous study the uranium was added in the EDTA solution and then contacted with the C-S-H phase, while in this study the U(VI) was co-precipitated with the C-S-H during the cementitious solid preparation from the precursor materials. This is of particular interest because the distribution coefficients obtained from the two different approaches may indicate on similarities and differences regarding the sorption/desorption process.
Figure 3 summarizes partition coefficient values as a function of the EDTA concentration in solution under N2- and ambient atmosphere. Surprisingly, in the systems under N2-atmosphere the Kd values increase with increasing EDTA concentration in solution and are generally higher (4.9 < [log10Kd] < 5.8) than the corresponding Kd value (log10Kd = 4.9) determined in the EDTA-free suspension. This is in contradiction with the results obtained from sorption experiments, which revealed the opposite effect due to stabilization of U(VI) in solution in the form of U(VI)-EDTA complexes [15]. The increased Kd values, which are associated higher U(VI) amounts adsorbed by the C-S-H phase could be ascribed to enhanced sorption of EDTA through an interaction with calcium and uranium ions present at the C-S-H surface and the formation of ternary surface complexes [24].
It is notable that the highest Kd value (log10Kd = 5.8) and lowest U(VI) desorption from the C-S-H phase is observed at an initial concentration of 0.001 M EDTA under the existing experimental conditions (e.g., 0.2 g C-S-H in 20 mL solution). A similar effect was observed in a previous study, where increased EDTA sorption by the C-S-H phase was observed under similar experimental conditions [15]. In the latter case the retardation of EDTA was ascribed to the sorption of EDTA through an interaction with calcium ions at the C-S-H surface [24]. Similarly, EDTA could be sorbed in the C-S-H phase via interaction with the U(VI) cations present in the solid phase. This interaction retards U(VI) in the solid phase resulting in lower U(VI) levels in solution compared to EDTA-free solutions.
On the contrary, under ambient atmosphere the Kd values (3.4 < [log10Kd] < 4.5) obtained are generally lower than the Kd value determined in the EDTA-free suspension and significantly lower than the corresponding Kd values obtained under N2-atmosphere. This indicates that under ambient conditions the equilibrium is shifted towards the U(VI) species in solution. Under ambient atmospheric conditions and at pH 11, CO2 is extensively dissolved in the aqueous phase and exists predominantly in the form of the carbonate anion (CO32−) [15, 25]. U(VI) forms with carbonate anions very stable complexes (e.g. UO2(CO3)34−, logβ = 19.5) [25], which compete the U(VI) sorption by C-S-H, resulting in lower Kd values. Similar behavior was observed also in the case of experiments related to the U(VI) sorption by C-S-H in the presence of varying EDTA concentrations and the higher U(VI) levels had been ascribed to the formation of U(VI)-carbonato species [15, 25], which compete the sorption by C-S-H and stabilize U(VI) in solution as described by Eq. 2.
Conclusions
U(VI) in a C-S-H phase is stabilized in the presence of EDTA due to sorption of the organic molecule through interaction with U(VI) at the C-S-H surface. The effect is more pronounced when the experiments are performed under N2-atmosphere.
Under ambient conditions the formation of stable U(VI)-carbonato species results in higher U(VI) concentrations in solutions compared to corresponding systems under N2-atmosphere.
References
Grambow B (2016) Geological disposal of radioactive waste in clay. Elements 12:239–245
Richardson IG (2008) The calcium silicate hydrates. Cem Concr Res 38:137–158
Tits J, Stumpf T, Rabung T, Wieland E, Fanghänel T (2003) Uptake of Cm(III) and Eu(III) by calcium silicate hydrates: a solution chemistry and time-resolved laser fluorescence spectroscopy. Study Environ Sci Technol 37:3568–3573
Tits J, Walther C, Stumpf T, Mace N, Wieland E (2015) A luminescence line-narrowing spectroscopic study of the uranium(VI) interaction with cementitious materials and titanium dioxide. Dalton Trans 44:966–976
Ochs M, Mallants D, Wang L (2016) Radionuclide and metal sorption on cement and concrete. Springer, Switzerland. DOI https://doi.org/10.1007/978-3-319-23651-3
Pashalidis I, Runde W, Kim IJ (1993) A study of solid-liquid phase equilibria of Pu (VI) and U (VI) in aqueous carbonate systems. Radiochim Acta 61:141–146
Zhang W, Wang J (2017) Leaching performance of uranium from the cement solidified matrices containing spent radioactive organic solvent. Ann Nucl Energy 101:31–35
Atkins M, Glasser FP (1992) Application of portland cement-based materials to radioactive waste immobilization. Waste Manage 12:105–131
Hongbin T, Yuxiang L (2005) The existence state of uranium(VI) in portland cement matrix material immobilization body. Uranium Min Metall 38:86–90
Wang F, Chen G, Ji L, Yuan Z (2020) Preparation and mechanical properties of cemented uranium tailing backfill based on alkali-activated slag. Adv Mater Sci Eng 2020:1–7
Ochs M, Vriens B, Tachi Y (2018) Retention of uranium in cement systems: effects of cement degradation and complexing ligands. Prog Nucl Sci Technol 5:208–212
Macé N, Wieland E, Dähn R, Tits J, Scheinost AC (2013) EXAFS investigation on U(VI) immobilization in hardened cement paste: influence of experimental conditions on speciation. Radiochim Acta 101:379–389
Tits J, Geipel G, Macé N, Eilzer M, Wieland E (2011) Determination of uranium(VI) sorbed species in calcium silicate hydrate phases: a laser-induced luminescence spectroscopy and batch sorption study. J Colloid Interface Sci 359:248–256
Androniuk I, Landesman C, Henocq P, Kalinichev GA (2017) Adsorption of gluconate and uranyl on C-S-H phases: combination of wet chemistry experiments and molecular dynamics simulations for the binary systems. Phys Chem Earth 99:194–203
Maragkou E, Pashalidis I (2021) Investigations on the interaction of EDTA with calcium silicate hydrate and its impact on the U(VI) sorption. Coatings 11:1037
du Bois de Maquillé L, Renaudin L, Goutelard F, Jardy A, Vial J, Thiébaut D (2013) Determination of ethylenediaminetetraacetic acid in nuclear waste by high-performance liquid chromatography coupled with electrospray mass spectrometry. J Chromatogr A 1276:20–25
Paschalidou P, Pashalidis I (2019) Alpha-spectroscopic analysis of uranium in ground- and seawater samples after EDTA-masking of interfering cations. J Radioanal Nucl Chem 321:973–975
Paschalidou P, Pashalidis I (2019) Selective separation and determination of uranium in calcite and gypsum after EDTA-mediated sample dissolution and cation-exchange. J Radioanal Nucl Chem 320:807–812
Paschalidou P, Pashalidis I (2019) Recovery of uranium from phosphate rock with EDTA-mediated dissolution and cation exchange. Hydrometallurgy 189:105118
Maddalena R, Li K, Chater AP, Michalik S, Hamilton A (2019) Direct synthesis of a solid calcium-silicate-hydrate (C-S-H). Constr Build Mater 223:554–565
Kiliari T, Pashalidis I (2010) Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat Meas 45:966–968
Grangeon S, Claret F, Linard Y, Chiaberge C (2013) X-ray diffraction: a powerful tool to probe and understand the structure of nanocrystalline calcium silicate hydrates. Acta Crystallogr B 69:465–473
Matsumoto K, Nonaka R, Wang Y, Veryasov G, Hagiwara R (2017) Formation of a solid solution between [N(C2H5)4][BF4] and [N(C2H5)4][PF6] in crystal and plastic crystal phases. Phys Chem Chem Phys 19:2053–2059
Nalet C, Nonat A (2016) Ionic complexation and adsorption of small organic molecules on calcium silicate hydrate: relation with their retarding effect on the hydration. Cem Concr Res 89:97–108
Pashalidis I, Czerwinski KR, Fanghaenel T, Kim JI (1997) A study of solid–liguid phase equilibria of Pu(VI) and U(VI) in aqueous carbonate systems. determination of the carbonate stability constants. Radiochim Acta 76:55–62
Acknowledgements
The project leading to this application has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 847593.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maragkou, E., Pashalidis, I. The effect of EDTA on the desorption of uranium from calcium silicate hydrate matrices. J Radioanal Nucl Chem 331, 507–510 (2022). https://doi.org/10.1007/s10967-021-08089-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-08089-w