Abstract
The analytical technique has been developed for the extraction and determination of uranium(VI). This process is based on the ion-pair complex formation of uranium(VI) with 2-octylaminopyridine in xylene. Uranium(VI) is quantitatively extracted by optimizing the parameters of solvent extraction in presence of sodium acetate, such as pH, concentration of weak organic acids, strippant, shaking period, solvent study, and concentration of extractant. A mechanism of extraction was proposed based on the slope ratio analysis method. The interference of various cations and anions was also investigated. The versatility of the developed method was investigated by employing it to binary and ternary mixtures. The robustness of the method was demonstrated by determining uranium(VI) in the bone sample.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is the most vital element for nuclear energy [1]. It is universal that uranium is toxic and is being radioactive; thus the safety profiles for uranium compounds are well established [2]. Hence it is one of the elements for which world health organization (WHO) norms are most stringent (30 μg/L) in drinking water. This is because uranium is a relatively contaminating element in many surface or near-surface environments, its geological exploration requires the measurement of trace quantities of metal ions in water and other samples [3,4,5]. The natural source for uranium is monazite which contains a sizeable amount of uranium. Consequently, the separation purification of uranium is of great practical importance. The methodology adopted for extraction and purification from ore and nuclear fuel reprocessing has always attracted the attention of separation scientists. The efficient and selective extraction of uranium from aqueous solution has been a subject of significant interest because of the global shortage of uranium sources and radioactive contaminants in the environment, soil, water in which versatile new materials that can separate efficiently desired ore [6,7,8].
A large number of modern analytical tools have been available for the determination of uranium in a wide variety of samples, The increasing availability of powerful instrumental techniques such as neutron activation analysis (NAA), energy dispersive X-ray fluorescence (EDXRF), inductively coupled plasma atomic emission spectrometry (ICP-AES); inductively coupled plasma emission mass spectrometry (ICP-MS) has enabled the analysis of complex mixtures with high accuracy and precision [9,10,11,12,13,14]. Even though these techniques provide high sensitivity and favorable detection limit, their application requires rather expensive equipment and leads to higher running cost [15]. Generally, solvent extraction and spectrophotometric technique are widely used to separate and determine uranium(VI) [16,17,18,19,20].
In this context, attentions have been given for the development of soft donor complexing agents as extractants for Ln/An separation. Relatively soft donor atom such as nitrogen prefers 5f elements than 4f elements, providing the higher selectivity than harder oxygen atoms. Concerning this high molecular weight, amines act as better extractant to oxygen-containing extractants like tri-n-butyl phosphate (TBP), trioctyl phosphine oxide (TOPO), and dioctyl sulfoxide (DOSO). This difference allows for preferential coordination with actinides (5f) over lanthanides. Hence amines with soft nitrogen atoms are a subject of extensive research as a potential extractant of actinides from high-level liquid waste (HLLW) [21]. The literature survey demonstrates that the solvent extraction of uranium(VI) can be carried out by various extractants like tri-n-butyl phosphate, trioctyl phosphine oxide and dioctyl sulfoxide [22], cyanex-272 [23]. This manuscript describes the extraction and separation of uranium(VI) from bone sample and associated elements using 2-octylaminopyridine (2-OAP) in sodium acetate medium. The advantage of this method over the earlier method is that extraction is in acetate medium which is a relatively greener approach than the earlier where extraction was carried out in mineral acids. The concentration of 2-OAP is moderate and it is synthesized in a laboratory at a low cost. The method is rapid, simple, and applicable for extraction and determination of uranium(VI) from binary, ternary mixture and bone sample.
Experimental
Apparatus
Digital spectrophotometer optimized α was used for the absorption measurement using 1 cm quartz cells. An Elico digital pH meter model LI-127 is used to measure the pH. All weighing operations were carried out by METLER TOLEDO analytical single pan balance model ML-204/-01 having accuracy 1 × 10–4 g.
Reagents
Standard uranium(VI) solution
A solution of uranium(VI) (1 mg mL−1) was prepared by dissolving 2.109 g of uranyl nitrate hexahydrate [UO2 (NO3)2·6H2O]; (Analytical R. grade, BDH, Poole, UK) in water containing one mL of conc., HNO3. The solution was diluted with water to one liter in a volumetric flask. Standardized by precipitating ammonium diuranate and ignited to U3O8. and prepared working solution by appropriate dilution in 0.01 M HNO3 [24].
2-Octylaminopyridine (2-OAP)
2-OAP was synthesized by the Borsch and Petrukhin method [25] and the working extractant solution having molarity (0.05 M) was prepared in xylene.
Buffer solution: pH 8.6
Dissolved 2 g of boric acid and 2 g of potassium chloride in water followed by 6.5 mL of 1 M NaOH and diluted to 500 mL.
Arsenazo-I
Arsenazo-I (0.05% w/v) was prepared by dissolving 0.05 g of arsenazo-I (s. d. Fine –chem. limited) in water.
All reagents and metal salts used are of analytical grade and their solutions were prepared in water and mineral acid. Double distilled water was used throughout the experiment.
Recommended procedure
A solution containing 200 µg uranium(VI, was mixed with 0.01% w/v sodium acetate and pH was adjusted to 4.0 with dil. HCl and NaOH by maintaining total dilution volume to 25 mL and then transferred to 125 mL separatory funnel. 10 mL 0.05 M 2-OAP in xylene as an extractant was added in separatory funnel and equilibrated for 5 min, two phases were allowed to separate. The uranium(VI) extracted in the organic phase was back-extracted with 3 M HCl (3 × 10) mL as a strippant solution.
The stripped solution containing uranium(VI) was evaporated to moist dryness, then 0.5 mL of conc. perchloric acid was added to decompose organic matter and diluted to 30 mL with water. Again it was evaporated to moist dryness and extracted into 30 mL of water. The pH was adjusted to 4.0 and transferred it into 50 mL volumetric flask, 4 mL 0.05% w/v arsenazo(I) solution was added and followed by 10 mL of buffer solution (pH 8.6) and diluted up to the mark. The absorbance was measured at 600 nm against a reagent blank [27].
Results and discussion
Effect of pH
The extraction of uranium(VI) was carried by 10 mL of 0.05 M 2-OAP in xylene in the presence of 0.01 M sodium acetate from pH 1–10. The study elucidates that the quantitative extraction was achieved in the pH range 3.8–4.8 (Fig. 1). With an increase in pH, the extraction decreases because, at higher pH, uranium(VI) in acetate medium will form a less stable ion-pair complex with 2-OAP. The pH 4.0 was selected for further extraction.
Impact of weak organic acid concentration
The distribution ratio (D) of uranium(VI) was investigated at pH 4.0 with 10 mL of 0.05 M 2-OAP in xylene in the presence of varying concentrations of different weak organic anions like acetate, succinate, malonate, and citrate. Quantitative extraction of uranium(VI) was found from acetate and succinate media. However, acetate gives more reproducible results, and more importantly it is greener, cost-effective. The weak acid curve of sodium acetate indicates that quantitative extraction was taking place in the conc. range of 0.008 to 0.011 M. In general procedure 0.01 M sodium acetate was recommended throughout the experiment (Fig. 2). The salicylate, malonate, citrate, and oxalate do not give quantitative extraction of uranium(VI) as there was no formation of stable ion-pair complexes.
Effect of 2-OAP concentration
The concentration of 2-OAP was studied in a concentration range 0.001 to 0.5 M to optimize extraction conditions. The study reveals that extraction increases up to 0.035 M and remained constant up to 0.06 M. However further increase in reagent concentration, extraction decreases this might be due to the formation of stable 2-OAP-acetate species. Therefore for quantitative extraction 10 mL 0.05 M of 2-OAP was used throughout the experiment (Fig. 3).
Solvent study
The use of a suitable solvent is very vital in solvent extraction. The various solvents were studied such as amyl alcohol, 1,2-dichloroethane, dichloromethane, xylene, nitrobenzene, n-butanol, kerosene, methyl-isobutylketone, chloroform, toluene, benzene (Table 1). The extraction of uranium(VI) was found to be quantitative in xylene and nitrobenzene with 0.05 M 2-OAP. It was found that there is any significant relation between dielectric constant and percentage extraction, xylene was selected as solvent for extraction of uranium(VI) which has low cost and showed clear phase separation.
Influence of stripping agents
Stripping is the reverse of extraction or back extraction which directly affects the extraction of uranium(VI). The back extraction uranium(VI) from the loaded organic phase was performed with stripping agents such as HNO3,HCl, CH3COOH, NH3, NaOH for the quantitative extraction of uranium(VI) with 10 mL of 0.05 M 2-OAP. There was quantitative recovery of uranium(VI) with HCl in the range 2.5–4 M. Hence 3 M HCl, was recommended for further study (Fig. 4). The stripping mechanism of uranium(VI) is represented.
Effect of organic to aqueous volume ratio
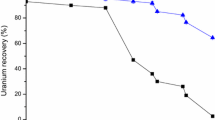
The different volumes of aqueous phase to organic phase was investigated by keeping organic phase volume constant. The investigation was carried out in the range of 1:1–1:30. It was found that 1:1–1:5 ratios give quantitative extraction of uranium(VI). Beyond 1:5 ratio the distribution ratio decreases because of lack of 2-OAP extractant due to an increase in volume (Fig. 5). Therefore 1:2.5 ratio of organic to aqueous was recommended for the proposed method for practical suitability to avoid the losses of chemicals.
Metal loading capacity
Extraction of uranium(VI) was examined as a function of metal loading capacity at various concentration of uranium(VI) in the range of 100–1500 µg per 10 mL. The quantitative extraction took place in the range 100–900 µg. It means that to extract up to 900 µg of uranium(VI), 10 mL 0.05 M 2-OAP was enough and thereafter extraction decreases. Illustrating that there was less availability of 2-OAP. Therefore the loading capacity of 10 mL of 0.05 M 2-OAP was 900 µg of uranium(VI).
Stoichiometry of extracted species
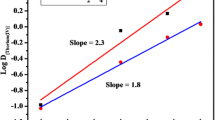
The extraction mechanism of uranium(VI) was proposed by examining the experimental data and based on the slope ratio analysis method. The plot of log D[U(VI)] versus log C[acetate] at fixed pH 3 and 6 was linear with slopes 1.97 and 2.16 respectively. This indicates that two acetate ions react with one UO22+ species (Fig. 6).
The plot of log D[U(VI)] versuss log C[2-OAP] at fixed pH 3 and 6 showed a slope 1.63 and 1.60 respectively. Which illustrates that two moles of 2-OAP take part in reaction with one mole of uranium(VI) (Fig. 7). Therefore the slope analysis method proposes the possible composition of species as 1:2:2 (Metal:acetate:2-OAP). The probable mechanism of extraction based on the slope ratio analysis method as follows
The effect of foreign ions
The interference of different foreign cations and anions on the extraction of uranium(VI) was examined with 0.01 M sodium acetate and at pH 4. The study showed that there was a sufficient tolerance limit for anions and cations. However the Th(IV), Zr(IV), Pb(II), Mn(VII) showed interference which was eliminated by the use of sequestering agent and tolerance limit was enhanced to cause allowed ± 2% error in the recovery of uranium(VI) (Table 2).
Applications
Separation of uranium(VI) from associated metal ions
The versatility of the proposed method was checked by carrying out extraction of uranium(VI) with various associated metal ions such as Zr(IV), Ce(IV), Y(III), Th(IV), La(III), Nd(III), Sm(III), Cd(II), Pb(II), Ba(II) and Ru(III) with 10 mL of 0.05 M 2-OAP in xylene from 0.01 M acetate medium. A very few metal ions like Zr(IV), Th(IV), Pb(II) have interfered which are masked by suitable masking agents. Added metal ion was determined by a reported method using chromogenic reagent spectrophotometrically [24, 26,27,28] whereas uranium(VI) was determined spectrophotometrically by aresnazo-I method (Table 3).
Separation of uranium(VI) from ternary mixture
A ternary mixture of uranium(VI) with Th(IV), Zr(IV); La(III), Ce(IV); Cd(II), Pb(II); Th(IV), Fe(II); Th(IV), Pd(II); Y(III), La(III); Ru(III), Pd(II); Sm(III), Nd(III); Pb(II), Th(IV); Zr(IV), Ru(III); were prepared and are subjected to extraction by general procedure. Associated metal ions remained in the aqueous phase whereas from loaded organic phase containing uranium(VI) was stripped with 3 M HCl (3 × 10) mL and determined spectrophotometrically by arsenazo-I (Table 4). The ions like Zr(IV) and Th(IV) have interfered but their interference was removed by using a suitable masking agent.
Determination of uranium(VI) from bone sample
The beauty of the proposed method was that we are successfully separated and determined uranium(VI) from a bone sample with 0.05 M 2-OAP as an extractant. The bone sample was collected carefully and crushed to suitable fineness, dried perfectly by keeping it in an oven at temperature 363 K for about two hours. The sample was cooled and 0.900 g was transferred in beaker, 2 mL conc. perchloric acid was added and followed by 5 mL conc. nitric acid and heated up to moist dryness on hot plate. The residue was extracted in water and diluted to 25 mL with water. One mL of sample solution was employed for the extraction of uranium(VI) by the proposed method and determined uranium(VI) by arsenazo-I method (Table 5).
Conclusion
The solvent extraction of uranium(VI) from weak organic acid media by 2-OAP as an extractant was investigated. The optimum conditions for the extraction of uranium(VI) were investigated. The extraction mechanism was proposed on the basis of slope ratio analysis method. The beauty of proposed method was that it was successfully employed for the determination of uranium(VI) from a synthetic mixture of associated metal ions and bone sample. The method is simple, reliable and reproducible. It does not require sophisticated instrumentation. The extraction of uranium(VI) was carried out from acetate medium, this shows that the method is eco-friendly belong to green chemistry approach.
References
Seaborg GT, Katz JJ (1957) The actinide elements. McGraw-Hill, New York
Madrakian T, Afkhami A, Mousavi A (2007) Spectrophotometric determination of trace amounts of uranium(VI) in water samples after mixed micelle-mediated extraction. Talanta 71:610–614
Miyake M, Sugimara Y, Mayeda M (1970) The Uranium content and the activity ratio 234U/238U in marine organisms and sea water in the western north pacific. J Oceanogr Soc Jpn 26:123–129
Shamsipur M, Saeidi M, Yari A, Yaganeh-Faal A, Mashhadizadeh MH, Azimi G, Naeimi H, Sharghi H (2004) UO22+ Ion-selective membrane electrode based on a Naphthol-Derivative Schiff’s Base 2,2’-[1,2-Ethandiyl bis(nitriloethylidene)]bis(1-naphthalene). Bull Korean Chem Soc 25:629
Shannon SS (1977) The HSSR programme and its relation to the nure effort symposium on hydrogeochemical and stream sediment reconnaissance for uranium in the unites states, grant junction, CO
Carboni M, Abney CW, Liu SB, Lin WB (2013) Highly porous and stable metal-organic frameworks for uranium extraction. Chem Sci 4:2396–2402
Sather AC, Berrymanb OB, Rebek J (2013) Jr. Selective recognition and extraction of the uranyl ion from aqueous solutions with a recyclable chelating resin. Chem Sci 4:3601–3605
El-Taher A (2010) INAA and DNAA for uranium determination in geological samples from Egypt. Appl Radiat Isot 68:1189–1192
El-Taher A, Nossair A, Azzam AH, Kratz KL, Abdel-Halim AS (2004) Determination of traces of uranium and thorium in some egyptian environmental matrices by instrumental neutron activation analysis (pp). J Environ Prot Eng 30:19–30
Afzal M, Hanif J, Saleem M, Hanif I, Ahmed R (1991) Estimation of titanium and iron in uranium by EDXRF using microdroplets on filter paper. J Radioanal Nucl Chem 152:251–259
Shrivastav P, Menon SK, Agrawal YK (2001) Selective extraction and inductively coupled plasma atomic emission spectrophotometric determination of thorium using chromogenic crown ether. J Radioanal Nucl Chem 250:459–464
Freitas MC, Hipolito CS (2007) NAA and PIXE for the determination of the contents of extractable sediment. J Radioanal Nucl Chem 271:179–183
Fujino O, Umetani S, Uenoa E, Shigeta K, Matsuda T (2000) Determination of uranium and thorium in apatite minerals by inductively coupled plasma atomic emission spectrometry with solvent extraction separation into diisobutyl ketone. Anal Chim Acta 420:65–71
Tomé FV, Blanco Rodríguez MP, Lozano JC (2001) Study of the representativity of Uranium and Thorium assays in soil and sediment samples by alpha spectrometry. Appl Radiat Isot 56:393–398
Nakashima T, Taketatsu YT (1992) Determination of uranium(VI) in seawater by ion-exchanger phase absorptiometry with arsenazo-III. Talanta 39:523–527
Gupta KK, Kulkarni PG, Singh RK (1993) Spectrophotometric determination of uranium using ascorbic acid as a chromogenic reagent. Talanta 40:507–510
Hirano Y, Ogawa Y, Ogama K (2003) Simultaneous spectrophotometric determination of uranium and thorium by flow injection analysis using selective masking. Anal Sci 19:303–307
Suresh A, Patre DK, Srinivasan TG, Vasudeva Rao PR (2002) A new procedure for the spectrophotometric determination of uranium(VI) in the presence of a large excess of thorium(IV). Spectrochim Acta Part A 58:341–347
Abbas MN, Homoda AM, Mostafa GAE (2001) First derivative spectrophotometric determination of uranium(VI) and vanadium(V) in natural and saline waters and some synthetic matrices using PAR and cetylpyridinum chloride. Anal Chim Acta 436:223–231
Alyapyshev MY, Babain VA, Antonov NG, Smirnov IV (2006) Extraction of Americium and Europium from perchloric acid solutions with N, N-Dialkyl and N, N, N, N-Tetraalkylpyridine-2,6-dicarboxamides. Russ J Appl Chem 79:1808–1835
Alyapyshev MY, Babain VA, Smirnov IV, Shadrin AY (2006) Separation of americium and europium from solutions of nitric and perchloric acid using dipicolinic acid diamides. Czch J Phys 56:D469–D475
Biswas S, Pathak PN, Singh DK, Roy SB, Manchanda VK (2010) Synergistic extraction of uranium with mixtures of PC88A and neutral oxodonors. J Radioanal Nucl Chem 284:13–19
Rajeshawari B, Dhavale BA, Bangia TR, Mathur JN, Page AG (2002) Role of Cyanex-272 as an extractant for uranium in the determination of rare earths by ICP-AES. J Radioanal Nucl Chem 254:479–483
Marckzenko Z (1976) Spectrophotometric determination of trace elements, 1st edn. Ellis Hardwood Ltd., John Wiley and Sons, Chichester
Bosch NA, Petrukhin OM (1978) 2-octylaminopyridine a new extractant. Zh Anal Chim 33:1805
Sandell EB (1965) Colorimetric determination of traces of metals, 3rd edn. Interscience Publishers Inc, New York
Flaschka HA, Bernard AJ (1972) Chelates in analytical chemistry of elements, vol 4. Marcel Dekker Inc, New York, p 140
Anuse MA, Chavan MB (1984) Studies on extraction separation of platinum metals and gold(III) with pyrimidine thiol. Spectrophotometric determination of palladium(II), osmium(VIII) and ruthenium(III). Chem Anal (Warsaw) 29:409–420
Acknowledgements
The author would like to thanks to UGC for providing teacher fellowship for completion of this work and special thanks to UGC-SAP-DRS-II and DST-FIST level-I for providing instrumentation facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kore, G.D., Zanje, S.B., Kokare, A.N. et al. Liquid–liquid extraction of uranium(VI) from weak sodium acetate medium using 2-octylaminopyridine: real sample analysis. J Radioanal Nucl Chem 329, 975–982 (2021). https://doi.org/10.1007/s10967-021-07828-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07828-3